Abstract
In order to develop a xenograft model to determine the efficacy of new therapies against primary human precursor-B acute lymphoblastic leukemia (ALL) stem cells (LSCs), we used the highly immunodeficient non-obese diabetic (NOD).Cg-PrkdcscidIL2rgtmlWjl/SzJ (NOD-severe combined immune deficient (scid) IL2rg−/−) mouse strain. Intravenous transplantation of 2 of 2 ALL cell lines and 9 of 14 primary ALL cases generated leukemia-like proliferations in recipient mice by 1–7 months after transplant. Leukemias were retransplantable, and the immunophenotypes, gene rearrangements and expression profiles were identical or similar to those of the original primary samples. NOD-scid mice transplanted with the same primary samples developed similar leukemias with only a slightly longer latency than did NOD-scid-IL2Rg−/− mice. In this highly sensitive NOD-scid-IL2Rg−/−-based assay, 1–100 unsorted primary human ALL cells from five of five tested patients, four of whom eventually experienced leukemia relapse, generated leukemias in recipient mice. This very high frequency of LSCs suggests that a hierarchical LSC model is not valuable for poor-outcome ALL.
Keywords: childhood acute lymphoblastic leukemia, leukemia stem cells, xenograft
Introduction
Precursor-B acute lymphoblastic leukemia (ALL) is the most common form of cancer in children. Although ∼80% of children can be cured with current chemotherapy regimens, cases with high relapse risk can be defined at diagnosis,1 and relapsed ALL remains a leading cause of cancer deaths in childhood.2 Moreover, adult ALL has a much poorer outcome.3 It is anticipated that new technologies such as next-generation sequencing of cancers4 will identify many new potential target molecules in ALL, and there will be an increasing need for predictive preclinical models of human ALL, to separate ‘driver' from ‘passenger' mutations and to investigate the biology of candidate target molecules.5 In addition, preclinical models can speed the evaluation of rationally targeted agents and their optimal incorporation into combination chemotherapies.6 Finally, the cancer stem cell theory7, 8, 9 predicts that efficacy against LSCs is required for cure, emphasizing the need for preclinical models that measure LSCs.
Human leukemia xenografts in immunodeficient mice have been used quite extensively in drug development.10 In ∼60–70% of primary ALL samples,11, 12, 13 human leukemia-like proliferations develop in non-obese diabetic (NOD).Cg-Prkdcscid (NOD-severe combined immune deficient (scid)) mice following intravenous injection, as in acute myeloid leukemia (AML).8, 14, 15, 16, 17 These human ALL cells generate clinical signs and findings in the NOD-scid mice that closely replicate human ALL. In addition to providing an operational definition for LSCs, the NOD-scid model has predicted patients' clinical responses to treatment with established11 and novel anti-leukemia agents,18 alone and in combination.19 Herein, we describe the biology after transplant of unpurified primary human leukemia cells from several cases of childhood precursor-B ALL into the highly sensitive NOD.Cg-PrkdcscidIL2rgtmlWjl/SzJ (NOD-scid IL2rg−/−) mouse strain.20, 21, 22 As our long-term goals were to develop new understanding and treatment of cases that cannot currently be cured, we obtained most of these samples from ALL cases at relapse and/or with high-risk features and/or poor eventual outcomes.
Materials and methods
Precursor-B ALL cell lines and primary patient samples
The REH and KOPN8 cell lines, derived initially from children with precursor-B ALL, were obtained from the American Tissue Culture Collection (Manassas, VA, USA) and the Deutsche Sammlung von Mikroorganismen und Zelkuturen GmbH (Braunschweig, Germany), respectively, and were cultured in RPMI 1640 medium containing 2 m-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin (all from Invitrogen, Carlsbad, CA, USA) and 10–20% fetal bovine serum (Gemini Bio-products, Calabasas, CA, USA).
Fourteen primary ALL blast samples from children with precursor-B ALL were obtained from the National Cancer Institute (NCI) and Johns Hopkins University (JHU) pediatric leukemia patient cell banks (Supplementary Table 1), under Institutional Review Board-approved research protocols. All cryopreserved patient samples contained ⩾94% CD19+CD22+ blast cells. Cells were thawed and resuspended in PBS before injection into mice. For five cases, cell dose titration and transplantation experiments were performed and LSC frequency was calculated (Table 1). Quintuplicate cell counts were performed for cells at the highest serial dilutions; no carrier cells were used. In addition to the actual titered transplants, two actual (Supplementary Table 5) and one statistical (Supplementary Table 6) simulation experiments were performed.
Table 1. High LSC frequencies in each of five primary childhood precursor-B ALL cases and two childhood precursor-B ALL cell lines.
| ALL primary case or cell line | Frequency of leukemia (post-transplant days to leukemia)a | LSC frequency (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 106 cells | 105 cells | 104 cells | 103 cells | 102 cells | 10 cells | 1 cell | 0 cells | ||
| ALL case no. 1 | 3/3 (35) | 3/3 (42) | 3/3 (50) | 3/3 (58) | 5/5 (66) | 3/10 (76) | 0/10 (207) | 0/3 (207)b | 1.95* |
| ALL case no. 2 | 3/3 (49) | 3/3 (55) | 3/3 (61) | 3/3 (67) | 5/5 (79) | 0/10 (194) | 0/10 (194) | 0/3 (194)b | 1.30** |
| ALL case no. 3 | 3/3 (72) | 3/3 (88) | 3/3 (95) | 3/3 (115) | 5/5 (143) | 10/10 (174) | 2/8 (208) | 0/3 (230)b | 23.90* |
| ALL case no. 4 | 3/3 (65) | 3/3 (80) | 3/3 (113) | 3/3 (135) | 4/5 (161) | 0/10 (243) | 0/10 (243) | 0/3 (243)b | 1.03* |
| ALL case no. 5 | 3/3 (90) | 3/3 (95) | 3/3 (116) | 3/3 (120) | 5/5 (140) | 0/10 (243) | 0/10 (243) | 0/3 (243)b | 1.30** |
| REH | 3/3 (28) | 3/3 (32) | 3/3 (38) | 3/3 (45) | 2/5 (55) | 0/10 (138) | 0/10 (138) | 0/3 (138)b | 0.74** |
| KOPN8 | 3/3 (28) | 3/3 (33) | 3/3 (40) | 3/3 (53) | 3/5 (61) | 0/10 (145) | 0/10 (145) | 0/3 (145)b | 0.86** |
Abbreviations: ALL, acute lymphoblastic leukemia; LSC, leukemia stem cell.
