Abstract
In Drosophila, intestinal stem cells (ISCs) respond to oxidative challenges and inflammation by increasing proliferation rates. This phenotype is part of a regenerative response, but can lead to hyperproliferation and epithelial degeneration in the aging animal. Here we show that Nrf2, a master regulator of the cellular redox state, specifically controls the proliferative activity of ISCs, promoting intestinal homeostasis. We find that Nrf2 is constitutively active in ISCs, and that repression of Nrf2 by its negative regulator Keap1 is required for ISC proliferation. We further show that Nrf2 and Keap1 exert this function in ISCs by regulating the intracellular redox balance. Accordingly, loss of Nrf2 in ISCs causes accumulation of reactive oxygen species and accelerates age-related degeneration of the intestinal epithelium. Our findings establish Keap1 and Nrf2 as a critical redox management system that regulates stem cell function in high-turnover tissues.
Introduction
A low intracellular concentration of reactive oxygen species (ROS) is emerging as a critical condition for stemness and pluripotency in neuronal and glial progenitors, in vertebrate hematopoietic stem cells (HSCs), as well as in Drosophila hematopoietic progenitors (Ito et al., 2004; Liu et al., 2009; Owusu-Ansah and Banerjee, 2009; Smith et al., 2000; Tothova et al., 2007; Tsatmali et al., 2005). In mice, loss of the cytoprotective transcription factor Foxo in HSCs results in elevated ROS levels, hyper-proliferation, and reduced long-term regenerative potential (Miyamoto et al., 2007; Miyamoto et al., 2008; Tothova and Gilliland, 2007; Tothova et al., 2007). Similarly, self-renewal is negatively influenced by elevated ROS in rat oligodendrocyte-type-2 astrocyte progenitor cells (Smith et al., 2000). In Drosophila, increased ROS concentration is observed endogenously in hematopoietic progenitors of the larval lymphgland, and this signal is required to prime these cells for differentiation (Owusu-Ansah and Banerjee, 2009). In addition to preserving general cell function by preventing cellular damage, a reducing intracellular environment is thus increasingly recognized to be critical for stem cell pluripotency and self-renewal, and to influence regulatory decisions in stem cells. How the intracellular redox balance is controlled in stem cells to regulate or modulate regenerative processes remains largely unknown.
Drosophila ISCs provide a reservoir for regeneration in the posterior midgut epithelium, where they give rise to enteroblasts (EBs), which in turn differentiate into either enterocytes (ECs) or enteroendocrine cells (EEs) (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). ISCs are the only cells in the adult Drosophila posterior midgut epithelium that are competent to undergo mitosis. The rate of proliferation in the intestinal epithelium is highly variable and is increased in response to a variety of signals, including oxidative challenges, activation of Jun-N-terminal Kinase (JNK), Jak/Stat signaling, as well as insulin and Wnt signaling (Amcheslavsky et al., 2009; Biteau et al., 2008; Buchon et al., 2009; Choi et al., 2008; Lee et al., 2009b; Lin et al., 2008). Stimulation of ISC proliferation represents the first step in a regenerative response that is required to recover intestinal integrity after a challenge (Biteau et al., 2008; Jiang et al., 2009). However, this process needs to be tightly regulated to ensure intestinal homeostasis, as excessive proliferation in the gut can result in disruption of the intestinal epithelium by the accumulation of misdifferentiated ISC daughter cells, as observed in conditions of excessive or chronic stress and in aging animals (Amcheslavsky et al., 2009; Biteau et al., 2008; Choi et al., 2008).
Modulation of the redox balance in the intestinal epithelium has significant consequences for ISC proliferation. Increased proliferation is observed in response to treatment with the ROS-inducing compound Paraquat (Biteau et al., 2008) (see also Figure 2D and Supplementary Information, Figure S2), as well as in mutants for the ROS detoxifying enzyme catalase (Choi et al., 2008), while treating flies with N-Acetyl-Cysteine and Glutathione is sufficient to limit ISC proliferation (Buchon et al., 2009). These observations suggest that, as in vertebrate stem cells, the intracellular redox balance of ISCs influences regenerative capacity. Little is known about mechanisms that might regulate this balance in ISCs endogenously.
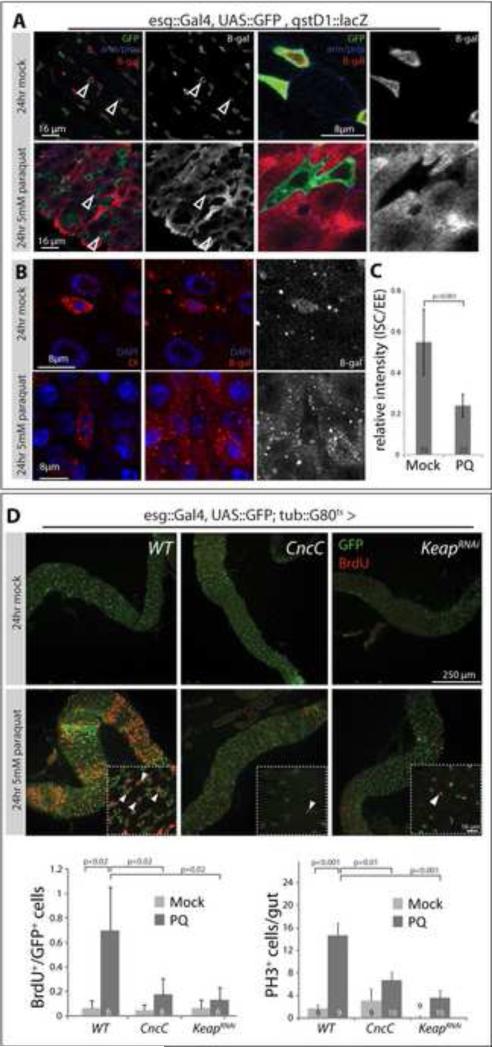
Figure 2. ISC-specific regulation of Nrf2/CncC controls ISC proliferation in response to stress.
A: gstD1::lacZ expression is repressed in esgGFP+ cells in response to oxidative stress. As shown in Figure 1, β-Galactosidase is expressed in ISCs and EBs of mock-treated gstD1::lacZ carrying flies (top panels; GFP, green; armadillo/prospero, blue; β-gal, red). After Paraquat exposure (5mM, 24 hours), β-Gal is strongly expressed in ECs, but cannot be detected in esgGFP+ cells (bottom panels, arrowheads). Overviews are shown left, higher magnification on the right. Single confocal sections are shown.
B: Intestines treated as in A, co-stained for the ISC marker delta (red, left panels) and β-gal (red, middle and right panels). Dl+ cells are also β-gal+ in mock treated intestines (top panels), but become β-gal- in response to paraquat (lower panels). See Figure S3 for more examples.
C: Quantification of relative β-gal fluorescence intensity of ISCs compared to EEs in mock and paraquat treated intestines. Note that gstD1::lacZ expression in EEs is not affected by Paraquat (Figure S3A). Only Dl+ cells were analyzed. Averages and standard deviation, Student's T-test.
D: BrdU incorporation in intestines of paraquat - treated flies expressing CncC or Keap1RNAi in ISCs and EBs using the TARGET system (transgene expression was induced by shifting flies to 29°C for 3 days prior to paraquat exposure). GFP (green), BrdU (red). Mock treated flies incorporate BrdU in very few cells during a 24hour exposure (upper panels, see also Figure S2). In response to Paraquat exposure, BrdU is widely incorporated in wild-type flies (WT), but not in flies expressing CncC or KeapRNAi (lower panels). A single confocal section is shown in each panel. Arrowheads point to selected BrdU+ cells. Frequency of BrdU incorporation (left; calculated as the fraction of GFP+ cells that are also BrdU+; averages and standard deviation, Student's T-test) and amounts of pH3+ cells per gut (right; averages and SEM; Student's T-test) are shown in the histograms (N, numbers of analyzed intestines, are shown in the bars).
See also Figure S2.
