Abstract
Aim
This study examines the effect of combining the antiangiogenic effect of αvβ3-targeted fumagillin nanoparticles with the conventional antirheumatic drug methotrexate for the treatment of inflammatory arthritis.
Method
Arthritis was induced in mice by K/BxN serum transfer, and disease activity was monitored by clinical score and change in ankle thickness. Groups of mice received nanoparticles or methotrexate as single therapy or nanoparticles and methotrexate as combination therapy.
Results
We found that animals treated with a pulse dose of fumagillin nanoparticles followed by methotrexate had significantly improved and sustained clinical response compared with those treated with either agent alone. Histological analysis confirmed a significant decrease in inflammatory cell influx, bone erosions, cartilage damage and angiogenesis with the combination therapy.
Conclusion
Analysis of plasma cytokine levels suggests that fumagillin nanoparticles enhanced the systemic anti-inflammatory effects of methotrexate. Antiangiogenic nanotherapy may represent a promising approach for the treatment of inflammatory arthritis when combined with a conventional antirheumatic drug.
Keywords: antiangiogenics, combination therapy, K/BxN arthritis model, nanotherapeutics, rheumatoid arthritis
Growing evidence in recent years suggests that early treatment of rheumatoid arthritis (RA) with a combination of disease-modifying anti-rheumatic drugs (DMARDs) may induce long-term and sustained functional and clinical outcomes in RA patients [1]. This early aggressive treatment can substantially reduce the inflammatory process, after which disease activity can be maintained with a conventional DMARD at a significantly reduced dose. Different types of induction therapy have been attempted in RA, however, with varying degrees of success [1,2].
Perfluorocarbon (PFC) nanoparticles represent a platform technology with flexible molecular imaging and local drug-delivery potential. We have previously shown that αvβ3-targeted PFC nanoparticles accumulate specifically in the inflamed arthritic paws of mice, whereas nontargeted nanoparticles did not [3]. Serial doses of αvβ3-targeted PFC nanoparticles conjugated to the antiangiogenic drug fumagillin suppressed ongoing inflammatory arthritis in the K/BxN mouse model, while αvβ3-targeted PFC nanoparticles without drug did not prevent the progression of arthritis [3]. The question remains as to whether antiangiogenic nanotherapy results in long-lasting therapeutic efficacy. Recent findings, however, suggest that antiangiogenics alone cannot maintain vascular regression [4]. More often it is used to enhance the effectiveness of standardized therapies, such as the use of bevacizumab and 5-fluorouracil for colon cancer [5]. Antiangiogenics work by ‘preconditioning’ or ‘pruning’ the vasculature [6], an effect that transiently normalizes blood supply, thereby augmenting the delivery of cytotoxic drugs [4]. We hypothesize that induction therapy with αvβ3-targeted fumagillin nanoparticles may increase the effectiveness of or synergize with a second conventional agent to durably impede the progression of inflammatory arthritis, even after the discontinuation of nanotherapy. Indeed, we have data indicating that αvβ3-targeted antiangiogenic nanoparticle injection followed by oral statin maintenance therapy leads to a sustained reduction in angiogenesis in an atherosclerosis model [7]. In the present study we show that a single dose of αvβ3-targeted fumagillin nanoparticles, given during the early but established phase of arthritis, followed by a reduced dose of methotrexate (MTX; a conventional DMARD used as first-line therapy for RA), induces a s ustained anti-inflammatory effect.
Materials & methods
Synthesis of nanoparticles
The nanoparticles were directed to the αvβ3-integrin with a peptidomimetic vitronectin antagonist developed by Bristol-Myers Squibb Medical Imaging (Billerica, MA, USA; [101] and related patents), as previously described [3]. In general, nanoparticles were comprised of 20% (v/v) perfluorooctylbromide (PFOB; Exfluor Research Corp., TX, USA) and 2.0% (w/v) of a surfactant comixture, and 1.7% (w/v) glycerin and water for the balance. The surfactant co-mixtures included 97.9 mole% highly purified egg yolk lecithin (Avanti Polar Lipids, Inc., AL, USA), 0.1 mole% peptidomimetic αvβ3-integrin antagonist conjugated to PEG2000-phosphatidylethanolamine (Kereos, Inc., MO, USA) and 2 mole% phosphatidylethanolamine (PE; Avanti Polar Lipids, Inc.). The surfactant components were dissolved in chloroform/methanol and dried in a 50°C vacuum oven overnight. Fumagillin (Fum; producted by the National Cancer Institute) was substituted to the surfactant mixture (1.5 mole%) at the expense of lecithin on an equimolar basis (i.e., molecule for molecule).