Values shown represent numbers of mice with leukemia/all mice transplanted with the dose of ALL cells specified for that column (mean post-transplant days to leukemia; for every experimental group, all mice in the group developed clinical signs within 3 days after the first mouse). For each case, the data are in bold for the lowest cell dose resulting in leukemias.
Day at which the experiment was terminated. Each mouse that had not developed clinical signs of leukemia was killed at the end of the experiment for that group (i.e. 22–131 days after the day when the last mouse transplanted with cells from that ALL case developed clinical signs of leukemia); absence of leukemia was confirmed in each of these mice by necropsy showing normal-sized spleen and flow cytometry detecting no human ALL blasts.
*P-value of trend (P value of the F statistic) <0.001.
**P-value of trend (P value of the F statistic) <0.01.
Titered doses of unpurified precursor B-ALL primary cells or cell lines were transplanted into NOD-scid IL2rg−/− mice.
Human ALL-immunodeficient mouse chimera models
The use of mice in this study was approved by the Institutional Animal Care and Use Committees of Johns Hopkins University and the University of Maryland. NOD-scid IL2rg−/− and NOD-scid mice, obtained initially from the Jackson Laboratory (Bar Harbor, ME, USA), were bred and housed in the animal facilities of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins or the University of Maryland School of Medicine. Mice were handled under sterile conditions in a laminar flow hood. Sublethally irradiated (250 cGy) 6–8-week-old NOD-scid IL2rg−/− and NOD-scid mice were injected intravenously (lateral tail vein) with human ALL cells. Mice were monitored daily and euthanized if severely ill. For serial transplantation studies, spleens of euthanized mice were harvested and dissociated into single-cell suspensions. In all experiments, the percentage of human CD19+CD22+ blastic leukemia cells in these splenocyte preparations (splenocytes) was 94–99%.
Histology, flow cytometry, immunoglobulin heavy chain (IgH) gene rearrangements, molecular karyotypes, statistics and gene expression microarrays
Please see Supplementary Material.
Results
Human ALL cell lines generated clinical signs of human ALL and fatal, retransplantable, leukemia-like proliferations in NOD-scid-IL2Rg−/− mice
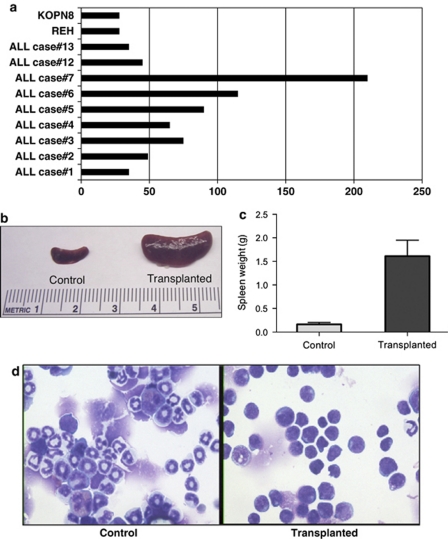
Two childhood precursor-B ALL cell lines were intravenously transplanted into groups of sublethally irradiated young adult NOD-scid IL2rg−/− mice. At approximately 1 month after transplant of 106 cells per mouse of either the REH or KOPN8 ALL cell lines, all recipient mice died suddenly or had severe clinical signs suggestive of leukemia (Figure 1a), including pallor, bleeding, weight and hair loss, hunched posture, and rear limb paralysis. All mice had massive spleens (Figures 1b and c) and hyperplastic bone marrows at necropsy. Blood, spleens and marrows were essentially replaced with cells with morphology (Figure 1d) and human-leukocyte differentiation antigen immunophenotype matching those of the transplanted human ALL cell lines (Supplementary Figure 1). Splenocytes (⩾94% human blasts) of transplanted mice were successfully secondarily transplanted into naive NOD-scid IL2rg−/− mice. No significant differences were observed in times-to-(fatal clinical) leukemias after secondary, as compared with primary, transplants (Supplementary Table 2 and Supplementary Figure 5).
Figure 1.
Nine of fourteen primary childhood precursor-B ALL cases and two of two cell lines generated leukemia-like proliferations in NOD-scid IL2rg−/− mice 1–7 months after intravenous transplantation of one million cells (a). Massive splenomegaly was the most prominent gross pathologic feature in all the mice (b, c), accompanied by replacement of blood, spleen and marrow by human cells with the blast morphology of the original precursor-B ALL cases and cell lines (d).
Characteristics of the 14 primary human ALL cases tested
We selected only cases with at least 10 vials of cryopreserved cells in the bank, so that it would be possible to perform additional experiments using aliquots of the identical specimen. Eight ALL samples (ALL cases 1–3, 8, 9 and 12–14) were from multiply relapsed, chemotherapy-refractory patients, all of whom died of ALL. ALL case 1 harbored the BCR-ABL translocation, a powerful poor prognostic factor. ALL cases 12 and 13 harbored the ETV6-RUNX1 and MYC-IGH translocations, respectively. Six samples (ALL cases 4–7, 10 and 11) were from patients at initial diagnosis, before chemotherapy. ALL cases 4 and 7 relapsed after 0.9 and 3.5-year continuous complete remissions (CCRs), respectively, and died of ALL. ALL cases 5, 6, 10 and 11 remain in CCR at >5, >5, >3.5 and >4 years, respectively (Supplementary Table 1).
Out of 14 primary ALL cases tested, 9 generated fatal, retransplantable human ALL in NOD-scid-IL2Rg−/− mice
All mice transplanted with 106 ALL cells from each of 9 of the above 14 (64.3%) primary cases tested (that is, ALL cases 1–7, 12 and 13) died or developed severe clinical signs suggestive of leukemia and requiring euthanasia (Figure 1a). Clinical signs and necropsy results in these mice were similar to those observed in mice transplanted with the REH and KOPN8 ALL cell lines. Times-to-leukemia in transplanted mice varied from 1 to 7 months after transplant, depending on the primary ALL case (Figure 1a). Splenocytes from these cases were successfully secondarily transplanted into naive NOD-scid IL2rg−/−mice (Supplementary Table 2 and Supplementary Figure 5).