A central regulator of the intracellular redox state in vertebrates and invertebrates is Nrf2, a member of the ‘cap-and-collar’ (Cnc) family of transcription factors. Nrf2 counteracts excessive ROS accumulation in cells by inducing genes encoding key antioxidant molecules, such as enzymes involved in glutathione (e.g. Gamma-glutamyl-cysteine-ligase catalytic subunit, gclc) and thioredoxin metabolism (Hayes and McMahon, 2009; Lee et al., 2005; McMahon et al., 2001; Motohashi et al., 2002), and antioxidant enzymes such as Peroxiredoxins (Lee et al., 2005; Maher and Yamamoto, 2010; Motohashi et al., 2002). This function of Nrf2 can significantly influence overall stress sensitivity and lifespan of the animal. Thus, oxidative stress tolerance and lifespan are increased in gain-of-function conditions for the Drosophila homologue of Nrf2, CncC, and for the C. elegans homologue SKN1 (Inoue et al., 2005; Sykiotis and Bohmann, 2008). It has been proposed that SKN1 increases tissue homeostasis and extends lifespan by promoting a germcell - like environment in somatic cells (Curran et al., 2009).
In vertebrates and in Drosophila, Nrf2 is negatively regulated by the cytoplasmic repressor Kelch-like ECH-associated protein 1 (Keap1) (Hayes and McMahon, 2009; Nguyen et al., 2009; Sykiotis and Bohmann, 2008; Toledano, 2009). Keap1 acts as an adaptor of a Cul3-ubiquitin ligase complex that promotes Nrf2 degradation. Accordingly, loss of Keap1 results in lifespan extension and increased oxidative stress tolerance in flies (Sykiotis and Bohmann, 2008). Interestingly, Keap1 mutant mice show significant hyperkeratosis of the esophageal epithelium, suggesting that Keap1/Nrf2 influences cell differentiation and proliferation in intestinal epithelia (Wakabayashi et al., 2003). This function is reminiscent of the requirement for SKN1 in intestinal development in C.elegans (An and Blackwell, 2003).
Here we show that Keap1 and CncC regulate ISC proliferation rates in the posterior midgut epithelium of Drosophila by influencing the intracellular redox state, and that this regulation is required to limit ISC hyperproliferation and intestinal degeneration in aging flies. We find that CncC is constitutively active in ISCs of young, unchallenged flies, and is repressed in response to oxidative stress, a response that is opposite to the well-known stress-induced activation of Nrf2-like proteins in differentiated cells. Strikingly, Keap1-mediated repression of CncC is required for ISC proliferation, while constitutive expression of CncC inhibits stress and mitogen-induced proliferation of ISCs in a reversible manner. Using in vivo imaging, we show that loss of Keap1, or over-expression of CncC, decreases intracellular ROS levels in ISCs, and that CncC is required to maintain low ROS levels in resting ISCs. We further show that this anti-oxidant function of CncC is required to limit ISC proliferation rates. Our results establish the Keap1/CncC regulatory module as central in the control of ISC proliferation and intestinal regeneration and further highlight the importance of intracellular redox control in the biology of somatic stem cells.
Results
Keap1 and CncC regulate proliferation of ISCs
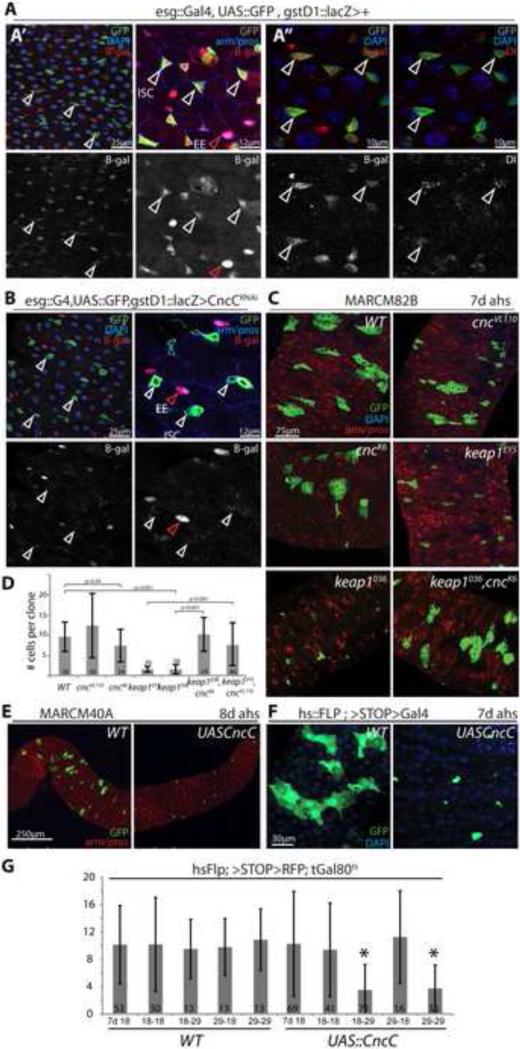
To test whether Nrf2/CncC, as a major regulator of the cellular redox milieu, might influence proliferation and differentiation of ISCs, we assessed CncC activity in the posterior midgut using a specific lacZ reporter based on the upstream regulatory sequences of the CncC target gene gstD1 (gstD1::lacZ (Sykiotis and Bohmann, 2008)). Interestingly, we found that under homeostatic conditions, i.e. in the absence of external stress, gstD1::lacZ was expressed highly in ISCs and EEs (Figure 1A; ISCs were labeled by escargot::Gal4 driven GFP (A’A”), which labels both ISCs and EBs, and further identified by immunostaining against the ISC marker Delta (Dl; A”); EEs were identified by immunostaining against the EE marker prospero (pros, A’)). The expression of gstD1::lacZ in ISCs was dependent on CncC gene function, as it was suppressed when CncC was knocked down using a UAS::CncCRNAi transgene (Sykiotis and Bohmann, 2008) (Figure 1B). To ask whether the activity of CncC in ISCs is required for ISC function, we generated GFP-marked stem cell clones homozygous for the cnc loss-of-function alleles cncVL110 (a deletion covering the cnc locus and disrupting all Cnc isoforms (Sykiotis and Bohmann, 2008; Veraksa et al., 2000)) and cncK6 (a point mutation affecting the CncC isoform, which is the only Cnc isoform that interacts with Keap1 (Sykiotis and Bohmann, 2008; Veraksa et al., 2000)) by somatic recombination using the MARCM system (Lee and Luo, 2001) (Figure 1C,D). Similarly, we induced clones homozygous for the keap1 null-alleles keap1EY5 and keap1036 (Sykiotis and Bohmann, 2008; Veraksa et al., 2000), thus increasing endogenous CncC activity. Surprisingly, cnc mutant ISC cell clones grew at the same rate as wild-type clones, and showed no obvious differentiation defects of ISC daughter cells (the same ratio, 20%, of clones derived from cncVL110 homozygous or wild-type ISCs contained 1 to 2 pros+ entero-endocrine cells), suggesting that CncC activity is dispensable for ISC proliferation and EB differentiation (the same phenotype was observed when cncC activity was knocked down in clones using CncCRNAi (Supplementary information, Figure S1). keap1 mutant clones, however, grew significantly slower than wild-type clones, indicating that repression of Cnc activity by Keap1 is required for ISC division. This interpretation is supported by the fact that loss of cncC was sufficient to rescue the growth deficiency of keap1 mutant clones (in keap1/cnc double-mutant clones; Figure 1C, D). We confirmed that increased CncC activity inhibits ISC proliferation using either the MARCM system (Figure 1E) or a FLP-out strategy (Theodosiou and Xu, 1998) (Figure 1F) to generate GFP-marked ISC clones of CncC over-expressing cells (by over-expression of a UAS::CncC transgene (Sykiotis and Bohmann, 2008)). Similar to keap1 mutant cell clones, CncC over-expressing clones induced using either strategy grew slower compared to wild-type controls (Figure 1E, F). Interestingly, this CncC-mediated repression of ISC proliferation is reversible: using conditional expression of CncC in ISC clones (achieved by co-expression of the temperature-sensitive Gal4 inhibitor Gal80ts (McGuire et al., 2003)), we found that flies that were maintained at the restrictive temperature throughout the experiment (29°C; thus over-expressing CncC in ISCs for 14 days), exhibited markedly reduced clone growth, while growth recovered in animals that were maintained at the restrictive temperature for one week, and then shifted to the permissive temperature (18°C, restoring wild-type levels of CncC expression after one week; Figure 1G). Similarly, clones in flies carrying the UAS::CncC transgene grew to about 10 cells when flies were maintained at 18°C for 7 days after heat shock (Figure 1G, 7d 18), but became smaller when flies were shifted to 29°C for another 7 days, while clones in corresponding wild-type controls maintained an average size of 10 cells (Figure 1G, 18-29; note that EC turnover time is about 7 days; (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006)). The CncC/Keap1 complex thus acts as a reversible “switch” to regulate ISC proliferation, without permanently impairing ISC function. Importantly, CncC over-expression does not affect the expression of ISC-markers such as Delta, further confirming that ISC identity is not affected by CncC gain-of-function conditions (Supplementary Information, Figure S1). Moreover, CncC does not affect the growth of clones in imaginal discs of developing larvae (Supplementary Information, Figure S1), indicating that the anti-mitotic function of CncC is specific to stem cells and confirming that CncC does not simply impair cell function.