Arthritis induction & treatment
All animal experiments were performed in strict accordance with the guidelines established by the Division of Comparative Medicine at Washington University (MO, USA). Arthritis was induced using the K/BxN mouse model of inflammatory arthritis as previously described [3]. Male C57BL/6 mice aged 6–8 weeks (The Jackson Laboratory, ME, USA) were injected intraperitoneally with 150 μl of serum from K/BxN mice on day 0 to induce arthritis. Clinical manifestation of arthritis was assessed daily on a scale of 0–3 (0 = no swelling or erythema, 1 = slight swelling or erythema, 2 = moderate erythema and swelling in multiple digits or entire paw, 3 = pronounced erythema and swelling of entire paw, maximum score of 12 per mouse). Change from baseline in paw thickness was determined daily by dial calipers, and an average change in ankle thickness was determined for each mouse from the two hind paw measurements. Mice were also weighed prior to, and every day after, K/BxN serum transfer. Percent weight change was calculated by the formula:
In Figures 1–4, mice were assigned to one of four treatment groups:
-
■
No treatment (n = 9)
-
■
αvβ3-targeted nanoparticles with Fum (2.5 μg/g body weight; n = 9)
-
■
MTX (1.0 μg/g body weight; n = 7)
-
■
αvβ3-targeted nanoparticles with Fum (2.5 μg/g body weight) and MTX (1.0 μg/g body weight; n = 9)
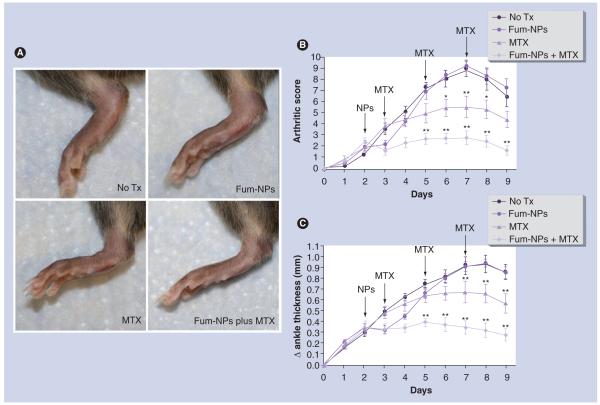
Figure 1. Synergistic effect of fumagillin nanoparticles and methotrexate in inflammatory arthritis.
Arthritis was induced by K/BxN serum transfer on day 0. NPs and MTX were provided on the indicated days (arrows). (A) Photograph of a hind paw of a representative mouse from each treatment group taken on day 9 after serum transfer. Arthritic score (B) and change (Δ) in ankle thickness (C) were monitored daily. Values represent mean ± SEM (n = seven to nine mice per treatment group derived from two independent experiments).
*p < 0.05.
**p < 0.001 compared with no Tx group.
Fum-NP: Fumagillin-nanoparticle; MTX: Methotrexate; Tx: Treatment.
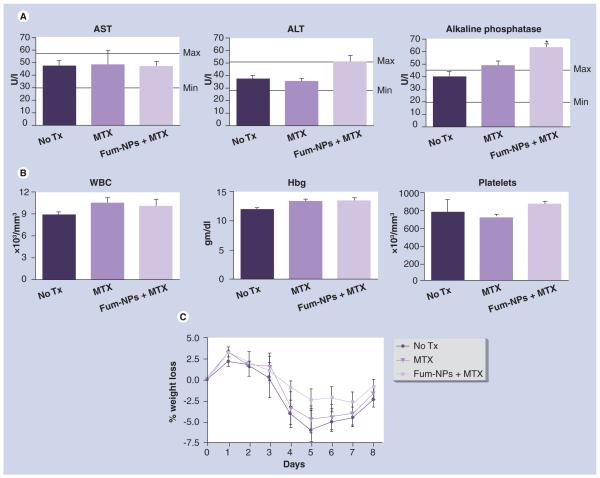
Figure 4. Analysis of toxicity profile of fumagillin nanoparticles and methotrexate combination therapy.
On day 9 following K/BxN serum transfer, blood samples were drawn and (A) liver enzymes (including serum concentration of aspartate amino transferase, alanine amino transferase and alkaline phosphatase) and (B) hematologic parameters (white blood cells, hemoglobin and platelets) were assessed. Values represent mean ± SEM (n = seven to nine mice per treatment group). (C) Mice were weighed daily and weight change from baseline weight (prior to serum transfer) was calculated, as detailed in the Materials & methods section.