Mice transplanted with 106 cells from 5 of the 8 (62.5%) primary samples obtained from patients at relapse (ALL cases 1–3, 12 and 13) developed leukemias, at 1–3 months post-transplant. Mice transplanted with 4 of the 6 (67%) primary samples obtained from patients at initial diagnosis (ALL cases 4–7) developed leukemias more slowly, at 2–7 months post-transplant, but the difference was not significant in this small sample (P=0.15). There were no consistent immunophenotypic differences between the set of ALL cases (1–7, 12 and 13) that generated leukemias in mice and the set of cases (8–11 and 14) that did not engraft.
Immunophenotypes of splenocytes from transplanted immunodeficient mice were similar to those of the pre-transplant ALL samples
Primary cells from each of the childhood precursor-B ALL cases strongly expressed human CD45 and the human CD10, CD19 and CD22 B-lymphoid markers (Supplementary Figure 1). ALL cases 1 and 3–7 expressed human CD20; cases 1, 3, 4, 6, 7 and 13 expressed human CD34. For ALL cases 1–4, 6, 7 and 12, the human-leukocyte differentiation antigen immunophenotypes of the cells harvested from the blood, spleens and marrows of recipient mice closely matched those of the transplanted human primary patient samples, qualitatively and quantitatively for every tested monoclonal antibody (Supplementary Figure 1). Cells from mice transplanted with ALL case 5 had decreased CD19 intensity, as compared with the primary sample (Supplementary Figure 1). Stably decreased CD19 intensity was observed in ALL case 5 cells from secondarily transplanted mice. The immunophenotype of cells from mice transplanted with ALL case 13 had somewhat increased CD34 intensity, as compared with the primary sample (Supplementary Figure 1). None of the tested primary cases or human cells from mice expressed either the CD13 or the CD33 human myeloid markers.
The patient-specific clonal gene rearrangements of the primary samples were observed in splenocytes from the immunodeficient mice
In all five primary cases tested (ALL cases 1–5), immunoglobulin heavy chain VH to JH, or VH to DH to JH, rearrangements were identical in primary samples and in the corresponding splenocytes from the recipient mice (Supplementary Figure 2). Molecular karyotyping of ALL cases 1–5 revealed that the BCR–ABL translocation in primary cells was obtained directly from the patient and from splenocytes of the transplanted mice.
In four of five cases, global gene expression patterns closely matching those of the primary samples were observed in splenocytes from immunodeficient mice
We performed cDNA microarray analyses on ALL cases 1–5 before and after transplant into NOD-scid IL2rg−/− mice. By unsupervised hierarchical clustering using all probes on the array, four of the five tested primary samples clustered nearest to their counterpart splenocytes from transplanted mice (Supplementary Figure 3). Exceptionally, the global gene expression of splenocytes from mice transplanted with ALL case 5 differed somewhat from that of the primary sample (Supplementary Figure 3). In the data from all five ALL cases, expression levels of only a small number of genes (<5%) differed by twofold or more between primary cells and splenocytes (Supplementary Figure 4).
Titrations of transplanted cell dose to quantitate LSC frequencies
All NOD-scid IL2rg−/− mice transplanted with ⩾1000 REH or KOPN8 cells developed clinically severe leukemias by ⩽53 days (mean) post-transplant. In all, 60 or 40% of mice transplanted with 100 REH or KOPN8 cells developed leukemias by 61 or 55 days, respectively (Table 1). Mice scored as not developing leukemia were observed for at least 3 weeks beyond the time that the final mouse developed leukemia; at this time, necropsy revealed normal organ size and appearance, and flow cytometry detected no human ALL blast cells. Calculated LSC frequencies were 0.74% for the REH and 0.86% for the KOPN8 cell line. In both REH and KOPN8 cell lines, the times-to-leukemia correlated with the number of cells transplanted, following an inverse log(cell dose)–linear(time) model (P<0.01 for the trend) (Supplementary Figure 5).
Next, we transplanted titered doses of unpurified primary childhood precursor-B ALL cells from five of the primary cases that had generated leukemias in mice by ⩽100 days after transplant of 106 ALL cells (ALL cases 1–5); this 100-day criterion was selected because it was anticipated that lower cell doses would require very long times to generate leukemias. For each ALL case tested, times-to-leukemia in the transplanted mice followed an inverse log-linear model (P<0.01) (Table 1 and Supplementary Figure 5). As above, the NOD-scid IL2rg−/− mice within each experimental group (that is, transplanted with the same dose of the same case) developed clinical signs of leukemia with little variation in time (<3 days). In all five of these primary ALL cases, 100% of the NOD-scid IL2rg−/− mice transplanted with 103–106 unpurified cells developed leukemias by 135 days (Table 1), and secondary transplants resulted in leukemias with times-to-leukemia closely similar to those of the initial transplants (P<0.05) (Supplementary Table 2 and Supplementary Figure 5).
All mice transplanted with ⩾100 unpurified cells from primary ALL case 1 developed severe leukemias by ⩽66 days post-transplant, and 3 of 10 mice transplanted with 10 cells developed leukemias by 76 days post-transplant. However, none of the 10 mice transplanted with one cell developed clinical leukemia by 207 days; at this time point, the experiment was terminated and flow cytometry detected no leukemia in the mouse splenocytes. The calculated LSC frequency in ALL case 1 was 1.95% (even higher than that for the REH and KOPN8 cell lines). Secondary transplant of 10 splenocytes from mice that had been transplanted with 10 primary cells from ALL case 1 generated leukemias, with time-to-leukemia closely similar to that observed after the initial transplant of 10 primary cells (P<0.01) (Supplementary Table 2 and Supplementary Figure 5).