Figure 1. Keap-mediated repression of CncC activity is required for ISC proliferation.
A: Intestines of flies carrying the gstD::lacZ reporter and esg::Gal4, UAS::GFP (ISC/EB marker; green). A’: Intestines are stained for β-galactosidase (β-gal, red) and DNA (DAPI, blue) or armadillo and the enteroendocrine (EE) marker prospero (arm/pros, blue). β-gal channel is shown separately in the lower panels. White arrowheads indicate ISCs, red arrowheads point to EEs. β-Gal is expressed in esgGFP+ cells and EEs in wild-type flies. A”: Intestines are stained for β-gal (red), the ISC marker delta (red), and DNA (DAPI, blue) as indicated. Lower panels show β-gal and delta separately. Note co-localization of β-gal and Dl expression, indicating that CncC activity is present in ISCs.
B: gstD::lacZ reporter activity (β-gal) is absent from CncCRNAi - expressing ISCs. Note that β-gal expression in EEs is not affected by esg-mediated expression of CncCRNAi. GFP, green; β-gal, red; DAPI or arm/pros, blue.
C: GFP-marked (MARCM) ISC clones mutant for cncVL110; cncK6; keap1EY5; keap1036; and keap1036, cncK6 at 7 days after heat shock. Single confocal sections: GFP, green; arm/pros, red; DAPI, blue.
D: Quantification of clone sizes at 7 days after heat shock (averages and standard deviations of numbers of cells per clone; Student's T-test).
E: MARCM clones over-expressing only GFP (left, WT) or GFP and CncC (right). Intestines were dissected 8 days after heat-shock. GFP, green; arm/pros, red.
F: “Flp-out” clones over-expressing CncC at 7 days after heat-shock (GFP, green; DAPI, blue).
G: Inhibition of ISC proliferation by CncC is reversible. CncC over-expressing clones were induced using the Flp-out system in the presence of the temperature-sensitive Gal4 inhibitor Gal80ts. After heat-shock, flies were maintained at the permissive (18°C) or the restrictive (29°C) temperatures for 14 days (18-18, 29-29, respectively) or switched from one to the other temperature after 7 days (18-29, 29-18). Intestines were dissected at 7d (7d 18) or after 14 days (all others). In wild-type control flies, the restrictive temperature does not affect clone growth (at 7 or 14 days after heat shock), while in CncC over-expressing flies incubation at 29°C for 14 days inhibits growth (only in these flies, CncC is over-expressed throughout the experiment). In CncC over-expressing flies maintained at 29°C for 7 days (in which clones fail to grow, F), but then shifted to 18°C (29-18), clone size recovers, indicating that CncC-mediated repression of ISC proliferation can be reversed. Conversely, clone size decreases in CncC over-expressing flies maintained at 18°C for 7days and then transferred to 29°C (18-29). Averages and standard deviations of clone cell numbers are plotted; *p<0.001, Student's T-test. Numbers of clones assessed (n) is shown as insert in each bar.
See also Figure S1.
Stress-mediated induction of ISC proliferation requires repression of CncC
The data described above suggest that high levels of CncC activity might limit ISC proliferation in response to stress and growth factors, and that, to initiate a proliferative response to stress, CncC activity has to be inhibited. To test this idea, we assessed the activity of CncC in animals exposed to Paraquat, which induces widespread activation of ISC proliferation (Figure 2, see also Biteau et al., 2008). As expected based on the Nrf2 response in other differentiated cells and tissues, gstD1::lacZ is widely induced in ECs under these conditions (Figure 2A). Strikingly, however, gstD1::lacZ is strongly repressed in esg+ cells, suggesting that CncC is inactivated in ISCs and EBs in response to oxidative stress (Figure 2A). To assess CncC activity in ISCs specifically, we quantified b-Gal fluorescence intensity in esg+/Dl+ ISCs relative to esg-/pros+ EEs in both mock and Paraquat-treated flies, confirming that CncC activity is reduced in ISCs of stressed intestines (Figure 2B, C and Supplementary information, Figure S3; note that gstD1::lacZ expression in EEs remains high in challenged flies, Supplementary information, Figure S3A). This “reverse” response of Nrf2/CncC to stress is unique to ISCs and EBs among the different intestinal cell types of the fly, and has, to our knowledge, not been described before in other systems.
We tested whether this repression of CncC might be required for the stress-induced proliferation of ISCs by asking whether sustained CncC expression or loss of Keap1 would be sufficient to dominantly suppress ISC proliferation. We used esg::Gal4 in combination with Gal80ts to alter the expression of Keap1 or CncC in ISCs and EBs in an inducible manner (Figure 2D; we suppressed Keap1 using a UAS::Keap1RNAi transgene; expression of this construct reduces Keap1 expression in the gut, supplementary information, Figure S2B, and results in increased endogenous CncC activity (Sykiotis and Bohmann, 2008)). We determined ISC proliferation rates in the intestinal epithelium by assessing BrdU incorporation as well as the frequency of pH3+ cells. While BrdU marks cells undergoing S-phase, but is also incorporated in endoreplicating EBs, pH3 is only detected in mitotic cells. In the posterior midgut epithelium of Drosophila, only ISCs undergo mitosis: in lineage - traced ISC clones, only one or (when just concluding Mitosis) two pH3+ nuclei are found regardless of the experimental condition, confirming the absence of transit amplifying cells in this lineage (Supplementary information, Figure S8. See also Ohlstein and Spradling, 2007). Young, unstressed wild-type animals exhibit very low proliferation rates in the intestinal epithelium (less than 10% of all esg-GFP+ cells incorporate BrdU within 24 hours, and only about 2-3 pH3+ cells can typically be found; Figure 2D). In flies exposed to Paraquat, BrdU is widely incorporated in esg+ cells (within 24 hours, BrdU is incorporated in between 60 and 100% of all esg+ cells, representing both proliferating ISCs and endoreplicating EBs), and significantly increased numbers of pH3+ cells are detected in each intestine (Figure 2D). Strikingly, when CncC expression was induced before Paraquat exposure, this stress-induced proliferation was inhibited (Figure 2D, and Supplementary Information, Figure S2). The same result was obtained when keap1 transcript levels were reduced, suggesting that Keap1 is required for CncC repression in response to stress and that sustained activity of endogenous CncC is incompatible with ISC division under stress conditions (Figure 2D).
