Abstract
Background
The functional significance of myocardial bridging remains controversial. The bridge morphology and structure of the tunneled vessels may modify its ultimate clinical effects.
Objective
To describe the morphological characteristics of myocardial bridges and their influence on coronary histology.
Design
A descriptive cross-sectional study.
Methods
One hundred and nine formalin-fixed adult hearts were evaluated by dissection for these data: prevalence, site, lengths and depths of myocardial bridges. Vessel segments proximal and distal to the bridged portion were also processed for histology and stained to elaborate smooth muscle, collagen and elastic fibers. Stereology was also employed to compare the relative sizes of the tunica intima and the vessel wall to lumen ratios.
Results
Myocardial bridges were found in 40.4% of the hearts, most commonly in the left anterior descending artery (LAD). The average length of the bridges was 22.66 +11.94 mm while the depth was 1.83+ 0.98mm, with only 11% being long (34.87mm – 50mm) and 9% of them being deep (3.46mm – 5.00mm). The tunica intima was thickest proximal to and thinnest under the myocardial bridge. The intima of the proximal segment was also more elastic. An elaborate perivascular ‘cushion’ of adipose tissue intervened between the intramural coronary and the surrounding myocardium.
Conclusions
Most myocardial bridges are superficial and short. Tunica intima under myocardial bridges is spared from “atherogenesis”. The thick perivascular space around the bridged segment may protect it from extreme compression.
Keywords: Myocardial Bridge, Morphology, Histological organization
Introduction
Myocardial bridges may compress the coronary vessel underneath and compromise myocardial blood supply. Cases of sudden cardiac death where myocardial bridging is the only postmortem finding have been reported1. There are reports of pathological observations indicating the virtual absence of atheromatous changes in the tunneled coronary segments while affecting the pre-bridged segments of the coronary1, 2. However, only a few bridges are reported to cause angina or myocardial infarction3. These patients (6% of patients with myocardial infarction) lack evidence of atherosclerosis on autopsy, with myocardial bridges as the only present culprit4. Although the postmortem prevalence of myocardial bridges may approach 30–40%, a much lower proportion of about 5% of the general population is reported to have coronary narrowing5. Akinesia of the myocardium in the setting of myocardial infarction may reduce the detection rate of myocardial bridges4. It is unclear whether the structure of the myocardial bridge, the structural relationship between the overlying myocardium and the tunneled coronary vessel may determine which bridge will compress the coronary artery. It is not known whether the bridged vessel is beset with structural elements that make it resilient to haemodynamic stresses imposed by such compression. The aim of the present study was to describe the morphological characteristics of the myocardial bridges and the structural composition of the coronary vessel under, before and beyond the myocardial bridges.
Methods
One hundred and nine formalin-fixed hearts were studied. These were all adult hearts obtained from consecutive autopsies of non-cardiac deaths at the Chiromo mortuary and the Nairobi city mortuary. Ethical approval was sought from the ethical review committee of the Kenyatta National Hospital - the University of Nairobi teaching hospital. The perivascular fat tissue was dissected to expose the coronary vessels which were then traced from the sinus of Vasalva to their terminations. Presence or otherwise of intramyocardial segments (coronary tunneling) were noted, photographed and recorded. The lengths and the depths (at the intermediate zone) of each bridge as well as their distances from the sinus of Vasalva were measured directly using a patchymeter (0.01mm precision).
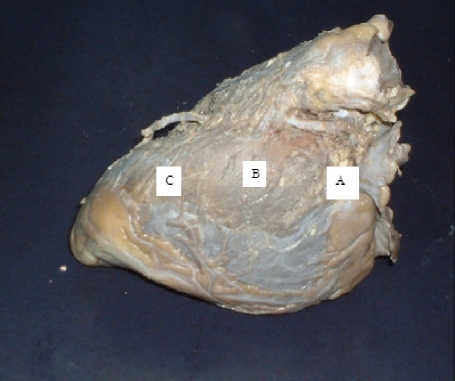
Five out of the 44 hearts beset with bridges were used for light microscopy and morphometry. These hearts were collected within 72hrs after death, and deemed to present the least effects of autolysis. Samples were taken from each of the three segments of the LAD namely 8mm proximal to the bridge (A), with the bridge (B) and 8mm distal to the bridge (C) (Fig 1) from each of the five hearts. The samples were removed enblock, dehydrated through increasing grades of alcohol and prepared for paraffin wax embedding. Many representative 7µm thick sections (on slides) were made and stained with Weigert resorcin fuschin and Van Geison stain, Mason's trichrome and Haematoxylin & Eosin to demonstrate structural details. The bridge fibers were denoted transverse when they ran perpendicular to the long axis of the involved vessel.