With primary ALL case 2, all mice transplanted with ⩾100 unpurified cells developed severe leukemias by ⩽79 days post-transplant. However, none of 10 mice transplanted with 10 cells or 1 cell developed clinical leukemia by termination of the experiment at 194 days, and no leukemia cells were detected in the mouse splenocytes by flow cytometry. Thus, in ALL case 2, the time-to-leukemia was somewhat longer than in ALL case 1, and the calculated LSC frequency was also somewhat lower (1.30%). Secondary transplants of splenocytes from mice that had been transplanted with 100 primary cells from ALL case 2 generated leukemias, with time-to-leukemia being similar to that observed after the initial transplant of 100 primary cells (P<0.01) (Supplementary Table 2 and Supplementary Figure 5).
With primary ALL case 3, all mice transplanted with ⩾10 unpurified cells developed severe leukemias by ⩽174 days post-transplant. Two of eight mice transplanted with one primary ALL cell developed leukemia by 208 days; however, the remaining six mice in this experimental group transplanted with one cell did not develop clinical leukemia by termination of the experiment at 230 days, when no leukemia was detected in the mouse splenocytes by flow cytometry. Secondary transplant of splenocytes from mice that had been transplanted with one primary cell generated leukemias, with time-to-leukemia being similar to that observed after the initial transplant of one primary cell (P<0.05) (Supplementary Table 2 and Supplementary Figure 5). ALL case 3 had the highest LSC frequency (23.9%) among the five titered cases, but the second longest time-to-leukemia, indicating lack of correlation between these two variables. Experimental (using REH cells) and statistical simulations were performed to model the likelihood of possible cell dose variations among mice in the experimental group intended to receive one ALL case 3 cell. The results suggested that it was unlikely that only two mice in the simulated experimental group received all the cells intended to be evenly distributed among the 10 mice transplanted (P<0.05) (Supplementary Tables 5 and 6).
With primary ALL case 4, all mice transplanted with ⩾1000 unpurified cells and four of five mice transplanted with 100 cells developed severe leukemia by ⩽161 days. Neither the remaining one mouse in the experimental group transplanted with 100 cells nor any of the mice transplanted with 10 cells or 1 cell developed clinical leukemia by termination of the experiment at 243 days, and no leukemia cells were detected in the mouse splenocytes by flow cytometry. Secondary transplant of splenocytes from mice that had been transplanted with 100 cells generated severe leukemias, with similar time-to-leukemia to that observed after the initial transplant of 100 primary cells (P<0.01) (Supplementary Table 2 and Supplementary Figure 5). ALL case 4 had a calculated LSC frequency of 1.03%.
With primary ALL case 5, all mice transplanted with ⩾100 cells developed severe leukemias by ⩽140 days. None of the mice transplanted with 10 cells or 1 cell developed clinical leukemia by termination of the experiment at 243 days, and no leukemia cells were detected in the mouse spleens by flow cytometry. Secondary transplant of splenocytes from mice that had been transplanted with 100 cells generated leukemias, with similar time-to-leukemia to that observed after the initial transplant of 100 primary cells (P<0.01) (Supplementary Table 2 and Supplementary Figure 5). ALL case 5 had a calculated LSC frequency of 1.30%.
Transplants in classical NOD-scid mice
To examine whether our observations might be dependent on the use of the new highly immunodeficient NOD-scid IL2rg−/− mouse strain, a somewhat more restricted number of cell doses was simultaneously titered into classical NOD-scid mice. As with the NOD-scid IL2rg−/− mice, time-to-leukemia in the transplanted NOD-scid mice was dependent on (1) ALL case and (2) ALL cell dose. The onset of leukemia was delayed by up to 3 weeks in the NOD-scid model compared with the NOD-scid IL2rg−/−, but both immunodeficient mouse models indicated that low numbers of transplanted ALL cells generated leukemias (Supplementary Table 3 and Supplementary Figure 6). Several of the transplanted NOD-scid mice had to be censored because of deaths due to murine thymic lymphomas that occur spontaneously in this strain.23
Discussion
In most cases, primary precursor-B ALL cells undergo rapid apoptosis in vitro. Although addition of cytokines and stromal feeder cells to cultures can prolong ALL cell survival for hours to days, few cases proliferate and expand ex vivo.24, 25, 26, 27, 28, 29 In contrast, many previous studies have shown that primary cells from the majority of cases of human acute leukemias can overcome the histocompatibility barrier after intravenous transplant into immunodeficient mice to engraft, survive, proliferate and disseminate, especially in the bone marrow and other hematopoietic organs. The resulting human leukemia-like cell proliferations in this xenogeneic environment are quite similar to human clinical leukemias.24, 30, 31, 32 Thus, the growth of primary human leukemia cells in immunodeficient mice offers an attractive assay for studies of leukemia biology and treatment, and such xenograft assays in NOD-scid mice provide the current ‘gold-standard' assay for LSCs.7, 9 It should be noted that most of these leukemia xenograft studies have been done with human AML, and many AML cases proliferate in a relatively benign manner in NOD-scid mice without causing death of the mice.8, 14, 33
Because of absent IL15 signaling, NOD-scid IL2rg−/− mice completely lack even the low levels of natural killer cells present in classical NOD-scid mice. As compared with classical NOD-scid mice (and principally ascribed to their complete natural killer cell deficiency), NOD-scid IL2rg−/− mice may allow higher-level engraftment of normal human hematopoietic stem cells20, 21, 22 and acute leukemias.34, 35, 36 Therefore, we characterized the generation of human ALL in highly immunodeficient NOD-scid IL2rg−/− mice. The choice of recipient mouse strain can affect measured LSC frequencies,21, 36, 37 and Quintana et al.38 reported that NOD-scid IL2rg−/− mice provide a significantly more sensitive assay of human melanoma stem cell engraftment and growth than classical NOD-scid mice. In our experiments, ALL cases 1–5 generated leukemias at low transplanted cell doses in both NOD-scid IL2rg−/− and classical NOD-scid mice. Whereas in ALL cases 3–5 the times-to-leukemia were not substantially different in NOD-scid IL2rg−/− versus NOD-scid mice, the slightly longer times-to-leukemia observed in ALL cases 1 and 2 in NOD-scid mice suggest that the presence of greater immune function (for example, natural killer cells) in the NOD-scid mice may slow the growth of some leukemia cases. In addition, several of the transplanted NOD-scid mice had to be censored because of deaths due to endogenous mouse thymic lymphomas known to occur spontaneously in this strain.23 Thus, although we found pragmatic advantages to use of NOD-scid IL2rg−/− mouse recipients for modeling the engraftment and growth of primary human ALL cells, especially for cases with long times-to-leukemia, our limited comparisons in NOD-scid versus NOD-scid IL2rg−/− mouse recipients suggest that use of classical NOD-scid mice would not have affected our overall scientific conclusions discussed below.