*p < 0.01 compared with no treatment group. Minimal (min) and maximal (max) values for liver enzymes in C57BL/6 mice were indicated.
ALT: Alanine amino transferase; AST: Aspartate amino transferase; Fum-NP: Fumagillin-nanoparticle; Hbg: Hemoglobin; MTX: Methotrexate; Tx: Treatment; WBC: White blood cell.
In Figure 5, mice were assigned to one of three treatment groups:
-
■
No treatment (n = 7)
-
■
αvβ3-targeted nanoparticles without drug (2.5 μg/g body weight) and MTX (1.0 μg/g body weight; n = 9)
-
■
αvβ3-targeted nanoparticles with Fum (2.5 μg/g body weight) and MTX (1.0 μg/g body weight; n = 10)
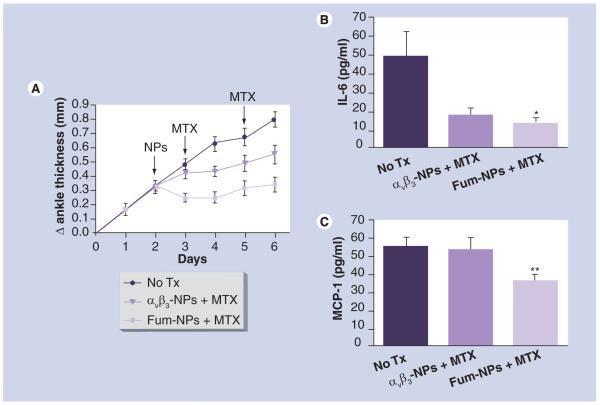
Figure 5. Fumagillin nanoparticles enhance the anti-inflammatory effects of methotrexate.
Arthritis was induced by K/BxN serum transfer on day 0. NPs (αvβ3-NPs and Fum-NPs) and MTX were administered on the indicated days (arrows). (A) Change (Δ) in ankle thickness was monitored daily. Values represent mean ± SEM (n = seven to ten mice per treatment group derived from two independent experiments). On day 6 after K/BxN serum transfer, plasma was obtained and assayed for IL-6 (B) and MCP-1 (C). Values represent mean ± SEM (n = five mice per treatment group).
*p < 0.05.
**p < 0.01 compared with no treatment group.
Fum: Fumagillin; MTX: Methotrexate; NP: Nanoparticle.
αvβ3-targeted nanoparticles (αvβ3-NPs) or αvβ3-targeted Fum nanoparticles (Fum-NPs) were administered intravenously once on day 2 after K/BxN serum transfer. MTX was administered intraperitoneally on days 3, 5 and 7 after serum transfer.
Histological analysis
Mouse paws were harvested on day 9 after serum transfer, fixed in 10% formalin for 48 h, decalcified in EDTA solution, embedded in paraffin and sectioned at 5 μm. The sections were stained with hematoxylin and eosin (H&E) or toluidine blue. Digital images of five random areas per H&E-stained paw section were acquired at ×400 and the number of exuded inflammatory cells enumerated. The number of bone erosions was enumerated per mm of bone surface using the ImageJ program [201]. Proteoglycan content, an indication of cartilage integrity, was graded on toluidine blue-stained sections on a scale of 0–4 as previously described [8] (0 = fully stained cartilage; 1 = less than 25% unstained; 2 = 25–50% unstained; 3 = 50–75% unstained; and 4 = greater than 75% unstained cartilage). Quantitative scoring was performed by an observer blinded to the treatment. Each value represents the average per animal derived from the cumulative scoring of two hind paws.
Evaluation of angiogenesis
Angiogenesis was evaluated as previously described [3]. Briefly, mouse paw sections were incubated with rabbit anti-von Willebrand factor antibody (1:200 dilution, Chemicon International, CA, USA) for 2 h at room temperature, followed by biotinylated antirabbit antibody and alkaline phosphatase conjugated streptavidin. Color was visualized using a substrate kit (Vector Laboratories, CA, USA). The slides were counterstained with 1% methyl green. Digital images of five random areas per paw were acquired at ×400 and the number of blood vessels enumerated by an observer blinded to the treatment.
Cytokine analysis
Plasma was collected in EDTA-containing tubes on day 6 following K/BxN serum transfer and cytokine levels (IL-1β, IL-6, IL-10, IL-12, TNF-α, IFN-γ and MCP-1) were measured by Cytometric Bead Array as recommended by the manufacturers (BD Biosciences, CA, USA).
Blood cell count & serum chemistry
Blood samples were drawn from the inferior vena cava by a single stick after the mice were euthanized on day 9 following K/BxN serum transfer and analyzed by the Washington University Department of Comparative Medicine using routine clinical procedures. Minimal and maximal values for liver enzymes in C57BL/6 male mice were compared with previously published reference ranges [9].
