Abstract
Objective:
To explore the relationship between severity of obesity at age 7 and age 15, age at onset of obesity, and parental body mass index (BMI) in obese children and adolescents.
Design:
Longitudinal cohort study.
Subjects:
Obese children (n=231) and their parents (n=462) from the Swedish National Childhood Obesity Centre.
Methods:
Multivariate regression analyses were applied with severity of obesity (BMI standard deviation score (BMI SDS)) and onset of obesity as dependent variables. The effect of parental BMI was evaluated and in the final models adjusted for gender, parental education, age at onset of obesity, severity of obesity at age 7 and obesity treatment.
Results:
For severity of obesity at age 7, a positive correlation with maternal BMI was indicated (P=0.05). Severity of obesity at this age also showed a strong negative correlation with the age at onset of obesity. Severity of obesity at age 15 was significantly correlated with both maternal and paternal BMI (P<0.01). In addition, BMI SDS at age 15 differed by gender (higher for boys) and was positively correlated with severity of obesity at age 7 and negatively correlated with treatment. Also, a negative correlation was indicated at this age for parental education. No correlation with age at onset was found at age 15. For age at onset of obesity there was no relevant correlation with parental BMI. Children within the highest tertile of the BMI SDS range were more likely to have two obese parents.
Conclusion:
The impact of parental BMI on the severity of obesity in children is strengthened as the child grows into adolescence, whereas the age at onset is probably of less importance than previously thought. The influence of parental relative weight primarily affects the severity of childhood obesity and not the timing.
Keywords: childhood obesity, parental BMI, maternal BMI, paternal BMI, age at onset of obesity, severity of obesity
Introduction
Parental obesity has been identified as a predominant risk factor for childhood obesity, probably owing to a combination of genetic, epigenetic, social and environmental factors.1, 2 Children with two obese parents have a higher risk of obesity than those with one or no obese parent.2 The majority of studies on the risk factors associated with childhood obesity have investigated populations with a relatively normal distribution of weight and thus a small proportion of obese children.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 Some of these studies have presented results on the correlation between the severity of obesity of the parents and of their children in different age brackets.3, 4, 6, 7, 11, 13, 16 A correlation between maternal and child body mass index (BMI) has been observed in many studies,4, 5, 7, 13, 16 whereas the effect of paternal BMI is less certain, especially among younger children.7, 13 The correlation between the severity of overweight in parents and in obese children has not been thoroughly studied previously. In one study a correlation between the BMI z score of the children and maternal BMI was reported, but the impact of paternal BMI was not studied.16 It has been suggested that early-onset obesity is associated more with hypothalamic obesity or with a high genetic obesity vulnerability.17
There is an urgent need to specifically study cohorts of obese children as they are in the greatest need for healthcare. The treatment of obesity is a very challenging task and knowledge of early prognostic factors affecting the severity of obesity in children would be beneficial. To the best of our knowledge, studies looking at the correlation between the severity of child obesity and parental relative weight, and other family risk factors in a large cohort of obese children are lacking so far.
The main objective of the present study was to analyse the severity of obesity at ages 7 and 15 in relation to parental BMI in a large cohort of obese children, exploring the impact of gender, birth weight, age at onset of obesity, parental cardio-metabolic co-morbidities, family socio-economic status and treatment. A further aim was to address the question of whether age at onset of obesity is correlated with parental BMI.
Materials and methods
Data collection
In this longitudinal cohort study, 921 obese children enrolled at the National Childhood Obesity Centre at Karolinska University Hospital, Huddinge, Sweden during 1997–2006 were reviewed for inclusion. The Centre receives patients with severe obesity from all parts of Sweden, but the majority come from the Stockholm region. The age at enrolment varied from 3 to 18 years, and the treatment was planned for 5 years.
All patients at the Centre were registered in the unique Swedish national quality registry for childhood obesity, BORIS. The database includes background data, recorded at first visit, and annual longitudinal visit data. From this database, data for all subjects were extracted in 2006: gender, birth weight, pregnancy length, age at enrolment, age at onset of obesity, BMI SDS (BMI standard deviation score) at age 7, BMI SDS from all visits, diagnoses that can cause secondary obesity, parental BMI at enrolment, parental cardio-metabolic co-morbidities (cardiovascular disease, hypertension, hyperlipidaemia, type 2 diabetes mellitus and gestational diabetes mellitus) and socio-economic status (parental occupation). Ethnicity data were available in the database but could not be used owing to missing items. Additional BMI SDS data were extracted from the database in 2010 in order to increase the number of individuals with complete data at both age 7 and age 15.