Figure 1.
A wide myocardial bridge over the middle third of the left anterior descending branch of the left coronary artery. The pre-bridge, bridged and post-bridge segments are labeled A, B and C
Morphometry was done by point counting to determine the relative thickness of the tunica intima, media and luminal area. Three best slides from each of the three segments of the coronary artery of each of the five hearts (15 slides per heart) were used for morphometry, giving a total of 45 slides. In each case, stained segments were projected to a screen (final magnification of x800) and a grid superimposed on to the image. The grid points falling on the tunica intima, media and lumen were counted and recorded. These were analyzed for means and variances using the statistical package of social sciences (SPSS) for windows version 11.5.0 Chicago, Illinois 2002. One way ANOVA was used to compare the mean thickness of the intimae of the pre, juxta and post-bridged segments of the LAD. A P value < 0.05 was considered significant.
Results
Branching pattern of coronary arteries
Ninety five (87.2%) of the hearts studied had the standard branching pattern of coronary arteries. The right coronary artery (RCA) fitted the standard description in all cases. Eight hearts had trifurcation of the left coronary artery (LCA) (7.3%), Four hearts (3.7%) had double LAD while one heart had trifurcated LAD. One heart had LCA that divided into four branches. This latter artery, half of the trifurcated LCA, and all the duplicated LAD had myocardial bridges related to the LAD.
Prevalence
Myocardial bridges (as shown in figure 1) were found in 44 (40.4%) hearts (32 males, 12 females). Most (n=39, 83.7%) of the bridges were found in the middle third of the left anterior descending (anterior interventricular) artery. Other bridged vessels included the right coronary artery (3, 6.8%) and the left circumflex, arteria ramus intermedius, posterior interventricular and the right marginal arteries had one bridge each (2.3%).
Muscle bridges in the majority of hearts (n = 36) were formed of broad transverse muscle fibers covering the vessel. Some coronary arteries disappeared into the myocardium not to resurface again. Three such vessels were observed on the left anterior descending, right coronary and the left circumflex arteries as shown in Figure 2.
Figure 2.
An extensive myocardial bridge (arrow heads) covering the whole middle and distal left circumflex artery - “disappearing circumflex coronary artery”.
For the left anterior descending artery, single bridges were found in 33 vessels while double bridges were recorded in 2 vessels. Five LADs were characterized by three or more bridges as shown in Table 1. Most of the bridges were located in its middle third at a point 43.51 + 12.24mm (range 10mm to 70mm) from the coronary sinus. The mean length of the MBs was 22.66 + 11.94mm (range 4.60mm–50mm).
Table 1.
Number and position of myocardial bridges on coronary vessels
| Characteristic | LAD | Mgl | Cx | RCA | Other |
| Number of bridges | |||||
| One | 33 | 0 | 1 | 1 | 2 |
| Two | 2 | 1 | 0 | 0 | 0 |
| Three and more | 5 | 0 | 0 | 0 | 0 |
| Position from sinus | |||||
| Proximal third | 1 | 1 | 0 | 1 | 0 |
| Middle third | 39 | 1 | 1 | 0 | 2 |
| Lower third | 3 | 0 | 0 | 0 | 0 |
LAD- left anterior descending artery, Mgl- marginal artery, Cx- left circumflex artery, RCA- right coronary artery
The bridge length was characterized as short (4.60mm –19.73mm; 46%), intermediate (19.74mm – 34.86mm; 43%) and long (34.87mm – 50mm; 11%). The depth of the bridges averaged 1.83 + 1mm (range 0.30 mm – 5.00 mm). They were superficial (0.30mm –1.87mm; 68.3%), intermediate (1.88mm – 3.45mm; 22.7%) and deep (3.46mm – 5.00mm; 9%). However the length-depth correlation was not significant (p= 0.28). The results for length and depths of the bridges were similar for male and female subjects as shown in table 2.