CncC controls ISC proliferation in a variety of mitogenic conditions
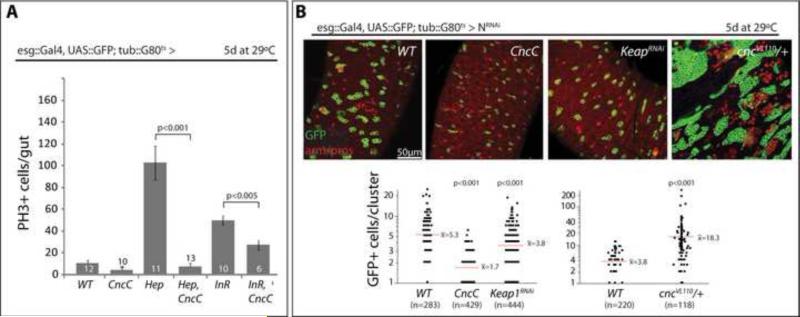
To explore how general the inhibition of ISC proliferation by CncC is, we asked whether CncC would influence ISC proliferation induced by JNK and Insulin signaling, by exposure to bacterial antigens, as well as ISC and EE hyperplasia in Notch signaling loss-of-function conditions (Figure 3). Activation of the JNK pathway by expression of the JNK Kinase Hemipterous, Hep, under the control of esg::Gal4 is sufficient to initiate widespread ISC divisions in the intestinal epithelium (Biteau et al., 2008; Buchon et al., 2009). Similarly, over-expression of the insulin receptor (InR) can promote accelerated cell divisions in this lineage (Amcheslavsky et al., 2009). Exposure to enteropathogenic bacteria, such as Serratia Marcescens, also stimulates ISC proliferation (Buchon et al., 2009), a condition that can be replicated by feeding Lipopolysaccharides (LPS) from S. Marcescens (Supplemental information, Figure S4). In all three conditions, co-expression of CncC was sufficient to inhibit ISC proliferation (Figure 3A, Supplemental information, Figure S4), revealing a general and dominant anti-mitotic function of CncC in stem cells. Loss of Notch signaling in ISCs and EBs perturbs EB differentiation, resulting in the progressive accumulation of ISC and EE tumors in the intestinal epithelium (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2007). Loss of Keap1 (by expression of KeapRNAi) or over-expression of CncC was sufficient to limit ISC tumor growth in Notch loss-of-function conditions (Figure 3B, Notch was suppressed by expression of NotchRNAi). In cncVL110 heterozygous conditions, on the other hand, Notch-mutant tumor growth was significantly increased (Figure 3B).
Figure 3. CncC inhibits ISC proliferation in response to mitogenic signaling and in Notch loss-of-function conditions.
A: CncC represses JNK- and InR- induced ISC proliferation. Quantification of pH3+ cells in intestines expressing CncC, InR and/or the JNKK Hep in ISCs and EBs using the TARGET system. Intestines were analyzed 5 days after shift to the restrictive temperature (Average and SEM; Student's T-test; N shown in each bar).
B: Expression of CncC or repression of Keap (Keap1RNAi) limits growth of ISC and ee tumors, while reduced cnc genedose (cncVL110 heterozygosity) enhances tumor growth in Notch loss-of-function conditions. Expression of NRNAi and other transgenes was induced by shifting flies to restrictive temperature (29°C). Intestines were analyzed 5 days after induction. Representative intestines are shown (GFP, green and armadillo/prospero, red). Quantification of tumor sizes is shown below.
See also Figure S3.
Regulation of ISC redox state by Keap1 and CncC
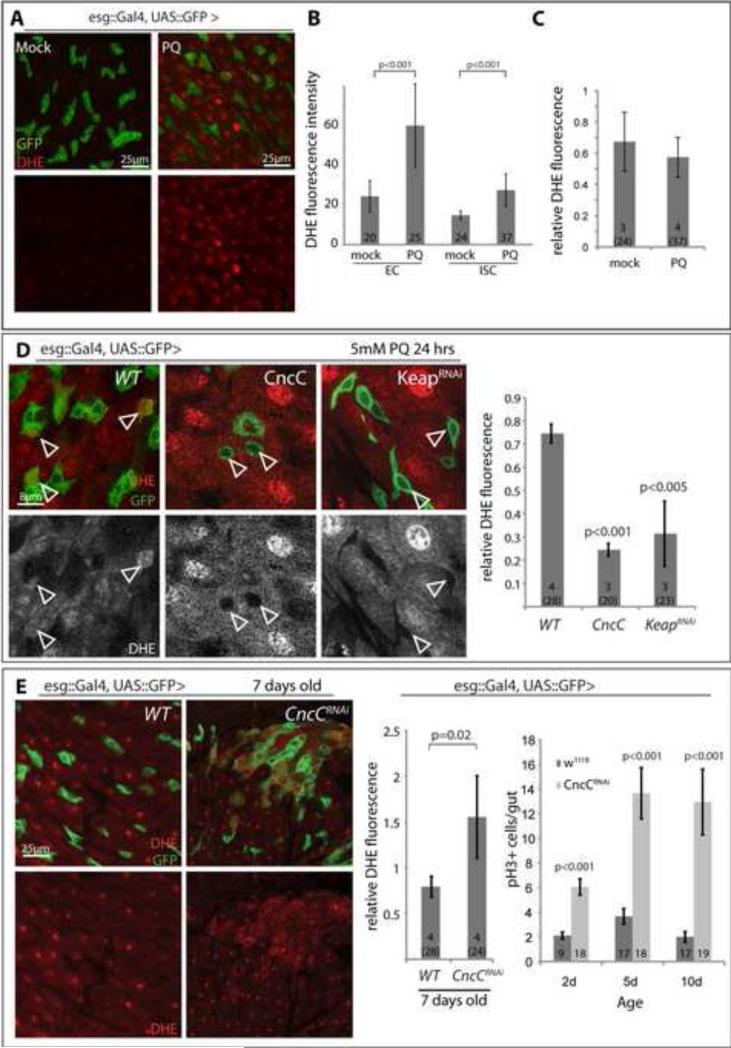
Control of ISC proliferation by Keap1 and CncC is thus critical to restrain ISC proliferation in a variety of stress and mitogenic contexts. We hypothesized that this function of Keap1 and CncC might be mediated by their regulation of the intracellular redox state. To test this idea, and to analyze the effect of Keap1 and CncC on the redox state in ISCs specifically, we monitored endogenous ROS levels in vivo using Dihydro-Ethidium (DHE), a redox sensitive dye that exhibits increased fluorescence intensity when oxidized (Owusu-Ansah et al., 2008). When flies are fed paraquat, increased DHE fluorescence is observed throughout the intestinal epithelium, including ECs and ISCs (Figure 4A-C and Supplementary Information, Figure S5A). Elevated CncC activity in ISCs strongly prevented Paraquat-induced oxidation of ISCs (Figure 4D, and Supplementary Information, Figure S5C), while loss of CncC (by expressing CncCRNAi under the control of esg::Gal4) increased ROS levels in ISCs of unchallenged flies (Figure 4E). This increased oxidation of ISCs in CncC loss of function conditions was accompanied by elevated proliferation rates, suggesting that increasing intracellular ROS levels is sufficient to promote ISC proliferation and that CncC controls ISC proliferation by maintaining a reduced intracellular environment (Figure 4E). Interestingly, these results contrast with the observation that proliferation rates of ISCs did not change when cncC was lost by somatic recombination in MARCM clones (Figure 1C, D). An important difference between the two experimental approaches is that somatic recombination occurs in G2 of the cell cycle, and cncC is thus only lost in cells that are actively cycling (and thus already have reduced levels of cnc activity) when Flp recombinase is expressed, while esg::Gal4 induces expression of CncCRNAi in all (including resting) ISCs of the intestinal epithelium. Further illustrating this distinction, reduction of cnc activity in all cells by cncVL110 heterozygosity is sufficient to cause increased ISC proliferation early in life (Supplementary information, Figure S5D). CncC thus appears to be required in resting ISCs to prevent entry into the cell cycle, maintaining a state of proliferative quiescence, but is dispensable once ISCs are actively proliferating.