Subjects with the following diagnoses that can cause secondary obesity were excluded from the primary analyses (in total 132 individuals, some having several diagnoses): obesity-related syndromes, n=32 (Prader–Willi, Laurence–Moon–Bardet–Biedl, Fragile-X, Morbus Down, melanocortin 4 receptor deficiency), psychological diagnoses, n=86 (developmental delay, attention-deficit hyperactivity disorder, Asperger's syndrome, autism), brain tumour or other brain damage, n=11, myelomeningocele or other disability conditions, n=16. A part of this excluded sub-cohort was used in a comparison of the age at obesity onset between the primary study cohort and the subjects with obesity syndromes. This sub-cohort constituted of 32 subjects with either a diagnosed obesity syndrome (Prader–Willi syndrome, Laurence–Moon–Bardet–Biedl syndrome, Fragile X or Morbus Down) or melanocortin 4 receptor deficiency. Subjects lacking data for any of the three dependent variables and for parental BMI were excluded from the study. Children with an unknown maternal pregnancy length or born earlier than at 37 weeks of gestation were excluded from the analyses involving birth weight. The resulting study cohort for the primary analyses consisted of 231 children and their parents, that is, 693 subjects.
Data definitions and processing
We used the international age- and gender-specific BMI cut-off points defined by the International Obesity Task Force to define childhood obesity.18 According to this definition, at age 7, 76% of the children in the study cohort were classified as obese and at age 15 the proportion of children with obesity was 81%. The term ‘severity of obesity' is used for referring to a BMI standard deviation score, according to Rolland-Cachera, and it was calculated in the BORIS database based on weight, height, age and gender.19 BMI SDS at age 7 was based on extrapolated weight and height measurements from the growth charts. BMI SDS at age 15 was based on the clinical visit records of weight and height between 14.5 and 15.9 years (children with missing weight records in this age bracket were excluded). Parental BMI data were calculated in the BORIS database based on the weight and height data supplied by the parents in the questionnaire completed at enrolment. Based on the self-reported BMI, parents were also classified as normal weight, overweight or obese, according to the international cut-off points (normal weight 18–24.9 kg m−2, overweight 25–29.9 kg m−2, obese >30 kg m−2).20 Age at onset of obesity was derived from growth charts collected at referral and was defined as the age at which the BMI of the child for the first time exceeded the BMI for the specific age and gender corresponding to BMI 30 kgm−2 for adults.18 The data on parental cardio-metabolic co-morbidities (cardiovascular disease, hypertension, hyperlipidaemia, type 2 diabetes mellitus and gestational diabetes mellitus) was used to define the variables ‘number of maternal co-morbidities' and ‘number of paternal co-morbidities', respectively, included in the analyses. The socio-economic status was based on parental occupation. This was coded into a proxy for parental education, based on official Swedish socio-economic categories and the Swedish standard classification of occupations, provided by Statistics Sweden, and presented in three categories: (1) at least one parent with an academic degree, (2) at least one parent with post-secondary education and (3) others (unemployed, early/disability retired, long-term sick-listed, housewives and participating in study program). Birth weight was used as a numerical variable and in addition coded into three categories: less than 3000 g, between 3000–3500 g and above 3500 g. Categorical variables were coded into multiple dummy variables. Gender was coded 1/0 for boys/girls.
Analyses and statistics
Unadjusted correlations between the dependent variables and parental BMI were calculated. Multivariate regression analyses were run for each of the dependent variables—age at onset of obesity, severity of obesity at age 7 and severity of obesity at age 15, testing the following factors with potential impact on the associations between the dependent variables and parental BMI: gender, parental cardio-metabolic co-morbidities, socio-economic status, birth weight, age at obesity onset (in the models for severity of obesity), severity of obesity at 7 years and at least 1 year of treatment (the latter two included in the model for severity of obesity at age 15). Interaction effects between maternal and paternal BMI, as well as between parental BMI and child gender, were also included in the regression models. Gender-specific correlations between the severity of obesity for boys and girls and parental BMI were calculated as well.
We also compared children with various severities of obesity—the range of BMI SDS at age 7 and 15, respectively, was divided into tertiles—in relation to having none, one or two obese parents, using Pearson's chi-square test. P-values <0.05 were regarded as statistically significant. Descriptive statistics were presented as the mean (s.d.). Variables that were not approximately normally distributed were square root transformed and normalized. Statistical analyses were performed using STATISTICA, version 9.1 (StatSoft, Inc., Tulsa, OK, USA, http://www.statsoft.com). The study was reviewed and accepted by the Stockholm regional ethics committee.