As we had observed multiple radiation-related deaths in the first few weeks after transplant in experiments involving radiation doses ⩾300 cGy (data not shown), we chose to administer a slightly lower dose (250 cGy) to recipient mice before transplant, and we observed few deaths in radiation-control mice. We have found that small numbers of ALL case 1 cells generated leukemias in mice treated with only 100–200 cGy (data not shown); however, we did not systematically investigate the reduction in radiation dose. Spiegel et al.39 found that radiation-induced upregulation of stromal cell-derived factor-1 (SDF-1) reduced engraftment of ALL blasts in NOD-scid recipients, and engraftment of precursor B-ALL cases has been reported in non-irradiated mice.32, 36 Thus, future experiments using non-irradiated hosts might lead to an increase in the frequency of ALL cases that generate leukemias upon transplant in mice and/or the calculated frequency of LSCs. Similarly, our use of the intravenous route for transplant of ALL cells might have led to an underestimation of the true LSC frequency because of inefficient homing of cells to a lymphopoietic niche; thus, intra-femoral transplantation40, 41, 42 and/or introduction into neonatal mouse liver43 might increase the measured LSC frequency by as much as 10-fold. Nevertheless, such technical modifications designed to enhance leukemia engraftment would not alter our data interpretation that ALL LSCs are frequent.
Both the REH and KOPN8 precursor B-ALL cell lines generated clinical leukemias at ∼1 month after intravenous transfer of 106 cells into sublethally irradiated NOD-scid IL2rg−/− mice. Preliminary experiments (not shown) demonstrated that human leukemia cells could be detected by immunostaining and fluorescence-activated cell sorting (FACS) analysis in mouse blood as early as 2 weeks post-transplant, considerably before the onset of clinical signs, as in human clinical ALL. Massive splenomegaly was the most prominent gross pathological feature in all the mice, accompanied by replacement of blood, spleen and marrow by human cells with the blast morphology and immunophenotype of the original cell lines. Secondary transplants of splenocytes from transplanted leukemic mice regenerated the characteristic human leukemias. When we titered the transplanted cell dose of these two ALL cell lines, we observed an inverse log-linear proportionality between cell dose and time-to-leukemia. This model should facilitate future quantitative comparisons of the effects of gene modifications or drug treatments with the transplantation efficiency of ALL cell lines. We were not surprised that low cell doses (⩽100 cells) of these cell lines generated leukemias in the mice, as we expected that the frequency of LSCs might be quite high in established autonomous cell lines.44 Based on these observations using cell lines, we proceeded to characterize primary B-precursor ALL cells in this same assay.
Of the eight tested cases of childhood precursor-B ALL obtained at relapse, 5 (62.5%) generated leukemias in NOD-scid IL2rg−/− mice after intravenous transplant of 106 primary cells. A similar frequency (67%) of the six cases of childhood precursor-B ALL obtained at initial diagnosis generated leukemias in NOD-scid IL2rg−/− mice; cases 5 and 11 had standard risk features based on patient age and white blood cell count at diagnosis (and case 11 harbored the good-risk ETV6–RUNX1 translocation) and remain in CCR; cases 6 and 10 had poor prognostic features, yet remain in CCR; cases 4 and 7 had poor prognostic features and have relapsed and died. Previous publications30, 32 have reported that similar overall frequencies (40–80%) of ALL cases generated leukemias in immunodeficient mice. In AML, it has been shown that poor prognostic ‘virulence' features, such as FLT3 mutations, correlate with the ability to generate leukemias in immunodeficient mice8, 14, 15 and that, for unknown reasons, some AML subtypes such as acute progranulocytic leukemia (FAB (French–American–British) M3) rarely, if ever, engraft.8 In ALL, samples from patients at relapse tend to generate leukemias more rapidly than samples from patients at initial diagnosis,12, 45 and short length of first remission has been shown to predict for particularly rapid leukemia generation in NOD-scid mice.12 Conceivably, failure to generate leukemia could be due to not only the low ‘virulence' of the given case but also factors such as display of recognition factors susceptible to residual NOD-scid IL2rg−/− mouse (innate) immunity on blast cells or the dependence of the LSCs on human microenvironmental survival or absence of proliferation signals in the mouse host environment,46, 47 irrespective of the true LSC frequency. Investigation of factors associated with LSC engraftment and growth in this xenogeneicmodel was not our main goal and will require testing of large numbers of the diverse subtypes of ALL cases.