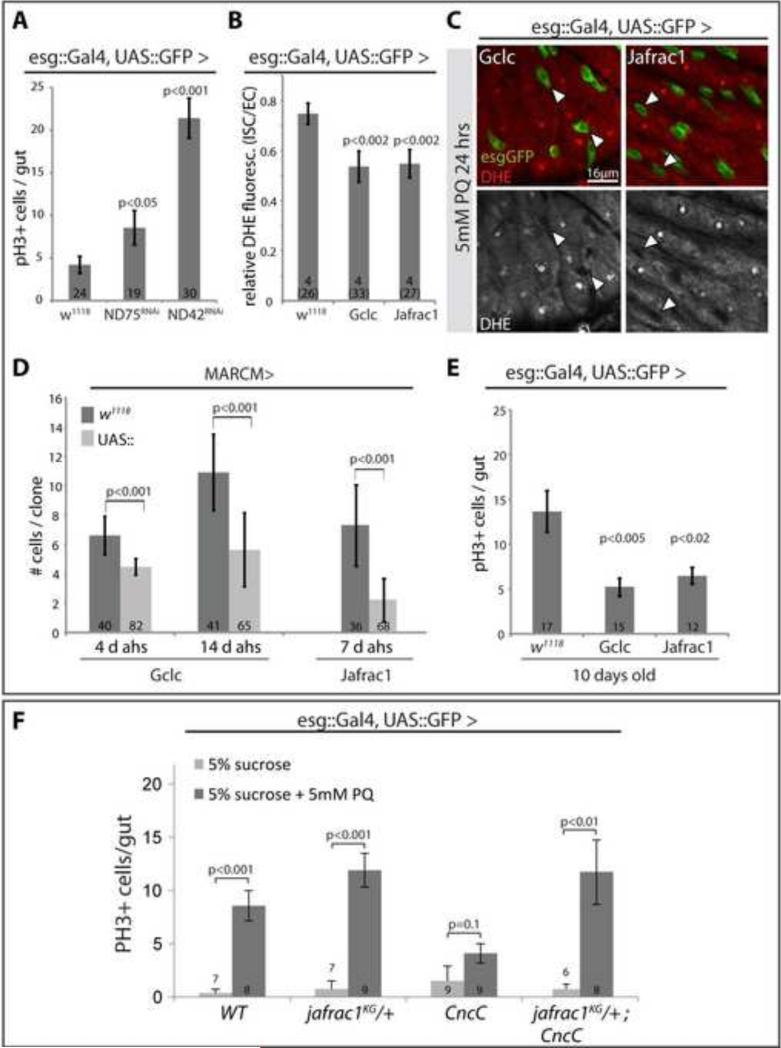
Figure 4. CncC influences the intracellular redox state of ISCs.
A-C: Di-hydro-ethidium (DHE) staining of live intestines of flies treated with Paraquat (PQ, 5mM in 5% sucrose) or mock (5% sucrose) for 24 hrs. Representative images are shown in A (single confocal section (GFP, green; DHE, red; DHE channel is shown alone in lower panels). B: Quantification of fluorescence intensity of the DHE channel (Average and standard deviation; Student's T-test; N shown in each bar). Note that fluorescence intensities increase in both ISCs and ECs after paraquat treatment. C: relative DHE fluorescence in ISCs, calculated as ratio of fluorescence intensity in ISCs and nearby ECs. N=number of analyzed guts (total analyzed cells in parenthesis) shown in each bar.
D: DHE staining of PQ-treated intestines over-expressing CncC or Keap1RNAi in ISCs and EBs. DHE channel is separated in grayscale in lower panels. Images are from single confocal sections. Arrowheads point to individual ISCs for orientation (basal location in the epithelium, isolated esg+ cells in CncC or KeapRNAi expressing guts). Quantification of relative DHE fluorescence of ISCs compared to surrounding ECs is shown in the graph (Average and standard deviation; Student's T-test; N=number of analyzed guts (total analyzed cells in parenthesis) shown in each bar).
E: Loss of CncC results in increased ROS levels in the ISC lineage and increases ISC proliferation. DHE fluorescence in intestines from flies expressing CncCRNAi and GFP (CncCRNAi) or only GFP (w1118) under the control of esg::Gal4 (representative images left, quantification middle). Fluorescence intensity in GFP+ cells is normalized to DHE fluorescence in adjacent ECs. (Average and standard deviation; Student's T-test; N=number of analyzed guts (total analyzed cells in parenthesis) shown in each bar).
Quantification of pH3+ cells in intestines of 2, 5, and 10 day-old flies is shown in the histogram on the right (average and SEM; Student's T-test; N shown in each bar).
See also Figure S4.
The intracellular redox state influences proliferation of ISCs
To test further whether CncC regulates ISCs proliferation through its control of the intracellular redox state, and to ask whether changing the intracellular redox state directly would be sufficient to influence ISC proliferation, we knocked down components of Complex I of the mitochondrial electron transport chain in ISCs and EBs using esgGal4. Knock-down of ND42 and ND75 using RNAi constructs is sufficient to significantly increase the intracellular ROS concentration (Owusu-Ansah et al., 2008). Supporting the notion that increased intracellular ROS promotes ISC proliferation, expression of ND42RNAi and ND75RNAi stimulated ISC proliferation significantly (Figure 5A). To promote a reducing environment in ISCs and EBs, we further over-expressed selected anti-oxidant genes using esg::Gal4 as well as the MARCM system, and assessed the frequency of mitotic cells in the intestine as well as clonal growth rates under homeostatic (unstressed) conditions: Gamma-glutamyl-cysteine-ligase catalytic subunit (gclc) is the rate-limiting enzyme for glutathione biosynthesis, reducing the intracellular environment, and increasing stress tolerance and lifespan of flies (Orr et al., 2005), while jafrac1 (also called peroxiredoxin 4783 or thioredoxin peroxidase 1) encodes a thioredoxin peroxidase that protects cells from oxidative stress (Lee et al., 2009a; Radyuk et al., 2003). Gclc is regulated by an antioxidant response element (ARE), to which CncC transcription factor complexes bind in vivo and in vitro (Rahman and Bohmann, in preparation), and jafrac1 expression can be stimulated by over-expression of CncC in the gut (Supplementary Information, Figure S6). Over-expression of these genes resulted in markedly reduced ROS levels in ISCs even under stress conditions (Figure 5B, C). Strikingly, and reminiscent of the effects of CncC activation, this reduced state correlates with decreased ISC proliferation, as over-expression of gclc or jafrac1 in ISCs results in delayed growth of stem cell clones (Figure 5D). Similarly, the age-associated increase in ISC proliferation (Biteau et al., 2008) is delayed when gclc and jafrac1 gene expression is increased in ISCs, as significantly less frequent mitotic figures are observed in the gut at 10 days of age (Figure 5E, see also Biteau et al., 2010). Combined, these results suggest that promoting a reduced intracellular environment is sufficient to limit ISC proliferation. We further tested whether the CncC-mediated reduction in the intracellular ROS concentration is also required for repression of ISC proliferation by assessing the ability of CncC to inhibit stress-induced proliferation in a jafrac1 mutant background (Figure 5F). Supporting a requirement for redox control downstream of CncC, over-expression of CncC was not sufficient to limit ISC proliferation when jafrac1 gene dose was reduced (Figure 5F).
Figure 5. Regulation of ISC proliferation by modulating the intracellular redox state.
A: Increased ISC proliferation in response to loss of mitochondrial electron transport chain components (NADH:ubiquinone reductase 75kD and 42kD subunit precursors; ND75RNAi and ND42RNAi). PH3+ cells per gut are quantified (Average and SEM, Student's T-test, N in bars).
B, C: Representative images and quantification of DHE fluorescence in intestines over-expressing Gclc or Jafrac1 in ISCs (7 day old flies, Average and SEM, Student's T-test, N in bars; esgGFP, green; DHE, red).
D: Cell numbers in MARCM clones over-expressing Gclc or Jafrac1 quantified at 4 and 14 days, or at 7 days after heat shock (average cell numbers and standard deviations; Student's T-test).
E: Quantification of mitotic figures in intestines of 10 day-old flies over-expressing Gclc or Jafrac1 under the control of esgG4::UASGFP (Average and SEM, Student's T-test).
F: Cnc-mediated repression of ISC proliferation in response to Paraquat exposure requires jafrac1 gene function. CncC was over-expressed under the control of esgGal4 in wild-type or jafrac1KG05372 heterozygous backgrounds (Average and SEM, Student's T-test).
See also Figure S5.