Results
Mean age at obesity onset was 4.9 (2.9) years. The mean severity of obesity at age 7 (BMI SDS) was 4.9 (1.9) and that at age 15 (BMI SDS) was 4.5 (1.5) (Table 1). The severity of obesity at either age was not significantly different in the study cohort compared with subjects excluded due to missing parental BMI data (data not shown). The mean age at registration at the National Childhood Obesity Centre was 12.8 (2.3) years. At age 15, 70% of the children had been treated for obesity at the Centre for at least 1 year.
Table 1. Characteristics of the children and each of the dependent variables (mean (s.d.)).
| All children, n=231 | Boys, n = 114 (49 %) | Girls, n = 117 (51 %) | |
|---|---|---|---|
| Age at onset of obesity (years) | 4.9 (2.9) | 4.8 (2.6) | 5.0 (3.2) |
| Severity of obesity at age 7 (BMI SDS) | 4.9 (1.9) | 4.9 (1.9) | 4.9 (1.8) |
| Severity of obesity at age 15 (BMI SDS) | 4.5 (1.5) | 4.7 (1.6) | 4.4 (1.4) |
| Age at enrolment to clinic (years) | 12.8 (2.3) | 12.9 (2.2) | 12.8 (2.5) |
Abbreviation: BMI SDS, body mass index standard deviation score (according to Rolland-Cachera et al.19).
The mean BMI of the mothers was 28.5 (5.8) kg m−2 and the mean BMI of the fathers was 28.5 (4.6) kg m−2 (Table 2). Of the mothers, 32% were normal-weighted, 34% had overweight and 34 % were obese. Among the fathers, 21% were normal-weighted, 48% had overweight and 31% were obese. Compared with the adult Swedish general population, a considerably higher proportion of the mothers and fathers were obese and fewer were normal-weighted: about 10–15% of Swedish men and women are obese and about 40–45% of Swedish men and 60–65% of Swedish women are normal-weighted.21 The analysis of the socio-economic status of the families showed that 20% of the children had at least one parent with an academic education, 28% had at least one parent with post-secondary education and 37% had parents with no post-secondary education or no professional occupation at all. The socio-economic status of 15% of the children was unknown.
Table 2. Weight category and BMI of the parents, according to international cut-off points.
| Mother, n=231 | Father, n=231 | |
|---|---|---|
| Normal weight, BMI 18–24.9 kg m−2; n (%) | 73 (32) | 49 (21) |
| Overweight, BMI 25–29.9 kg m−2; n (%) | 79 (34) | 111 (48) |
| Obese, BMI >30 kg m−2; n (%) | 79 (34) | 71 (31) |
| BMI; mean (s.d.) | 28.5 (5.8) | 28.5 (4.6) |
Abbreviation: BMI, body mass index.
The distribution of age at onset of obesity was skewed in our cohort. A total of 77% became obese at age ⩽6 and 58% were obese at age ⩽4. The mean age at obesity onset for the small cohort of children and adolescents with obesity syndromes (age at obesity onset was known for 22 of these 32 subjects) was 5.0 (3.4) years. This did not differ from the mean age at obesity onset of the primary study cohort, 4.9 (2.9) years.
The unadjusted correlations (Pearson's r) between the dependent variables and parental BMI, and between the three dependent variables, by gender, are reported in Table 3. All the correlations at age 15 are significant and of medium strength (r=0.22–0.36). At age 7, the correlation between severity of obesity and paternal BMI is significant (r=0.17). The correlation between severity of obesity at age 7 and age at onset of obesity is significant and strong (r=−0.74). The correlation coefficients between boys'/girls' BMI SDS and maternal/paternal BMI did not diverge considerably from the correlations between these variables for all children.