Statistical analysis
Comparisons between groups were done by two-way ANOVA followed by the Bonferroni post hoc test to compare all groups of data. Comparisons between two groups were performed using student’s t-test. p-values of less than 0.05 were considered significant. Numerical values are reported as mean ± SEM.
Results
Combination of antiangiogenic nanotherapy and MTX leads to sustained clinical response in the K/BxN mouse model of inflammatory arthritis. On day 2 after serum transfer, when arthritis was clearly established in all animals, mice were randomly assigned to receive no treatment or a single intravenous dose of Fum-NPs. On day 3, mice were again randomly assigned to receive no further treatment or MTX (1.0 μg/g body weight intraperitoneal every other day). Another group of mice that did not received Fum-NPs was also assigned to receive MTX. Fum-NPs led to a noticeable but transient reduction in clinical score and ankle thickness in the injected mice (Figure 1B–C). Treatment with MTX led to a significant decrease in both clinical score and ankle thickness, with a peak difference seen on day 7 after K/BxN serum transfer (p < 0.001 compared with the no treatment group). Treatment with a combination of Fum-NPs plus MTX led to a further decrease in clinical score and ankle thickness (p < 0.001 starting on day 5 after serum transfer compared with the no treatment group). Of note, the clinical response was sustained even though nanotherapy was discontinued after the single pulse treatment on day 2 (Figure 1).
Combination of antiangiogenic nanotherapy and MTX reduces inflammation, cartilage destruction, bone erosions and angiogenesis. To elucidate the effects of treatment histologically, day 9 sections of paws from different groups of untreated and treated animals were examined. Synovial inflammation, cartilage damage and bone erosions were noted to be most severe and consistent across the midfoot region, in the tarsal and metatarsal joints (Figure 2A–L). Therefore, these joint sections were assessed and scored quantitatively.
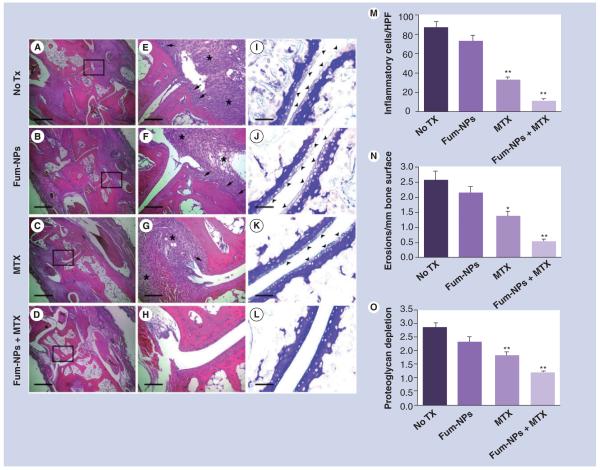
Figure 2. Combination of fumagillin nanoparticles and methotrexate suppresses joint inflammation and damage.
On day 9 after K/BxN serum transfer, mice were sacrificed and their paws sectioned and stained with hematoxylin and eosin (A–H) to assess for the number of inflammatory cells (A–D) and erosions (arrows, E–H). Sections were also stained with toluidine blue to assess the proteoglycan content in cartilage (arrowheads, I–L). Scale bars = 0.5 mm (A–D), 0.1 mm (E–H) and 0.05 mm (I–L). The number of inflammatory cells/HPF (×400) (M), erosions (N) and degree of proteoglycan depletion (O) were assessed in a blinded fashion. Values represent mean ± SEM (n = 7–9 mice per treatment group).
*p < 0.01.
**p < 0.001 compared with no Tx group.
Fum-NP: Fumagillin-nanoparticle; MTX: Methotrexate; Tx: Treatment.
Synovial inflammation
Treatment with MTX limited the influx of inflammatory leukocytes into the subsynovial space (32.5 ± 1.9 cells per high power field [HPF] in the MTX group versus 87.3 ± 6.2 cells/HPF in the no treatment group; p < 0.001), while treatment with one single dose of Fum-NPs on day 2 did not lead to a sustained anti-inflammatory effect. Fum-NPs plus MTX combined therapy provided the greatest reduction in inflammation (11.3 ± 1.5 cells/HPF in the Fum-NPs plus MTX group, p < 0.001, compared with the no treatment and Fum-NPs groups) (Figure 2M).