Table 2.
lengths, depths and positions of myocardial bridges
| Measure | Male | Female | ||
| Mean | SD | Mean | SD | |
| MB from coronary sinus |
43.63 | 11.85 | 43.18 | 11.67 |
| Length of MB | 22.49 | 11.67 | 23.22 | 13.52 |
| Depth of MB | 1.70 | 0.65 | 2.22 | 0.98 |
MB- myocardial bridge, all distances are in millimeters
Microscopic organization
Figure 3 shows the typical organization of the muscular bridge. The muscle fibers were arranged in longitudinal, transverse and oblique layers over the intramural coronary vessel. Interspersed between the muscle fibers were blood vessels and nerves.
Figure 3.
Light micrograph of a myocardial bridge. Note the connective tissue trabeculae and vascular elements within them. Masson's Trichrome (Mag. X 100)
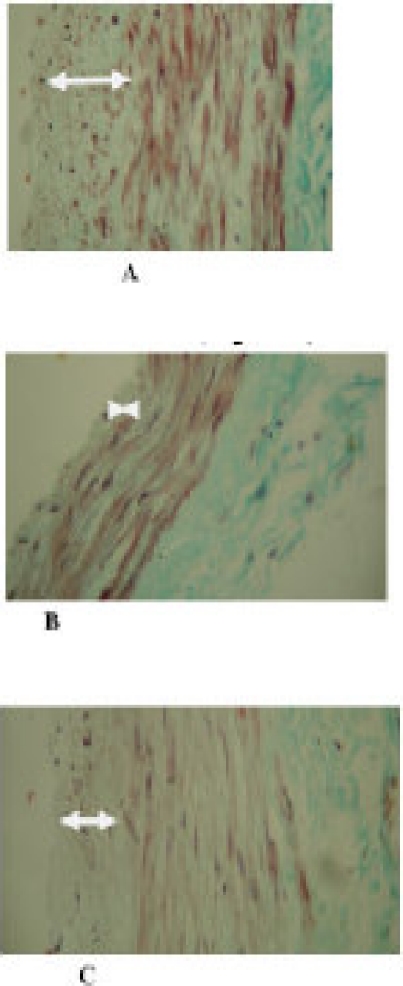
The tunica intimae of the pre-bridge and that of the post-bridge coronary vessel appeared thicker (thickest proximally) than that of the tunneled segment as shown in Figure 4. When compared with the subendothelial zone of the prebridged zone, the bridged vessel as segment was flimsy while that of the prebridged zone contained abundant connective tissue including elastic fibers as shown in Figure 4 and 5. The vessel segment under the myocardial bridge was surrounded by a prominent perivascular space characterized by preponderance of adipose tissue in many places as shown in Figure 3. Nerves and blood vessels were found in this space.
Figure 4.
Sections through the coronary artery at different segments showing the differential dimensions of the vessels tunica intima. Section A (before the bridge), B (under the muscle bridge), C (distal to the bridge). Masson's Trichrome stained (Mag. X 400)
Figure 5.