CncC activity promotes proliferative homeostasis in aging intestinal epithelia
The constitutive activity of CncC in ISCs of unchallenged flies (as shown in Figure 1A) is thus likely required to maintain low levels of mitotic activity in young, healthy intestinal epithelia, ensuring epithelial homeostasis. We tested this hypothesis, asking whether CncC influences the age-associated loss of epithelial homeostasis observed in wild-type flies, where ISCs progressively over-proliferate in aging animals, causing the accumulation of misdifferentiated daughter cells and disruption of the apico-basal organization of the intestinal epithelium (Biteau et al., 2008, Biteau et al., 2010)(Figure 6). Interestingly, and consistent with the age-related increase in ISC proliferation rates, old intestinal epithelia exhibit significantly elevated ROS levels in both ECs and ISCs (Figure 6A, B, Supplementary Information, Figure S7). As in Paraquat-treated animals, this is accompanied by wide-spread activation of gstD1::lacZ expression in ECs, but repression of this CncC reporter in ISCs (Figure 6C – E; note that, in contrast to Paraquat treated guts, a small fraction of ISCs retain gstD1::lacZ expression, Figure 6C), suggesting that ISC-specific CncC activity is reduced in aging flies. To test if changing CncC expression levels in the ISC lineage is sufficient to influence the age-related loss of epithelial homeostasis, we aged flies expressing CncC or CncCRNAi, and compared the progression of intestinal degeneration in these flies and in wild-type control animals. We measured both the number of pH3+ cells in the gut, as well as the extent of intestinal degeneration within these populations, using a scoring system that allows classifying gut phenotypes according to the extent of accumulation of mis-differentiated esg+ cells (Figure 6F, G; the morphological classification and the amount of pH3+ cells in each gut correlate, suggesting that both measurements are accurate quantitative representations of age-related degeneration in the intestinal epithelium). Strikingly, aging flies expressing CncCRNAi in ISCs and EBs showed dramatic acceleration of intestinal degeneration, while intestinal morphology and low ISC proliferation rates were maintained throughout the experiment when CncC was over-expressed (Figure 6H). These findings suggest that CncC activity in ISCs is critical for long-term homeostasis in the intestinal epithelium.
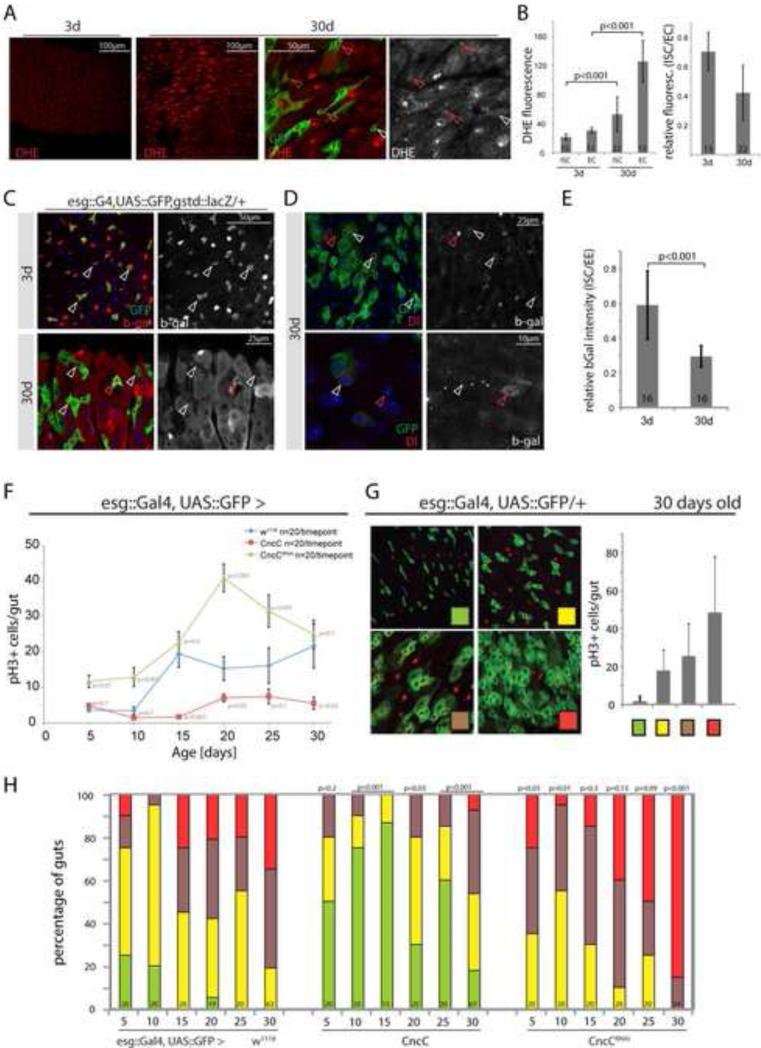
Figure 6. CncC/Nrf2 maintains intestinal homeostasis in aging flies.
A, B: Elevated ROS concentration in the intestinal epithelium of aging flies. Images (A) and quantification (B) of DHE fluorescence in intestines of young (3 day) and old (30 day) flies (DHE, red). Intestines were co-stained in the same well to ensure equal DHE staining, young and old guts were identified by GFP expression (3d: OreR, 30d: esg::G4;UAS::GFP; Average and standard deviation, Student's T-test, N in bars; GFP, green; DHE, red. Normalized fluorescence (ISC/EC ratio) is shown on the right. See Figure S7 for additional images.
C: Reporter activity in young (3 day) and old (30 day) flies (GFP, green; β-gal, red). gstD1::lacZ expression is reduced in most ISCs of old flies (white arrowheads). Infrequently, single esg-GFP+ cells expressing β-gal are detected (red arrowheads).
D: Reporter activity detected in 30-day-old flies counterstained with Dl antibody to identify ISCs (Dl, red, left panels; β-gal, red, right panels). White arrowheads point to ISCs for orientation, red arrowheads to EEs.
E: quantification of β-gal fluorescence intensity. As in D, ISCs detected as esg-GFP+, Dl+ cells. Average and standard deviation, Student's T-test, N in bars.
F: ISC proliferation in aging wild-type flies (esg::Gal4, UAS::GFP, blue) and in flies expressing CncC (red) or CncCRNAi (green) in ISCs and EBs. Flies were analyzed by counting PH3+ cells in the gut every 5 days. Data points represent averages +/- SEM.
G: Four categories based on the extent of accumulation of esg+ cell clusters used for quantification of intestinal degeneration (representative pictures). The four categories correlate with increasing numbers of PH3+ cells (graph on right).
H: Quantification of intestinal degeneration in aging flies (5-30 days of age) by blind scoring of individual intestines. In addition to containing fewer proliferating cells, guts with elevated levels of CncC in ISCs/EBs show significantly reduced intestinal degeneration compared to wild-types, whereas CncC knock-down in ISCs/EBs accelerates the progression of intestinal degeneration. P-values from Pearson χ2 test (compared to wild-types of same age), N shown in each bar.
See also Figure S6.