Bone erosions
Treatment with MTX reduced the number of bone erosions by approximately 50% compared with the no-treatment group (p < 0.01; Figure 2N), while treatment with Fum-NPs only did not significantly change the bone erosion score. Treatment with Fum-NPs plus MTX further reduced the number of bone erosions by approximately 80% compared with the no-treatment group (p < 0.001).
Cartilage damage
Consistent with the decrease in synovial inflammation and bone erosions, we saw approximately 35% decrease in proteoglycan depletion (a sign of cartilage damage) in animals treated with MTX (p < 0.001 compared with the no treatment group; Figure 2O). Combination therapy treatment with Fum-NPs plus MTX further reduced the extent of cartilage damage by 60% (p < 0.001 compared with the no-treatment group).
Angiogenesis
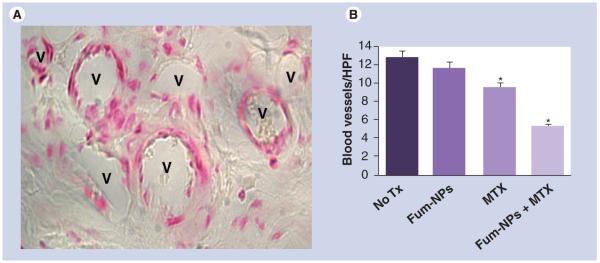
To enumerate blood vessels, paw sections were stained for von Willebrand factor, a molecule synthesized by endothelial cells (Figure 3A) [10]. Treatment with MTX significantly reduced the number of blood vessels in the paw sections (9.4 ± 0.5 blood vessels/HPF in the MTX group compared with 12.6 ± 0.7 in the no-treatment group and 11.5 ± 0.7 in the Fum-NPs group, p < 0.0001). Treatment with Fum-NPs plus MTX had the biggest impact on the degree of angiogenesis (5.2 ± 0.3 blood vessels/HPF, p < 0.0001 compared with no treatment, Fum-NPs and MTX groups), decreasing the number of blood vessels by 60% in paw sections (Figure 3B).
Figure 3. Combination of fumagillin nanoparticles and methotrexate suppresses angiogenesis.
(A) Representative micrograph of blood vessels (V) visualized with an antibody to von Willebrand factor. (B) Number of blood vessels/HPF on day 9 was assessed in a blinded fashion. Values represent mean ± SEM (n = seven to nine mice per treatment group).
*p < 0.0001 compared with no Tx and Fum-NPs groups.
Fum-NP: Fumagillin-nanoparticle; HPD: High power field; MTX: Methotrexate; Tx: Treatment.
Antiangiogenic and MTX combination therapy has a favorable safety profile. We previously found that serial intravenous injections of high-dose nanoparticles caused a mild, transient transaminitis, likely due to the accumulation of nanoparticles in the reticulo-endothelial system, leading to increased intrahepatic pressure [3]. In the present study, we demonstrated that the administration of a single dose of Fum-NPs did not increase serum levels of aspartate amino transferase and alanine amino transferase above the upper limit of normal, even when combined with repeated MTX injections (Figure 4A, left and middle panels). 1 week after the administration of Fum-NPs plus MTX, the serum level of alkaline phosphatase was mildly increased to 1.4-times the upper limit of normal (Figure 4A, right panel), which may reflect biliary elimination of the nanoparticles reported in rodents previously [11]. White blood cell (WBC) counts, hemoglobin (Hbg) and platelet values did not differ with the administration of MTX without or with Fum-NPs compared with the no-treatment group (Figure 4B). With the induction of arthritis, all the mice lost weight (2.5–7.5% decrease in body weight at the nadir on day 5; Figure 4C). While no significant difference in weight loss between the no treatment, MTX, or Fum-NPs plus MTX groups was appreciated, the Fum-NPs plus MTX group maintained their baseline weight better than the other groups. Taken together these results suggest a favorable safety profile for the nanotherapy and MTX combination.
Nanotherapy does not affect plasma or red blood cell (RBC) MTX levels. Polyglutamates of MTX are active metabolites that accumulate in cells, such as RBCs, and the intracellular concentration of MTX metabolites in RBCs serve as an indicator of MTX bioavailability [12]. To examine whether Fum-NP administration delayed the metabolism of MTX, leading to higher effective dosage over time, we measured MTX and MTX metabolite levels in the plasma and RBCs of mice treated with the combination of drugs. No elevated plasma or RBC MTX or MTX-polyglutamate metabolites were detected (1 ng/ml lower detection limit) on day 9 after K/BxN serum transfer, which confirmed that the enhanced clinical response to Fum-NPs plus MTX was not due to impaired clearance of MTX in the presence of NPs, which resulted in higher levels of drug accumulated in plasma or RBCs (see Supplementary data at www.futuremedicine.com/toc/nnm/5/7).