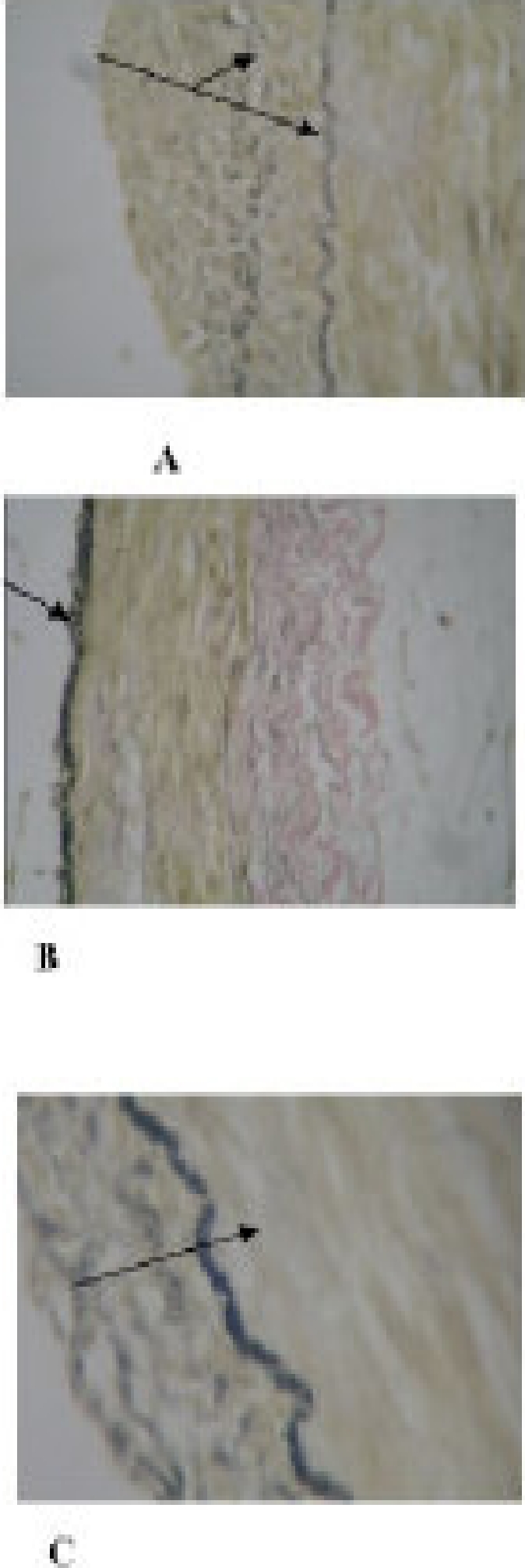
The internal elastic lamina (IEL) is duplicated (shown by the arrows) in A while prominent in B and C. The tunical intima is prominent in A and C but flimsy/nonexistent in B. the darker fibers within the tunica intima are elastic. Weigert Elastic stained (Mag. X 400)
Morphometry
Using one-way ANOVA there was a significant difference (p=0.01) between the thickness of the tunica intima at the position of the bridge (13.82+2.90) and both distal (17.67+3.68) and proximal (18.90+2.52) zones as shown in table 3.
Table 3.
Thickness of the three segments of the LAD
| Count | Components of the vessel | ||
| Tunica intima | Tunica media | Lumen | |
| Av points counted Proximal (N = 15) | 18.90 + 2.52 | 21.03 + 2.73 | 31.32 + 2.21 |
| Under the bridge (N = 15) | 13.82 + 2.90 | 16.74 + 1.36 | 30.00 + 3.14 |
| Distal (N = 15) | 17.67 + 3.68 | 13.50 + 2.70 | 28.64 + 3.56 |
However on Post-hoc analysis, there was no significant difference between the proximal and distal intimal thickness. As shown in the table, there was a progressive proximal to distal reduction in the thickness (21.03 + 2.73:13.50 + 2.7, p= 0.05) of the tunica media and the area (31.32 + 2.21:28.64 + 3.56, P= 0.06) of the lumen along the vessel.
Discussion
Although the prevalence of MB shown in this study is higher than the earlier report by Saidi et al. 2 among Kenyans (29%), the observations are consistent with the widely quoted prevalence of 15–85% at necropsy 3,5,6,7. Such disparate autopsy prevalence rates may result from the selection and preparation of the hearts as well as variations in definitions 5, 8.
Consistent with the literature in earlier necropsy studies 1, 3, 7, 9, 10, the middle LAD coronary artery was the most common location for myocardial bridges in the current study. In cases of duplication, triplication and even a quadriplication of LAD, MB occurred in only one of the branches. Similar observations have been made by Vanildo et al. 11. Presumably, such arrangement ensures collateral conduits for myocardial perfusion when one of the LADs is occluded. It remains to be determined whether the branching of the coronary was adaptive or merely coincidental. The factors dictating the preponderant middle location also remain unclear.
The variations in the lengths and breadths of the myocardial bridges in the present study are also consistent with literature 1,7,12. We however have noted that only 11% of the bridges were very long. These bridges are bound to significantly occlude the coronary artery compared to the short bridges. An intriguing finding in our study was that some coronary arteries took intramural courses never to resurface to the subepicardial layer again. Such a prolonged intra-myocardial course may predispose to myocardial ischemia in systole and diastole in the absence of atheroma due to the degree of systolic compression 7, 13, 14.
