Post-traumatic stress disorder (PTSD) is a debilitating psychiatric disorder caused by exposure to severe stress. An article by Tronson et al. in the current issue highlights new potential mechanisms involved in stress regulation of fear memory in an animal model of relevance to PTSD (1). PTSD is characterized by symptoms in three primary domains, 1) inappropriate and sustained hyperarousal, 2) re-experiencing phenomena in the form of flashbacks or nightmares, and 3) avoidance behaviors aimed at limiting exposure to reminders of the traumatic experience. In severe cases psychotic and dissociative symptoms can also occur. Current conceptualizations of PTSD emphasize a pathophysiology underscored by impaired extinction, or “inhibition”, of fear memories made during traumatic experiences (2). Current pharmacological treatment strategies are primarily aimed at symptom resolution, e.g. selective serotonin re-uptake inhibitors to treat anxiety and depressive symptoms, alpha-2 adrenergic agonists and mood stabilizers to target hyperarousal, and alpha-1 adrenergic antagonists to target sleep disturbances (3). The limited efficacy of currently available pharmacological treatments for PTSD, and the large numbers of returning combat troops exhibiting PTSD symptoms (4) has fueled resurgent research interest into novel treatment approaches for this devastating disorder.
Although severe and chronic stressors have long been known to disrupt learning and memory processes, it is also clear that low levels of stress can enhance cognitive functions, arousal, and memory formation (5). Indeed, pre-exposure to stress can enhance the acquisition of subsequent fear memories in animal models (6). Such models could provide insight into the mechanisms by which chronic exposure to stress, such as in military combat, could pre-dispose development of PTSD in response to subsequent exposure to severely traumatic experiences.
The hippocampus is a highly stress-sensitive brain region that is heavily involved in contextual memory formation and retrieval, and is critical for contextual fear learning. Thus, the hippocampus is a likely candidate to mediate effects of stress on contextual fear learning. Tronson et al. provide new insight into the molecular mechanisms by which stress exposure can enhance the acquisition of fear memories via modulation of metabotropic glutamate receptor (mGluR) signaling in the hippocampus, and in so doing suggest novel therapeutic strategies to possibly counteract the effects of stress on fear learning (1). Tronson et al. show that a brief episode of immobilization stress 3-6 hours prior to fear training resulted in an enduring and context-specific potentiation of subsequent fear upon re-exposure to the environment 1 to 28 days later. This potentiation was blocked when inverse agonists of mGluR5 (MPEP and MTEP) were either administered systemically or directly injected into the hippocampus 1-5 hours after the stressor, prior to conditioning. Intriguingly, with these doses of inverse agonists, only the stress enhancement of fear was blocked, normal fear conditioning was unaffected. Further, the effects of MPEP and MTEP were not mimicked by MCPG, a broad-spectrum competitive mGluR antagonist.
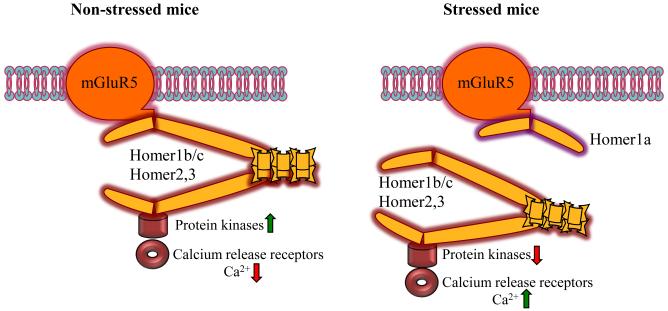
The differential effectiveness of inverse agonists and a competitive antagonist indicates the possibility that the participation of mGluR5 in stress enhancement of context-specific fear could involve constitutively activated forms of mGluR5 that operate independent of endogenous ligand. Previous studies have suggested that one way to produce constitutively active mGluR5 is through alterations in its constituent binding partners in the cell. The Homer family of scaffolding proteins has been characterized as an important regulator of glutamate synapses, and in particular, as binding partners that tether mGluR5 to some of its signaling machinery through two EVH1 binding domains (see Figure 1). An interesting feature of the Homer family is the existence of a short, Homer1a isoform that functions as an activity-dependent immediate-early gene, and contains only a single EVH1 domain. This short form can compete with long-form Homers to disrupt mGluR5 interactions with other synaptic molecules, and increase the receptor’s constitutive activity (7). Tronson et al. found that Homer 1a levels were increased and long forms of Homer displaced from mGluR5 in the hippocampus after forced swim stress at timepoints similar to when mGluR5 signaling enhances context-specific fear learning (see Figure 1).