Discussion
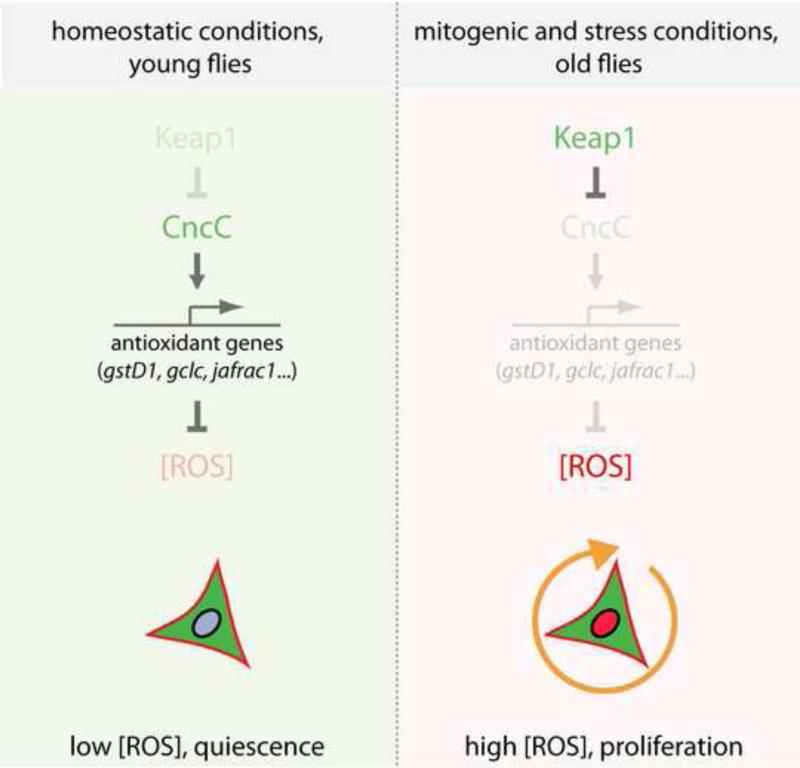
Our results establish Nrf2 and Keap1 as central components of a redox control mechanism that regulates stem cell proliferation in high turnover tissues (Figure 7). We propose that the Keap1/CncC regulatory complex establishes a switch that controls the rate of proliferation in the intestinal epithelium: while CncC is constitutively active in resting ISCs, maintaining low intracellular ROS levels and preventing excessive proliferation, ISC-mediated regeneration of differentiated cells requires repression of CncC function by Keap1. Since loss of CncC in all ISCs increases the number of proliferating cells in the intestine, but loss of CncC in already dividing ISCs (as in MARCM clones) does not influence the rate of ISC proliferation, CncC appears to maintain ISCs in a resting, quiescent state, preventing S-phase entry rather than influencing the speed of the cell cycle directly. Down-regulation of CncC in conditions that signal potential tissue damage therefore creates a permissive state in which ISCs can embark on an expansion, differentiation and regeneration program. A potential alternative explanation of these results could be that loss of cncC in esg+ cells (which include EBs) results in cell-non-autonomous induction of ISC proliferation by the cnc-deficient EB. Non-autonomous induction of ISC proliferation has been proposed in the proliferative response of ISCs following EC damage (Jiang et al., 2009). While such a mechanism cannot be fully ruled out, we believe that it is unlikely as an exclusive mechanism here, as a non-autonomous effect of CncC should also cause increased growth of cnc mutant clones. In such clones, mutant ISCs are in continuous close contact to cnc mutant EBs and ECs, and would thus be stimulated to proliferate faster if these mutant cells secreted mitogens. We have not observed such an effect. Furthermore, keap1 mutant ISCs do not form clones, remaining mostly as single cells unless cnc is also mutated, demonstrating that the keap1/cnc regulation of ISC proliferation has a significant cell-autonomous component. Supporting this interpretation, we find that CncC activity can be detected directly in ISCs and that it limits ROS accumulation in these cells.
Figure 7. Model for the role of Keap 1 and Nrf2/CncC in the control of ISC proliferation.
Nrf2/CncC induces anti-oxidant gene expression, promoting a reducing environment in ISCs that ensures cytoprotection and limits ISC proliferation. Keap1-mediated inhibition of CncC is critical to allow proliferation of ISCs in response to stress and mitogenic signaling. By limiting ISC proliferation, the Keap1/CncC signaling module thus balances the need for ISC-mediated tissue regeneration with the necessity for proliferation control, promoting proliferative homeostasis in the intestinal epithelium.
Our data further suggest that the regulation of ISC proliferation by CncC is mediated by its anti-oxidant function, since loss of CncC results in increased ROS accumulation, accompanied by enhanced proliferation, and since CncC-induced repression of ISC proliferation is prevented in a jafrac1 mutant background. These findings support the idea that the intracellular redox balance is a critical determinant of stem cell function in various systems (Ito et al., 2004; Liu et al., 2009; Owusu-Ansah and Banerjee, 2009; Smith et al., 2000; Tothova et al., 2007; Tsatmali et al., 2005) and suggest that this balance is dynamically regulated by Keap1 and Nrf2. The regulation of ISC proliferative activity by changes in the intracellular redox state is reminiscent of the regulation of differentiation in larval hematopoietic progenitors, where a transient increase in ROS concentration primes cells for differentiation (Owusu-Ansah and Banerjee, 2009), as well as of HSCs in mammals, where loss of antioxidant factors (such as Foxo) results in hyperproliferation and loss of regenerative capacity (Tothova et al., 2007). Interestingly, the ultimate consequence of changes in the redox milieu of stem cells appears to differ between ISCs, HSCs, and other stem cell populations. In several vertebrate stem cell lineages and in the Drosophila lymph gland, increases in ROS concentration “prime” stem and progenitor cells for differentiation (Ito et al., 2004; Owusu-Ansah and Banerjee, 2009; Smith et al., 2000; Tsatmali et al., 2005), while the same stimulus triggers ISCs to initiate proliferation. As mentioned above, however, Keap1 and CncC appear to control a shift from a resting, largely quiescent state of ISCs to a condition of active proliferation (Figure 7). The existence of defined “quiescent” and “active” states in ISCs is supported by the fact that young intestinal epithelia show very limited proliferative activity, while clonal analysis shows that once ISCs are actively proliferating, they rarely stop dividing (the size of ISC clones increases linearly before reaching a plateau where EC attrition and ISC proliferation are in equilibrium)(Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). Repression of CncC by Keap1 thus seems to “prime” resting ISCs for proliferation, suggesting that the regulation of stem cell function by the intracellular redox milieu is a widely shared, evolutionarily conserved phenomenon.
The molecular basis for the control of stem cell function by a reducing environment is not clear, but findings in other systems suggest that ROS accumulation is critical for signal transduction and signal stabilization in growth factor signaling pathways (Bashan et al., 2009). Thus, receptor tyrosine kinase signaling requires transient inactivation of peroxiredoxins to promote local ROS accumulation at the membrane (Woo et al., 2010). The increased local ROS concentration is believed to cause oxidation and inactivation of redox-sensitive protein tyrosine phosphatases, thus strengthening the signal downstream of the receptor (Lambeth, 2004; Toledano et al., 2010; Tonks, 2006). Repression of CncC activity by Keap1 in ISCs may thus be required to induce a permissive state in the cell that ensures sufficient ROS accumulation in response to a mitogenic signal. Supporting this view, we find that CncC over-expression inhibits insulin receptor-mediated proliferation of ISCs.
The appropriate regulation of ROS production and detoxification is thus critical to ensure proper cellular responses to mitogenic and survival signals (Bashan et al., 2009). This regulatory system is, however, vulnerable to external oxidative challenges, in particular in barrier epithelia like the intestinal epithelium, where a vigorous oxidative burst response is employed as a defense against pathogenic bacteria (Ha et al., 2009a; Ha et al., 2009b; Ha et al., 2005). Our results suggest that constitutive Nrf2/CncC activity in ISCs is critical to prevent excessive ROS accumulation in response to such challenges, and thus to set a threshold for ROS levels at which a regenerative response is warranted.
It is intriguing that the CncC response in stem cells is reversed compared to differentiated cells: CncC activity is suppressed in response to oxidative signals in ISCs, but activated by ROS in differentiated cells. ISCs are thus the only described cells in which Nrf2 activity is repressed in response to oxidative stress, highlighting the specificity and importance of dynamic redox control in this cell type. The mechanism of CncC repression is currently unclear, yet is likely to involve Keap1 function, since loss of Keap1 prevents stress-induced proliferation of ISCs.
The widely conserved anti-oxidant role of Nrf2, and the importance of the intracellular redox balance for stem cell function in other systems, suggest that this function of Nrf2 is also evolutionarily conserved. Accordingly, Keap1 mutant mice display proliferation and differentiation defects in the esophageal epithelium, although the underlying cellular defects causing this phenotype remain unexplored (Wakabayashi et al., 2003). In humans, constitutive activation of Nrf2 has been associated with cancer (Hayes and McMahon, 2009). At the same time, cancer stem cells have been shown to exhibit a low intracellular ROS concentration, suggesting that, as in endogenous stem cell populations, maintenance of a reduced intracellular environment is associated with an undifferentiated state (Diehn et al., 2009). It will be intriguing to test whether Keap1/Nrf2-mediated regulation of intracellular ROS levels is required to maintain stem-like properties in such cells.