The depth of MB in our study is in agreement with earlier reports depicting depths between 1mm to 10mm 2, 5, 7. As the depths and lengths of myocardial bridges are thought to impact on patient symptoms, the variable measurements documented in the current study explain the great discrepancy in the prevalence of clinically significant bridges. Now that only 9% of the bridges are very deep, they are bound to occlude the coronary more than the superficial ones. Additional aspect of bridge morphology that makes prediction for which bridge would cause symptoms is the muscle fiber arrangement over the tunneled vessel. We observed oblique, transverse and longitudinal patterns of muscle fibers forming the bridges without any predictable order/pattern. We have no explanation and the literature is unclear on what differential effects ensue for transverse, oblique or longitudinal pattern of bridge muscle fiber arrangements.
We observed that the bridged segment of the coronary artery tended to have a reduced tunica intima as compared to the proximal and the distal segments. This is consistent with the observations in bovines and earlier human observations 15. There have been suggestions that the myocardial bridge predisposes the proximal segment to atherosclerosis6. Conversely, the bridge protects the tunneled segment from atherosclerosis, manifesting with a reduced intima even in comparison to the distal zone. Compression of the tunneled segment may be associated with increased resistance to flow and a reduced mural tension for any given tension as deduced from Laplace principle 16, 17. Ge et al. 18 have recorded the highest intravascular pressure in the coronary artery just proximal to the bridge and a pressure gradient across the bridge with a distinctive negative pressure in late diastole proceeded with a peak beneath the bridge. They interpreted the existence of a central pressure chamber as a result of heterogeneous compression of the tunneled segment with higher proximal and distal forces compared with the central portion, potentially contributing to reduced coronary flow reserve 3.
We observed more elastic fibers in the intima of the proximal and distal segments while a minimal amount was found in the tunneled tunica intima. Whether the content of elastic fibers has a direct relation to the absence of atheromas in the bridged vessel is not clear. Elastic fibers endow blood vessels with critical properties of resilience, permitting long range deformability and passive recoil without energy input. It is conceivable that, the elastic tissue in muscular arteries serves not only to supply a certain level of maintenance of tension but also in cooperation with smooth muscle cells, make possible graded vasomotor responses 19, 20. By tugging on the smooth muscle, the two mural components form a single unit of structure whose principle function is to dampen tensile stresses during the pulsatile motion occurring in each cardiac cycle. The concentration of elastic fibers in the segments proximal and distal to the bridge may relate more to hemodynamics in these segments than mere atherosclerosis.
The distinct perivascular spaces on the bridged coronary artery, comprising adipose tissue, may constitute the ‘coronary cushion’ that limits the compressive forces on the tunneled vessels during systole. The distance between the MB and the intramural coronary artery may be a factor in determining the size of the force exerted 13. We contend that variability of the organization of this space may also be a factor in the difficult task of predicting which bridge would be symptomatic.
Conclusion
Myocardial bridging is common in this African population. Most of the myocardial bridges are superficial and short and may be asymptomatic. The presence of a myocardial bridge results in changes in the pre-bridge coronary segment akin to early atherosclerosis. The fibroreticular cushion might have a protective role on the intramural coronary artery. Functional studies are needed to determine which of the lengths, breadths and sizes of perivascular space relate to bridges with symptoms.