Although as discussed above, additional refinements may be advantageous, this NOD-scid IL2rg−/− model provides a robust and reliable pre-clinical assay to investigate LSC biology and to determine the efficacy of new agents in those cases that generate leukemias in this model—the majority of cases of precursor-B ALL.30, 32 In contrast to most cases of AML, all nine of the childhood precursor-B ALL cases that engrafted generated fatal leukemias, with human blasts essentially replacing hematopoietic organs and disseminating throughout other tissues of the mice. Times-to-leukemia varied from 1 to 7 months after transplant, despite the fact that these samples were all fully evolved leukemia cells from patients with clinical leukemias. The observation that the NOD-scid IL2rg−/− mice within an experimental group developed clinical signs of severe leukemia with minimal variability within experimental groups makes it unlikely that additional mutations in the leukemia cells are required for the development of leukemias in these xenografts, as the time taken to develop such necessary additional mutation(s) would be expected to vary among mice. Our cases of ALL obtained at relapse tended to generate leukemias more rapidly in mice than did our cases obtained at initial diagnosis (P=0.15). Although small numbers limit its statistical significance, this trend is consistent with the literature.12
In all five of the primary ALL cases tested, titered secondary transplants of ALL cells generated severe leukemias in NOD-scid IL2rg−/− mice, with times-to-leukemia being closely similar to the corresponding initial transplants (P<0.05). In some reports, shortened times-to-leukemia were observed after serial transplant.31 Perhaps, we would have seen such an evolution to more rapidly growing leukemias after multiple passages in mice, as Liem et al.11 observed that the times-to-leukemia were closely similar in initial versus secondary transplants of 10 ALL cases but that times-to-leukemia tended to decrease in tertiary transplants. On the other hand, most of our high-risk ALL cases might have evolved extensively already in the patients, such that further evolution during the course of their growth in mice produced insignificant additional proliferation/survival advantages. In any case, results similar to our own have been previously reported for secondary xeno-transplants of human AML,14 ALL,12, 30 colorectal cancer and breast cancer.9
The same IgH gene rearrangements were present in splenocytes from all five tested transplanted mice, as compared with samples obtained directly from the corresponding patients. In seven of the nine cases tested, comparisons of the initial patient sample with samples obtained after initial and secondary mouse transplants revealed that the leukemia cell immunophenotype was essentially unchanged by in vivo expansion of the leukemia in the mouse. In ALL cases 5 and 13, expression of CD19 or CD34 (respectively) was slightly different after transplant. The changed CD19 expression was stable in secondary transplanted ALL case 5 cells, suggesting a shift in gene expression possibly induced by the xenogeneic microenvironment. (Immunophenotype was not examined after secondary transplant of ALL case 13.) The mechanism of this potential artifact may be post-transcriptional, as the CD19 mRNA level was not altered in ALL case 5 (Supplementary Figure 4). Similar small changes in immunophenotype have been reported in a minority of ALL cases.11 Global gene expression profiles of ALL cases 1–4 were conserved between the primary sample and splenocytes of counterpart transplanted mice, consistent with previous reports.14 In ALL case 5, global gene expression was slightly altered after transplant. For all five cases tested, the times-to-leukemia were not significantly different between initial and secondary transplants; perhaps this characteristic is more important than gene expression, as engraftment and growth reflect complex functionality, as opposed to a specific marker(s). Therefore, we have listed, but not further investigated, the most differentially expressed genes (Supplementary Figure 4 and Supplementary Table 4).
Previous studies using classical NOD-scid mice have reported that LSCs were enriched in CD19+,24, 48, 49 CD34+CD19−24, 27, 28, 50 or CD133+CD19−51 subpopulations of childhood precursor-B ALL cells. More recent studies, using the more highly immunodeficient NOD-scid IL2rg−/− mouse strain, found LSCs in each of two (that is, CD34+CD19+CD38+ and CD34+CD19+CD38−)34 or three (that is, CD34+CD19−, CD34+CD19+ and CD34−CD19+) purified ALL cell subsets.32 We did not observe large cell subsets defined by immunological markers in our cases (Supplementary Figure 1), and chose not to re-study such immunological fractionation of LSCs. Our results, obtained by transplantation of unpurified ALL cells, were not confounded by any potential effects of monoclonal antibodies on LSC engraftment capacity.52
Our most important finding was that the LSC frequency in the five tested precursor-B ALL cases was 1–24%, much higher than has been described in AML.8, 33, 35 Although we did not verify the cell counts in samples actually injected into mice, we observed consistent times-to-leukemia within experimental groups of mice and consistent log-linear relationships to cell dose. In addition, the probability that recipient mice received approximately the intended dose was confirmed by experimental and statistical simulation for ALL case 3. Importantly, the times-to-leukemia and LSC frequencies were reproduced in repeated, independent initial and secondary transplant experiments. Moreover, two recent abstracts have described the engraftment of unsorted precursor-B ALL cases at similar low doses.41, 42 Thus, we have high confidence that the LSC frequency is high in these ALL cases. These findings are similar to those obtained via similar limiting-dose transplantation assays in genetic models in which ∼10–200 mouse or human model cancer cells generated leukemia/lymphomas53, 54, 55, 56 in syngeneic mice, as well as the previously mentioned results with primary human melanomas.38 A number of factors suggest that the true frequencies of LSCs may be perhaps 10–100-fold higher than those estimated in our study. All the primary samples tested had been obtained from patients in hospitals and required transport to a research laboratory, cryopreservation and thawing before injection into mice. After intravenous injection, LSCs presumably had to home to a suitable niche.46 Recent evidence suggests that the LSC model may be enhanced by experimental manipulations including omission of radiation,32, 36 and/or intrafemoral or intrahepatic transfer.40, 41, 42, 43 Consequently, the true LSC frequency in some ALL samples may approach 100%, especially in ALL cases 1 and 3. On the other hand, our finding that 9 of 14 primary human ALL cases generated leukemias in mice is comparable to the frequencies of leukemia reported with injections at other sites, and our observed generation of leukemias after intravenous injection of very low ALL cell doses suggests that homing was highly efficient in these ALL cases.
The hierarchical cancer stem cell model posits that primary human cancers are organized similarly to the normal hematopoietic system, with only the rare cancer stem cell possessing extensive self-renewal and differentiation capacities.37 In contrast, in stochastic cancer models, most or all cancer cells can self-renew and generate cancer heterogeneity.57 The results of our studies described above suggest that many, most or even all precursor-B ALL cells are functional LSCs. Thus, it seems that precursor-B ALL cells may follow a stochastic model, in which the cancer cells are biologically homogeneous with respect to their self-renewal capacity. As we assessed LSC frequencies mainly in ALL cases obtained either at relapse or at initial diagnosis from patients with poor prognosis and outcomes (of the five cases titered for LSC frequency, only ALL case 5 was cured), our findings require confirmation in a larger variety and number of ALL subtypes before generalization to the many clinically and molecularly defined subtypes of precursor-B ALL. Nevertheless, the very high frequency of LSCs observed herein suggests that a hierarchical LSC model is not valuable for poor-outcome ALL.