Antiangiogenic nanotherapy enhances the systemic anti-inflammatory effects of MTX. Although MTX was initially introduced for the treatment of RA for its presumed anti-proliferative properties, it has become clear that MTX has direct anti-inflammatory effects on many cell types, including neutrophils, macrophages and endothelial cells by suppressing the release of inflammatory cytokines [13]. To further explore the mechanism of synergy between antiangiogenic nanotherapy and MTX, we hypothesized that Fum-NPs potentiate the systemic antiinflammatory effects of MTX. To this end, we compared the systemic levels of inflammatory cytokines in the different treatment groups. Mice received K/BxN serum followed by NPs and MTX injections as detailed in the Materials & methods section. To ensure that the synergism observed between Fum-NPs and MTX requires the antiangiogenic effect of Fum and is not due to a nonspecific ‘carrier effect’ by NPs (i.e., MTX is adsorbed to NPs and carried to target tissues), we compared αvβ3-NPs (the control nanoparticles without drug) to Fum-NPs. We observed a decrease in ankle thickness in the group of mice that received αvβ3-NPs + MTX and this decrease was comparable to the one previously seen in mice that received MTX alone (Figure 5A). Analysis of cytokines in the plasma revealed that treatment with αvβ3-NPs plus MTX, suppressed the systemic level of IL-6, a potent inflammatory cytokine, but this difference did not reach statistical significance (p > 0.05 compared with the no treatment group; Figure 5B). The anti-inflammatory effect of MTX was further enhanced when mice were given Fum-NPs (p < 0.05, compared with the no-treatment group; Figure 5B). Furthermore, treatment with Fum-NPs plus MTX significantly decreased the systemic level of MCP-1, also known as CCL2 and a potent inflammatory chemokine, while the treatment with αvβ3-NPs plus MTX did not (p < 0.01 compared with the no-treatment group; Figure 5C). No substantial increases (above background) in the plasma levels of IL-1β, IL-10, IL-12, IFN-γ or TNF-α were measured (data not shown). These results confirm that antiangiogenic nanotherapy combined with MTX leads to enhanced suppression of systemic inflammation.
Discussion
The results of this study suggest that a single injection of Fum-NPs followed by MTX was a relatively safe and effective treatment in the early phase of inflammatory arthritis in the K/BxN mouse model. The mice treated with the combined therapy exhibit significantly less joint and systemic inflammation compared with mice treated with either agent alone.
The αvβ3 integrin is a well-characterized heterodimeric adhesion molecule expressed on a variety of cell types, including vascular endothelial cells, and is considered a marker of angiogenesis [14]. We have previously shown that αvβ3-targeted nanoparticles localize specifically to inflamed paws in arthritic mice, and suppress inflammation when combined with the antiangiogenic drug Fum [3]. Fum, a mycotoxin produced by Aspergillus fumagatus, targets the metalloprotease methionine aminopeptidase-2 [15], and this interaction mediates a cytostatic response of endothelial cells [16]. In animal models, TNP-470 – a soluble form of Fum – is moderately effective in a preventive protocol and minimally reduces clinical severity when given after the onset of disease [17]. However, the high doses of TNP-470 required for therapeutic effect pose significant risk of neurotoxicity in mice and humans [17,18]. In the present study the dose of Fum used was more than a 30-fold reduction in the equivalent TNP-470 dosage previously administered in mouse models of arthritis [17]. Moreover, we show that a single pulse dose of Fum-NPs in combination with MTX (versus serial Fum-NPs treatment) was sufficient to provide a sustained therapeutic effect, and did not induce the mild transient transaminitis measured with frequent NPs dosing [3]. Although MTX at 10 μg/g bodyweight administered three times per week suppresses arthritis development in a mouse collagen-induced arthritis model [19], the dose of MTX required in this study to halt inflammation and suppress joint damage was tenfold lower when combined with a single dose of Fum nanoparticles.