Table 3. Unadjusted correlations (Pearson's r) between the dependent variables and parental BMI, as well as between the three dependent variables, by gender.
| Mother BMI | Father BMI | Age at onset of obesity | Severity of obesity, age 7 | |||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Age at onset of obesity | ||||||||
| All | −0.00 | 0.96 | −0.14 | 0.04 | — | — | — | — |
| Girls | 0.00 | 0.99 | −0.13 | 0.15 | — | — | — | — |
| Boys | −0.01 | 0.91 | −0.14 | 0.13 | — | — | — | — |
| Severity of obesity, age 7 | ||||||||
| All | 0.11 | 0.10 | 0.17 | 0.01 | −0.74 | 0.00 | — | — |
| Girls | 0.14 | 0.12 | 0.2 | 0.03 | −0.76 | 0.00 | — | — |
| Boys | 0.07 | 0.45 | 0.14 | 0.13 | −0.73 | 0.00 | — | — |
| Severity of obesity, age 15 | ||||||||
| All | 0.29 | 0.00 | 0.27 | 0.00 | −0.22 | 0.001 | 0.34 | 0.00 |
| Girls | 0.28 | 0.002 | 0.24 | 0.01 | −0.22 | 0.02 | 0.33 | 0.00 |
| Boys | 0.32 | 0.00 | 0.31 | 0.001 | −0.22 | 0.02 | 0.36 | 0.00 |
Abbreviation: BMI, body mass index.
The unadjusted and adjusted correlations between the dependent variables and parental BMI are given in Table 4. Common co-variates in the final multivariate models were gender and parental education. In addition, the correlations with parental BMI were adjusted for age at onset of obesity in the final model for severity of obesity at age 7 (adjusted R2=0.53). In the final model for severity of obesity at age 15, the correlations with parental BMI were additionally adjusted for age at onset of obesity, severity of obesity at age 7 and treatment more than 1 year (adjusted R2=0.28). Variables for birth weight and parental co-morbidities were tested in the multivariate analyses, but not kept in the final models. Age at obesity onset was significantly correlated with paternal BMI, but as this relationship was very weak (adjusted R2=0.01), this result was considered not relevant. In the adjusted model for the severity of obesity at age 7, a positive correlation with maternal BMI was indicated (P=0.05). In addition, severity of obesity at age 7 was strongly negatively correlated with obesity onset (P<0.00001). The severity of obesity at age 15 was significantly correlated with maternal BMI (P=0.003) and paternal BMI (P=0.01) in the final model. Additional significant variables in the final model for this variable were gender (P=0.04), severity of obesity at age 7 (P<0.00001) and obesity treatment for at least 1 year at the Centre (P<0.0001), but not age at obesity onset. A negative correlation between severity of obesity at age 15 and parental education (at least one parent with an academic education) was also found (P=0.05).
Table 4. Unadjusted (a) and two-step adjusted (b and c) correlations between the dependent variables: age at onset of obesity, severity of obesity at age 7 and severity of obesity at age 15, and maternal and paternal BMI.
| Age at onset of obesitya | Severity of obesity, age 7 | Severity of obesity, age 15 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | P | R2 | β | P | R2 | β | P | R2 | |
| (a) Unadjusted | |||||||||
| Maternal BMI | −0.00 | 0.96 | 0 | 0.11 | 0.10 | 0.01 | 0.29 | 0.0000 | 0.08 |
| Paternal BMI | −0.14 | 0.04 | 0.01 | 0.17 | 0.01 | 0.03 | 0.27 | 0.0001 | 0.07 |
| (b) Adjusted, 1st step | 0.01 | 0.02 | 0.14 | ||||||
| Maternal BMI | 0.04 | 0.56 | 0.07 | 0.29 | 0.23 | 0.0004 | |||
| Paternal BMI | −0.14 | 0.04 | 0.15 | 0.02 | 0.20 | 0.001 | |||
| Gender | −0.00 | 1.00 | 0.00 | 0.99 | 0.10 | 0.10 | |||
| Parental academic degree | 0.07 | 0.31 | −0.02 | 0.82 | −0.14 | 0.02 | |||
| (c) Adjusted, 2nd step | 0.53 | 0.28 | |||||||
| Maternal BMI | — | — | 0.09 | 0.05 | 0.17 | 0.003 | |||
| Paternal BMI | — | — | 0.04 | 0.36 | 0.16 | 0.01 | |||
| Gender | — | — | −0.02 | 0.69 | 0.11 | 0.04 | |||
| Parental academic degree | — | — | 0.04 | 0.43 | −0.11 | 0.05 | |||
| Age at onset of obesity | — | — | −0.72 | <0.00001 | 0.05 | 0.60 | |||
| Severity of obesity, age 7 | — | — | — | — | 0.39 | <0.00001 | |||
| Treatment >1 year | — | — | — | — | −0.26 | <0.0001 | |||
Abbreviation: BMI, body mass index.
R2, adjusted R2, amount of explanation.
Normalized.