Acknowledgments
This research was supported in part by grants from the Leukemia & Lymphoma Society (Translational Grant 6082-08), National Foundation for Cancer Research, National Institutes of Health (P01CA070970) to CIC and CA34196 to the Jackson Lab. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute and Center for Cancer Research.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Trends in childhood cancer mortality--United States 1990-2004. MMWR Morb Mortal Wkly Rep. 2007;56:1257–1261. [PubMed] [Google Scholar]
- Larson S, Stock W. Progress in the treatment of adults with acute lymphoblastic leukemia. Curr Opin Hematol. 2008;15:400–407. doi: 10.1097/MOH.0b013e3283034697. [DOI] [PubMed] [Google Scholar]
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardelli A, Parsons DW, Silliman N, Ptak J, Szabo S, Saha S, et al. Mutational analysis of the tyrosine kinome in colorectal cancers. Science. 2003;300:949. doi: 10.1126/science.1082596. [DOI] [PubMed] [Google Scholar]
- Levis M, Murphy KM, Pham R, Kim KT, Stine A, Li L, et al. Internal tandem duplications of the FLT3 gene are present in leukemia stem cells. Blood. 2005;106:673–680. doi: 10.1182/blood-2004-05-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Park CY, Tseng D, Weissman IL. Cancer stem cell-directed therapies: recent data from the laboratory and clinic. Mol Ther. 2009;17:219–230. doi: 10.1038/mt.2008.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann PS, Lock RB. In vivo models of childhood leukemia for preclinical drug testing. Curr Drug Targets. 2007;8:773–783. doi: 10.2174/138945007780830809. [DOI] [PubMed] [Google Scholar]
- Liem NL, Papa RA, Milross CG, Schmid MA, Tajbakhsh M, Choi S, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103:3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- Lock RB, Liem N, Farnsworth ML, Milross CG, Xue C, Tajbakhsh M, et al. The nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model of childhood acute lymphoblastic leukemia reveals intrinsic differences in biologic characteristics at diagnosis and relapse. Blood. 2002;99:4100–4108. doi: 10.1182/blood.v99.11.4100. [DOI] [PubMed] [Google Scholar]
- Baersch G, Mollers T, Hotte A, Dockhorn-Dworniczak B, Rube C, Ritter J, et al. Good engraftment of B-cell precursor ALL in NOD-SCID mice. Klin Padiatr. 1997;209:178–185. doi: 10.1055/s-2008-1043947. [DOI] [PubMed] [Google Scholar]
- Lumkul R, Gorin NC, Malehorn MT, Hoehn GT, Zheng R, Baldwin B, et al. Human AML cells in NOD/SCID mice: engraftment potential and gene expression. Leukemia. 2002;16:1818–1826. doi: 10.1038/sj.leu.2402632. [DOI] [PubMed] [Google Scholar]
- Monaco G, Konopleva M, Munsell M, Leysath C, Wang RY, Jackson CE, et al. Engraftment of acute myeloid leukemia in NOD/SCID mice is independent of CXCR4 and predicts poor patient survival. Stem Cells. 2004;22:188–201. doi: 10.1634/stemcells.22-2-188. [DOI] [PubMed] [Google Scholar]
- Gaidzik V, Dohner K. Prognostic implications of gene mutations in acute myeloid leukemia with normal cytogenetics. Semin Oncol. 2008;35:346–355. doi: 10.1053/j.seminoncol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Ailles LE, Gerhard B, Kawagoe H, Hogge DE. Growth characteristics of acute myelogenous leukemia progenitors that initiate malignant hematopoiesis in nonobese diabetic/severe combined immunodeficient mice. Blood. 1999;94:1761–1772. [PubMed] [Google Scholar]
- Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- Crazzolara R, Cisterne A, Thien M, Hewson J, Baraz R, Bradstock KF, et al. Potentiating effects of RAD001 (Everolimus) on vincristine therapy in childhood acute lymphoblastic leukemia. Blood. 2009;113:3297–3306. doi: 10.1182/blood-2008-02-137752. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- McDermott SP, Eppert K, Lechman E, Doedens M, Dick JE. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood. 2010;116:193–200. doi: 10.1182/blood-2010-02-271841. [DOI] [PubMed] [Google Scholar]
- Prochazka M, Gaskins HR, Shultz LD, Leiter EH. The nonobese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proc Natl Acad Sci USA. 1992;89:3290–3294. doi: 10.1073/pnas.89.8.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castor A, Nilsson L, Astrand-Grundstrom I, Buitenhuis M, Ramirez C, Anderson K, et al. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat Med. 2005;11:630–637. doi: 10.1038/nm1253. [DOI] [PubMed] [Google Scholar]
- Gluck U, Zipori D, Wetzler M, Berrebi A, Shaklai M, Drezen O, et al. Long-term proliferation of human leukemia cells induced by mouse stroma. Exp Hematol. 1989;17:398–404. [PubMed] [Google Scholar]
- Ito C, Kumagai M, Manabe A, Coustan-Smith E, Raimondi SC, Behm FG, et al. Hyperdiploid acute lymphoblastic leukemia with 51 to 65 chromosomes: a distinct biological entity with a marked propensity to undergo apoptosis. Blood. 1999;93:315–320. [PubMed] [Google Scholar]
- Cobaleda C, Gutierrez-Cianca N, Perez-Losada J, Flores T, Garcia-Sanz R, Gonzalez M, et al. A primitive hematopoietic cell is the target for the leukemic transformation in human Philadelphia-positive acute lymphoblastic leukemia. Blood. 2000;95:1007–1013. [PubMed] [Google Scholar]
- Cox CV, Evely RS, Oakhill A, Pamphilon DH, Goulden NJ, Blair A. Characterization of acute lymphoblastic leukemia progenitor cells. Blood. 2004;104:2919–2925. doi: 10.1182/blood-2004-03-0901. [DOI] [PubMed] [Google Scholar]
- Campana D, Iwamoto S, Bendall L, Bradstock K. Growth requirements and immunophenotype of acute lymphoblastic leukemia progenitors. Blood. 2005;105:4150. doi: 10.1182/blood-2004-10-3933. [DOI] [PubMed] [Google Scholar]