The exact mechanism underlying the synergistic effect of combining antiangiogenic nanotherapy and MTX remains to be determined. However, the rapid decrease in ankle thickness (within 24 h) in the groups that received Fum-NPs supports the contention that antiangiogenic treatment acutely stabilized leaky blood vessels, especially in the inflamed joints [4,6], which was reflected as a reduction in paw edema. The ‘normalized’ and less leaky vasculature may augment the anti-inflammatory effect of MTX by allowing the drug to better penetrate endothelial cells where it suppresses the production/release of inflammatory cytokines and chemokines. This results in augmented anti-inflammatory effect, as evidenced by decrease in systemic levels of IL-6 and MCP-1, both of which are produced by endothelial cells and can promote/perpetuate recruitment of leukocytes to inflamed areas. Without augmentation of MTX treatment, the single dose effects of Fum on day 3 yielded to recrudescence of microvasculature on day 9 (i.e., no difference in the number of blood vessels between the no treatment group and the group treated with Fum-NPs only). Collectively, the results support the concept that pathologic angiogenesis is functionally abnormal [4], and normalization of blood vessels by antiangiogenic treatment, although transient, improves penetration and efficacy of drugs in target tissues.
Conclusion
In this study, the enhancement of conventional DMARD treatment with antiangiogenic nanotherapy was demonstrated in a preclinical model of inflammatory arthritis. Although the specific mechanistic basis for this synergism is undetermined, the data obtained suggest that the transient antiangiogenic effects of Fum were sustained by the anti-inflammatory benefits of MTX. Importantly, both the acute single dose of Fum administered by nanoparticles and the more chronic dosage of MTX required to suppress joint damage were decreased by 30- and 10-fold, respectively, compared with levels required in previous studies. These results offer a proof-of-concept for this emerging technology, and provide the rationale for further exploration into the use of antiangiogenic nanomedicine as induction therapy, perhaps in combination with other treatment modalities for RA and related inflammatory arthritides.
Future perspective
Although angiogenesis is a prominent feature of inflammatory arthritis, the clinical effectiveness of any single antiangiogenic therapy in human RA has not been confirmed [20,21]. Perhaps one of the challenges in the further advance of antiangiogenic therapy is the lack of validated ways to precisely monitor antiangiogenic response. PFC nanoparticles are detectable with MRI and may allow quantitative monitoring of early response using spectroscopy techniques. With PFC nanoparticles now approved for Phase I clinical studies as an imaging agent, the development of Fum nanotherapy should also progress to the clinic in a timely fashion, allowing the hypothesis of targeted antiangiogenic nanotherapy synergy with DMARD to be tested in patients.
Supplementary Material
Acknowledgements
We extend sincere appreciation to Ralph Fuhrhop for nanoparticle synthesis and Ying Hu for technical expertise. We thank Paul Allen and Fei Shih for generous gift of K/BxN serum.
Footnotes
Financial & competing interests disclosure
Samuel A Wickline and Gregory M Lanza are scientific cofounders of Kereos, Inc., and minority shareholders (<5%). Hui-fang Zhou designed and performed the arthritis model in addition to data analysis. Grace Hu designed and performed the MTX analysis. Samuel A Wickline, Gregory M Lanza and Christine TN Pham conceived and participated in the design of the study and helped draft the manuscript. This work was supported by grants from the NIH (AR056468, CA119342, HL073646, NS059302, and HL094470). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■ of considerable interest
- 1.Sizova L. Approaches to the treatment of early rheumatoid arthritis with disease-modifying antirheumatic drugs. Br. J. Clin. Pharmacol. 2008;66(2):173–178. doi: 10.1111/j.1365-2125.2008.03222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Der Bijl AE, Goekoop-Ruiterman YP, De Vries-Bouwstra JK, et al. Infliximab and methotrexate as induction therapy in patients with early rheumatoid arthritis. Arthritis Rheum. 2007;56(7):2129–2134. doi: 10.1002/art.22718. [DOI] [PubMed] [Google Scholar]
- 3.Zhou H-F, Chan HW, Wickline SA, Lanza GM, Pham CT. αvβ3-targeted nanotherapy suppresses inflammatory arthritis in mice. Faseb J. 2009;23(9):2978–2985. doi: 10.1096/fj.09-129874. ■■ First study to show that αvβ3-targeted nanoparticles conjugated to fumagillin accumulate specifically in inflamed joints and suppress the progression of experimental arthritis.
- 4.Duda DG, Jain RK, Willett CG. Antiangiogenics: the potential role of integrating this novel treatment modality with chemoradiation for solid cancers. J. Clin. Oncol. 2007;25(26):4033–4042. doi: 10.1200/JCO.2007.11.3985. ■■ Presents the rationale and current understanding of antiangiogenics and their therapeutic potential in combination with chemoradiotherapy.