References
- 1.Angelini M, Donis J, Leachman RD. Myocardial bridges: a review. Prog Cardiovasc Disease. 1983;26:75–78. doi: 10.1016/0033-0620(83)90019-1. [DOI] [PubMed] [Google Scholar]
- 2.Saidi HS, Olumbe AO, Kalebi A. The anatomy and pathology of coronary arteries in adult Kenyans. EAMJ. 2002;79(6):323–327. doi: 10.4314/eamj.v79i6.8853. [DOI] [PubMed] [Google Scholar]
- 3.Edward JC, Burnsil C, Swan RL, Canshi AJ. Artherosclerosis in intramural and extramural portions of coronary arteries in human heart. Circulation. 1956;13:235–241. doi: 10.1161/01.cir.13.2.235. [DOI] [PubMed] [Google Scholar]
- 4.Widmann JD, Cox SL. Roongsritong. Unappreciable Myocardial Bridge Causing Anterior Myocardial Infarction and Postinfarction Angina. South Med J. 2003;96(4):400–402. doi: 10.1097/01.SMJ.0000061161.32715.FD. [DOI] [PubMed] [Google Scholar]
- 5.Resar JR, Brinker JA. Bridge work. Cathet Cardiovascular diagnosis. 1997;41:421–422. doi: 10.1002/(sici)1097-0304(199708)41:4<421::aid-ccd18>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Mohlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation. 2002;106:2616. doi: 10.1161/01.cir.0000038420.14867.7a. [DOI] [PubMed] [Google Scholar]
- 7.Loukas M, Curry B, Bowers M, Loius RG, Bartczak A, Kiedrowski M, Kamionek M, Fudalejek M, Wagner T. The relationship of myocardial bridges to coronary artery dominance in the adult human heart. J Anat. 2006;209:43–50. doi: 10.1111/j.1469-7580.2006.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polacek P. Relationship of Myocardial bridging & loop to coronary occlusion. Am Heart Journal. 1961;61:44–52. doi: 10.1016/0002-8703(61)90515-4. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira AG, Trotter SE, Kong B, Decourt LV, Fox K, Olsen EJ. Myocardial bridge: morphological and functional aspects. Br Heart J. 1991;66:364–367. doi: 10.1136/hrt.66.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishimori T, Raizner AF, Chabli RA, Adek M, Luchi R. Myocardial bridging in man: clinical correlates & angiography accentuated with nitrogliceride catheterization. Cardiovasc Diagn. 1977;3:59–65. doi: 10.1002/ccd.1810030107. [DOI] [PubMed] [Google Scholar]
- 11.Vanildo ML, Cavalcant JS, Tashiro T. Myocardial Bridges and their relationship to the anterior interventricular branch of the left coronary artery. Arq Bras Cardiol. 2002;79(3):31–35. doi: 10.1590/s0066-782x2002001200002. [DOI] [PubMed] [Google Scholar]
- 12.Bourassa MG, Lesperance J, Butnaru A, Tardiff JC. Symptomatic myocardial bridges: Over view of ischaemic mechanisms and current diagnosis and therapeutic strategies. J Am Coll Cardiol. 2003;41:351–359. doi: 10.1016/s0735-1097(02)02768-7. [DOI] [PubMed] [Google Scholar]
- 13.Mays AE, McHale PA, Greenfield JC. Transmural coronary myocardial flow in a canine model of myocardial bridging. Circ Res. 1981;49:726–732. doi: 10.1161/01.res.49.3.726. [DOI] [PubMed] [Google Scholar]
- 14.Ozbag D, Kervancioglu P. The investigation of perivasculary space under the myocardial bridge in different species. Int J Clin Pract. 2004;58(11):1008–1013. doi: 10.1111/j.1742-1241.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 15.Ishii T, Asuwa N, Masuda S. The effects of a myocardial bridge on coronary atherosclerosis and ischaemia. J Pathol. 1998;85:4–9. doi: 10.1002/(SICI)1096-9896(199805)185:1<4::AID-PATH50>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Shotar A, Busutil A. Myocardial bars and bridges and sudden death. Forensic Sci Int. 1994;68:143–147. doi: 10.1016/0379-0738(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 17.Bandyopandhyay B, Kulkarni S, Rohia M, Cherian KM. Extrinsic obstruction of main coronary artery in a child with HOCM. India Heart J. 2002;54:202–205. [PubMed] [Google Scholar]
- 18.Ge J, Erbel R, Gorge G, Haude M, Meyer J. High wall shear stress proximal to myocardial bridging and arteriosclerosis. Intracoronary ultrasonography and pressure measurements. Br Heart J. 1995;73:462–465. doi: 10.1136/hrt.73.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimani JK. Some aspects of the structural organization of the carotid arterial system (Giraffe camelopardis) with special reference to the carotid sinus baroreceptor equivalent. 1979. PhD thesis of the University of Nairobi. [Google Scholar]
- 20.Klues HG, Swarz ER, Vom Dahl J. Disturbed intracoronary haemodynamics in myocardial bridging. Early normalization by intracoronary stent placement. Circulation. 1997;96:2905–2913. doi: 10.1161/01.cir.96.9.2905. [DOI] [PubMed] [Google Scholar]