Experimental Procedures
Fly lines and husbandry
Fly lines used: w1118, frt82B, frt40A, UAS::EGFP, tub::Gal80ts, and y1 P{SUPorP}Jafrac1KG05372 are provided by Bloomington Drosophila stock center. The RNAi lines ND75RNAi and ND42RNAi were obtained from the National Institute of Genetics Fly Stock Center (Japan). gstD1::lacZ, UAS::CncC, UAS::KeapRNAi, UAS::CncCRNAi, cncK6, cncVL110, keap1EY5, and keap1036 were described in (Sykiotis and Bohmann, 2008). y1,w1; esg::Gal4, UAS::GFP, gift from S. Hayashi. y1,w1,hs::FLP,UAS::GFP; tub::Gal4; FRT82B, tub::Gal80 (gift from Ben Ohlstein), y1w1,hs::FLP,UAS::GFP; FRT40, tub::Gal80; tub::Gal4 (gift from Leanne Jones), y1,w1,hsFLP; act:FRT-STOP-FRT:Gal4, UAS::GFP (gift from Willis X. Li), w, hsFLP; actin>y+>Gal4, UAS::RFP (gift from M. Uhlirova), UAS::Gclc (gift from W.C.Orr), UAS::Jafrac1 (gift from R. Lehmann).
Flies were reared on yeast/molasses-based food at 25°C with a 12hr light/dark cycle unless otherwise noted. For aging experiments, flies were reared at similar larval densities, then maintained in cages at populations of 50-100 flies. Food was changed every 3 - 4 days.
For TARGET experiments, flies were reared at 18°C. Progeny were allowed to emerge and then shifted to the restrictive temperature of 29°C for 3-6 days. For clone induction (MARCM and flp-out), adult flies were aged for 1-2 days then heat shocked at 37°C for 45 min. When Flp-out clones were combined with the TARGET system (Figure 1G), flies were heat-shocked at 37°C for 45 min to induce clones, then transferred to 18°C or 29°C for one week, and the corresponding second temperature for another week. Before dissection, all flies were shifted to 29°C for one day to allow RFP expression in all lineage-traced cells.
BrdU incorporation and paraquat exposure
For paraquat exposure, flies were dry starved for 6 hours and then fed 5% sucrose +/- 5mM paraquat (methyl viologen, Sigma Aldrich) on filter paper.
For BrdU incorporation, flies were dry starved for six hours, then fed 0.2mg/ml BrdU in 5% sucrose +/- 5mM paraquat. Dissected guts were fixed for 45 minutes in fixation buffer (see below) and treated with 3M HCl for 30 minutes to denature DNA. Intestines were then subjected to the standard immunostaining procedure described below.
Immunostaining and microscopy
Immunostaining was performed as described (Ohlstein and Spradling, 2006) Briefly, guts were fixed for 45 minutes at room temperature in 100 mM glutamic acid, 25 mM KCl, 20 mM MgSO4, 4 mM sodium phosphate (dibasic), 1 mM MgCl2, and 4% formaldehyde. Washes and antibody incubations were performed in Phosphate-buffered saline (PBS), 0.5% Bovine Serum Albumin, 0.1% Triton X-100 at 4°C (antibody incubations were overnight with 1 hour washings).
For staining with anti-Delta antibody, guts were prepared using a methanol-heptane fixation method as described in (Lin et al., 2008).
In vivo detection of ROS using DHE
ROS levels were detected in live tissue as described (Owusu-Ansah et al., 2008). Briefly, guts were dissected and handled throughout in Schneider's medium (HyClone). After incubation in 30 μM DHE (Invitrogen) for 3-7 minutes in the dark at room temperature, guts were washed three times with Schneider's medium and mounted. Where possible, experiments were performed within the same well (genotypes distinguished by expression of GFP) to eliminate differences in incubation times. Images were captured immediately using confocal microscopy (543nm excitation, 550-610nm detection, fixed laser power, gain and offset settings). Z-series spanning the intestinal epithelium were collected and single confocal sections were used to measure signal intensities using the histogram function in NIH ImageJ. ISCs were identified by their esg-GFP expression, their size, and their basal location within the intestinal epithelium. Where possible, only isolated esg-GFP+ cells were measured, which represent Dl+ ISCs (Figure S8). In wild-type PQ treated and in CncCRNAi-expressing flies small, basally located GFP+ cells were analyzed. Co-staining experiments confirm the sensitivity and reproducibility of the DHE experiments, Figure S5B and S7).
Supplementary Material
Acknowledgements
This work was supported by the National Institute on Aging (NIH RO1 AG028127 to H.J.), NYSTEM (grant # N08G-048), and the Ellison Medical Foundation (AGSS-2224-08), as well as an AFAR/Ellison postdoctoral fellowship to B.B. We would like to thank Mark Noble for comments and Olga Dunaevsky for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–1893. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. 2009;89:27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;14:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008 doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran SP, Wu X, Riedel CG, Ruvkun G. A soma-to-germline transformation in long-lived Caenorhabditis elegans mutants. Nature. 2009;459:1079–1084. doi: 10.1038/nature08106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EM, Lee KA, Park SH, Kim SH, Nam HJ, Lee HY, Kang D, Lee WJ. Regulation of DUOX by the Galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Dev Cell. 2009a;16:386–397. doi: 10.1016/j.devcel.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, Kim J, Lee WJ. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in drosophila gut. Nat Immunol. 2009b;10:949–957. doi: 10.1038/ni.1765. [DOI] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, Matsumoto K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005;19:2278–2283. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? Faseb J. 2005;19:1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- Lee KS, Iijima-Ando K, Iijima K, Lee WJ, Lee JH, Yu K, Lee DS. JNK/FOXO-mediated neuronal expression of fly homologue of peroxiredoxin II reduces oxidative stress and extends lifespan. J Biol Chem. 2009a doi: 10.1074/jbc.M109.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009b;136:2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, Liu H, Keyvanfar K, Chen H, Cao LY, et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387–392. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J, Yamamoto M. The rise of antioxidant signaling--the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol. 2010;244:4–15. doi: 10.1016/j.taap.2010.01.011. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. The Cap'n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Miyamoto T, Kato R, Yoshimura A, Motoyama N, Suda T. FoxO3a regulates hematopoietic homeostasis through a negative feedback pathway in conditions of stress or aging. Blood. 2008;112:4485–4493. doi: 10.1182/blood-2008-05-159848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H, O'Connor T, Katsuoka F, Engel JD, Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294:1–12. doi: 10.1016/s0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Orr WC, Radyuk SN, Prabhudesai L, Toroser D, Benes JJ, Luchak JM, Mockett RJ, Rebrin I, Hubbard JG, Sohal RS. Overexpression of glutamate-cysteine ligase extends life span in Drosophila melanogaster. J Biol Chem. 2005;280:37331–37338. doi: 10.1074/jbc.M508272200. [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009 doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat Genet. 2008;40:356–361. doi: 10.1038/ng.2007.50. [DOI] [PubMed] [Google Scholar]
- Radyuk SN, Sohal RS, Orr WC. Thioredoxin peroxidases can foster cytoprotection or cell death in response to different stressors: over- and under-expression of thioredoxin peroxidase in Drosophila cells. Biochem J. 2003;371:743–752. doi: 10.1042/BJ20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000;97:10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosiou NA, Xu T. Use of FLP/FRT system to study Drosophila development. Methods. 1998;14:355–365. doi: 10.1006/meth.1998.0591. [DOI] [PubMed] [Google Scholar]
- Toledano MB. The guardian recruits cops: the p53-p21 axis delegates prosurvival duties to the Keap1-Nrf2 stress pathway. Mol Cell. 2009;34:637–639. doi: 10.1016/j.molcel.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Toledano MB, Planson AG, Delaunay-Moisan A. Reining in H(2)O(2) for safe signaling. Cell. 2010;140:454–456. doi: 10.1016/j.cell.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1:140–152. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Tsatmali M, Walcott EC, Crossin KL. Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res. 2005;1040:137–150. doi: 10.1016/j.brainres.2005.01.087. [DOI] [PubMed] [Google Scholar]
- Veraksa A, McGinnis N, Li X, Mohler J, McGinnis W. Cap ‘n’ collar B cooperates with a small Maf subunit to specify pharyngeal development and suppress deformed homeotic function in the Drosophila head. Development. 2000;127:4023–4037. doi: 10.1242/dev.127.18.4023. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.