- 5.Culy C. Bevacizumab: antiangiogenic cancer therapy. Drugs Today (Barc) 2005;41(1):23–36. doi: 10.1358/dot.2005.41.1.875776. [DOI] [PubMed] [Google Scholar]
- 6.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc. Res. 2007;74(2–3):72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winter PM, Caruthers SD, Zhang H, Williams TA, Wickline SA, Lanza GM. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JAAC Cardiovasc. Imaging. 2008;1(5):624–634. doi: 10.1016/j.jcmg.2008.06.003. ■■ Shows that integrin-targeted fumagillin nanoparticles can induce sustained antiangiogenic response in a model of atherosclerosis when combined with a statin.
- 8.Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J. Clin. Invest. 2002;109(3):363–371. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnell MA, Hardy C, Hawley M, Propert KJ, Wilson JM. Effect of blood collection technique in mice on clinical pathology parameters. Human Gene Ther. 2002;13(1):155–161. doi: 10.1089/10430340152712700. [DOI] [PubMed] [Google Scholar]
- 10.Storgard CM, Stupack DG, Jonczyk A, Goodman SL, Fox RI, Cheresh DA. Decreased angiogenesis and arthritic disease in rabbits treated with an αvβ3 antagonist. J. Clin. Invest. 1999;103(1):47–54. doi: 10.1172/JCI3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulte JWM, Lanza GM, Fuhrhop RW, et al. Gd-DTPA perfluorocarbon emulsions as a novel paramagnetic particulate contrast medium: T1 and T2 relaxometry; Presented at: Proceedings of International Society for Magnetic Resonance in Medicine 6th Annual Meeting; Sydney, Australia. 18–24 April; 1998. Abstract 209. [Google Scholar]
- 12.Lena N, Imbert AM, Brunet P, Cano JP, Carcassonne Y. Kinetics of methotrexate and its metabolites in red blood cells. Cancer Drug Deliv. 1987;4(2):119–127. doi: 10.1089/cdd.1987.4.119. [DOI] [PubMed] [Google Scholar]
- 13.Chan ES, Cronstein BN. Methotrexate – how does it really work? Nat. Rev. Rheumatol. 2010;6(3):175–178. doi: 10.1038/nrrheum.2010.5. ■■ Details the complex and multifaceted mechanisms that make methotrexate efficacious in the treatment of inflammatory disorders.
- 14.Varner JA, Brooks PC, Cheresh DA. Review: The integrin αvβ3: angiogenesis and apoptosis. Cell Adhes. Commun. 1995;3(4):367–374. doi: 10.3109/15419069509081020. [DOI] [PubMed] [Google Scholar]
- 15.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99(13):1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 16.Brdlik CM, Crews CM. A single amino acid residue defines the difference in ovalicin sensitivity between type I and II methionine aminopeptidases. J. Biol. Chem. 2004;279(10):9475–9480. doi: 10.1074/jbc.M307246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Bandt M, Grossin M, Weber AJ, et al. Suppression of arthritis and protection from bone destruction by treatment with TNP-470/AGM-1470 in a transgenic mouse model of rheumatoid arthritis. Arthritis Rheum. 2000;43(9):2056–2063. doi: 10.1002/1529-0131(200009)43:9<2056::AID-ANR17>3.0.CO;2-2. ■■ Early report showing that TNP-470, a soluble form of fumagillin, can prevent the development of experimental arthritis.
- 18.Herbst RS, Madden TL, Tran HT, et al. Safety and pharmacokinetic effects of TNP-470, an angiogenesis inhibitor, combined with paclitaxel in patients with solid tumors: evidence for activity in non-small-cell lung cancer. J. Clin. Oncol. 2002;20(22):4440–4447. doi: 10.1200/JCO.2002.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Neurath MF, Hildner K, Becker C, et al. Methotrexate specifically modulates cytokine production by T cells and macrophages in murine collagen-induced arthritis (CIA): A mechanism for methotrexate-mediated immunosuppression. Clin. Exp. Immunol. 1999;115(1):42–55. doi: 10.1046/j.1365-2249.1999.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szekanecz Z, Besenyei T, Paragh G, Koch AE. New insights in synovial angiogenesis. Joint Bone Spine. 2009;77(1):13–19. doi: 10.1016/j.jbspin.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szekanecz Z, Besenyei T, Szentpetery A, Koch AE. Angiogenesis and vasculogenesis in rheumatoid arthritis. Curr. Opin. Rheum. 2010;22(3):299–306. doi: 10.1097/BOR.0b013e328337c95a. ■■ Provides a current understanding of the role of angiogenesis in rheumatoid arthritis, and summarizes the possibilities of therapeutic intervention.
References
■■ Patent
- 101.BRISTOL-MYERS SQUIBB PHARMA COMPANY US6511648. 2003
References
■■ Website
- 201.Image J: image processing and analysis in Java. http://rsb.info.nih.gov/ij.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.