- Kamel-Reid S, Letarte M, Doedens M, Greaves A, Murdoch B, Grunberger T, et al. Bone marrow from children in relapse with pre-B acute lymphoblastic leukemia proliferates and disseminates rapidly in scid mice. Blood. 1991;78:2973–2981. [PubMed] [Google Scholar]
- Barabe F, Kennedy JA, Hope KJ, Dick JE. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316:600–604. doi: 10.1126/science.1139851. [DOI] [PubMed] [Google Scholar]
- le Viseur C, Hotfilder M, Bomken S, Wilson K, Rottgers S, Schrauder A, et al. In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell. 2008;14:47–58. doi: 10.1016/j.ccr.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Kong Y, Yoshida S, Saito Y, Doi T, Nagatoshi Y, Fukata M, et al. CD34+CD38+CD19+ as well as CD34+CD38-CD19+ cells are leukemia-initiating cells with self-renewal capacity in human B-precursor ALL. Leukemia. 2008;22:1207–1213. doi: 10.1038/leu.2008.83. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- Agliano A, Martin-Padura I, Mancuso P, Marighetti P, Rabascio C, Pruneri G, et al. Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. Int J Cancer. 2008;123:2222–2227. doi: 10.1002/ijc.23772. [DOI] [PubMed] [Google Scholar]
- Dick JE. Looking ahead in cancer stem cell research. Nat Biotechnol. 2009;27:44–46. doi: 10.1038/nbt0109-44. [DOI] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A, Kollet O, Peled A, Abel L, Nagler A, Bielorai B, et al. Unique SDF-1-induced activation of human precursor-B ALL cells as a result of altered CXCR4 expression and signaling. Blood. 2004;103:2900–2907. doi: 10.1182/blood-2003-06-1891. [DOI] [PubMed] [Google Scholar]
- Mazurier F, Doedens M, Gan OI, Dick JE. Rapid myeloerythroid repopulation after intrafemoral transplantation of NOD-SCID mice reveals a new class of human stem cells. Nat Med. 2003;9:959–963. doi: 10.1038/nm886. [DOI] [PubMed] [Google Scholar]
- Rehe K, Wilson K, McNeill H, Schrappe M, Irving J, Vormoor HJ.Disease propagating blasts in standard and high risk acute lymphoblastic leukemia are frequent and of diverse immunophenotype Blood 200911422(abstract no. 1421). [Google Scholar]
- Schmitz M, Mirkowska P, Breithaupt P, Meissner B, Cario G, Schrauder A, et al. Leukemia-initiating cells are frequent in very high risk childhood acute lymphoblastic leukemia and give rise to relatively stable phenotypes in immunodeficient mice Blood 200911422(abstract no. 86). [Google Scholar]
- Cheung AM, Fung TK, Fan AK, Wan TS, Chow HC, Leung JC, et al. Successful engraftment by leukemia initiating cells in adult acute lymphoblastic leukemia after direct intrahepatic injection into unconditioned newborn NOD/SCID mice. Exp Hematol. 2009;38:3–10. doi: 10.1016/j.exphem.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun FM, Sather HN, Waurzyniak BJ, Sensel MG, Chelstrom L, Ek O, et al. Prognostic significance of B-lineage leukemic cell growth in SCID mice: a Children's Cancer Group Study. Leuk Lymphoma. 1998;30:503–514. doi: 10.3109/10428199809057563. [DOI] [PubMed] [Google Scholar]
- van Hennik PB, de Koning AE, Ploemacher RE. Seeding efficiency of primitive human hematopoietic cells in nonobese diabetic/severe combined immune deficiency mice: implications for stem cell frequency assessment. Blood. 1999;94:3055–3061. [PubMed] [Google Scholar]
- Vormoor HJ. Malignant stem cells in childhood acute lymphoblastic leukemia: the stem cell concept revisited. Cell Cycle. 2009;8:996–999. doi: 10.4161/cc.8.7.7984. [DOI] [PubMed] [Google Scholar]
- Hotfilder M, Rottgers S, Rosemann A, Jurgens H, Harbott J, Vormoor J. Immature CD34+CD19- progenitor/stem cells in TEL/AML1-positive acute lymphoblastic leukemia are genetically and functionally normal. Blood. 2002;100:640–646. doi: 10.1182/blood.v100.2.640. [DOI] [PubMed] [Google Scholar]
- Hong D, Gupta R, Ancliff P, Atzberger A, Brown J, Soneji S, et al. Initiating and cancer-propagating cells in TEL-AML1-associated childhood leukemia. Science. 2008;319:336–339. doi: 10.1126/science.1150648. [DOI] [PubMed] [Google Scholar]
- Hotfilder M, Rottgers S, Rosemann A, Schrauder A, Schrappe M, Pieters R, et al. Leukemic stem cells in childhood high-risk ALL/t(9;22) and t(4;11) are present in primitive lymphoid-restricted CD34+CD19- cells. Cancer Res. 2005;65:1442–1449. doi: 10.1158/0008-5472.CAN-04-1356. [DOI] [PubMed] [Google Scholar]
- Cox CV, Diamanti P, Evely RS, Kearns PR, Blair A. Expression of CD133 on leukemia initiating cells in childhood ALL. Blood. 2009;113:3287–3296. doi: 10.1182/blood-2008-04-154187. [DOI] [PubMed] [Google Scholar]
- Taussig DC, Miraki-Moud F, Anjos-Afonso F, Pearce DJ, Allen K, Ridler C, et al. Anti-CD38 antibody-mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood. 2008;112:568–575. doi: 10.1182/blood-2007-10-118331. [DOI] [PubMed] [Google Scholar]
- Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- Kennedy JA, Barabe F, Poeppl AG, Wang JC, Dick JE. Comment on ‘Tumor growth need not be driven by rare cancer stem cells'. Science. 2007;318:1722. doi: 10.1126/science.1149590. [DOI] [PubMed] [Google Scholar]
- Adams JM, Kelly PN, Dakic A, Nutt SL, Strasser A. Response to Comment on ‘Tumor growth need not be driven by rare cancer stem cells'. Science. 2007;318:1722d. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- Adams JM, Strasser A. Is tumor growth sustained by rare cancer stem cells or dominant clones. Cancer Res. 2008;68:4018–4021. doi: 10.1158/0008-5472.CAN-07-6334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.