Figure 1.
Schematic of mGluR5-Homer family interactions in non-stress (left panel) and stress (right panel) conditions. (Courtesy of Jelena Radulovic)
The investigators then performed a series of elegant molecular experiments to demonstrate the sufficiency of this hippocampal Homer1a up regulation in stress-potentiation of context-specific fear. First, they showed that increasing Homer1a expression in the hippocampus via viral transduction bypassed the need for stress to facilitate fear conditioning. Second, they utilized “decoy” peptides to displace mGluR5 from all Homers, which also mimicked the effects of stress on fear conditioning.
The results in total indicate an important role for hippocampal mGluR5 and Homer in stress enhancement of contextual fear responses. While the doses of mGluR5 antagonists used in the present study did not directly impact fear conditioning per se, previous studies using genetic deletions have demonstrated roles of the receptor in several aspects of contextual fear learning (8). Thus, a particularly interesting aspect of the new findings is that while the inverse agonists and Homer manipulation blocked and mimicked the stress enhancement, respectively, a competitive antagonist, MCPG, did not. This suggests the potential for a unique population of constitutively active receptors in the stress enhancement that might be amenable to a more targeted, perhaps prophylactic therapy. In future studies, it will be important to further solidify the contribution of constitutive signaling. For example, it is possible that MCPG is ineffective in this case because of increased levels of extracellular glutamate relative to other behaviors shown to be sensitive to this dose. Further examination of downstream signal transduction mechanisms and the use of additional pharmacology will likely be informative. For example, Homer family proteins are thought to interact with a number of additional proteins whose function could be altered by increased Homer 1a expression (9). In addition, a wealth of allosteric compounds targeting mGluR5 both at the MPEP site and other sites are in various stages of development (9). Regardless, the present observations are an important new step towards our understanding of the interactions between stress and learning, and further emphasize the mGluR system as an emerging molecular target for the treatment of neuropsychiatric disorders (10).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1).Tronson NC, Yomayra F, Guzman YF, Guedea AL, Huh KH, Gao C, Schwarz MK, Radulovic J. Metabotropic glutamate receptor 5/Homer interactions underlie stress effects on fear. Biological Psychiatry ???? 2010 doi: 10.1016/j.biopsych.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Javanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–62. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Norrholm SD, Jovanovic T. Tailoring therapeutic strategies for treating posttraumatic stress disorder symptom clusters. Neuropsychiatr Dis Treat. 2010;7:517–32. doi: 10.2147/NDT.S10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Thomas JL, Wilk JE, Riviere LA, McGurk D, Castro CA, Hoge CW. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. 2010;67:614–23. doi: 10.1001/archgenpsychiatry.2010.54. [DOI] [PubMed] [Google Scholar]
- 5).Bangasser DA, Shors TJ. Critical brain circuits at the intersection between stress and learning. Neurosci Biobehav Rev. 2010;34:1223–33. doi: 10.1016/j.neubiorev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, et al. Agonist independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–965. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- 8).Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29:3676–84. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Krystal JH, Mathew SJ, D’Souza DC, Garakani A, Gunduz-Bruce H, Charney DS. Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 2010;24:669–93. doi: 10.2165/11533230-000000000-00000. [DOI] [PubMed] [Google Scholar]