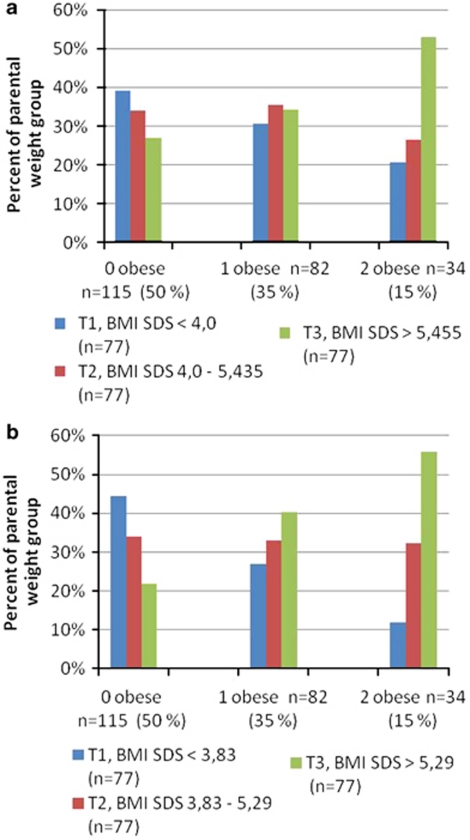
Of all the children, 50% had non-obese parents, 35% had one obese parent and 15% had two obese parents. We found that children within the highest tertile of severity of obesity were significantly more likely to have two obese parents, both at 7 years (P=0.01) and at 15 years (P=0.003) (Figure 1).
Figure 1.
The proportion of children in each tertile of the BMI SDS range at ages 7 and 15 having zero, one or two obese parents. (a) At age 7. (b) At age 15.
Discussion
Our results show that the correlation between severity of obesity and parental BMI is progressively strengthened from 7 years to 15 years, and that different factors explain the variance in the severity of obesity at 7 years compared with that at 15 years. The severity of obesity at age 7, adjusted for several relevant factors, related significantly with the mother's BMI, but not with paternal BMI, although the unadjusted correlation with paternal BMI was stronger than the correlation with maternal BMI. The adjusted severity of obesity at age 15 correlated significantly with both parents' BMI. At age 7, but not at 15, the dominant risk factor for severe obesity was the age at obesity onset.
Our study supports the theory that genetic factors have a stronger influence the older the child gets and affect the severity of obesity, especially during adolescence. Previous observations have suggested that there are three critical periods during childhood for the development of obesity: the prenatal period, the period of adiposity rebound (4–6 years) and adolescence.22 It has also been suggested that the genes influencing adiposity have differential effects at distinct childhood ages and may not be exerted equally throughout the life cycle.11, 23 Several twin studies have concluded that genetic factors influencing weight, BMI and body size become more apparent with age.24, 25, 26 It is not clear in our study whether the relationship between parental BMI and severity of obesity in their children is due to genetic or environmental factors. However, the fact that the correlation between parental BMI and severity of obesity is more pronounced when the children are 15 years old might indicate that genetic factors dominate, as during adolescence, the parental impact on daily life declines. As a recent twin research in children has shown, the genetic influence on childhood obesity persists despite the obesogenic environment.27
Our data suggest that the genetic influence has a limited effect on the timing of childhood obesity development and that obesity onset is of lesser importance than previously thought. We did not observe any relevant correlation between obesity onset and parental weight status. The strong negative correlation between the severity of obesity at age 7 and onset of obesity indicates that the earlier the obesity onset, the more severe the obesity at age 7. However, the age at onset was not related to the severity of obesity at age 15, despite the significant correlation between severity of obesity at age 15 and severity of obesity at age 7. This may simply be due to the fact that the effect of age at onset was diluted by other factors having a stronger impact on severity of obesity at age 15. To our knowledge, no similar research on obesity onset has been done.28 Population-based studies show that the effect of the parents' weight status on that of their children is not evident until the child grows into the pre-school years.11, 15, 23, 29 Previously an early childhood onset was closely associated with monogenetic forms of obesity and considered to be an important factor in the assessment of severely obese children.17 In the present study, the age at onset of obesity was the same in children with hypothalamic and syndrome obesity and the rest of the cohort, which is similar to the findings in other comparable studies.30, 31
The mother's influence in early childhood could be of epigenetic origin. Kral et al.32 showed that the prevalence of obesity was normalized among children born after their mothers had undergone obesity surgery with a resulting substantial weight loss. This supports the role of epigenetic factors in the aetiology of childhood obesity, indicating a gene–environment interaction influencing the obesity phenotype. A stronger or earlier relationship between mother and child weight than between father and child weight has been seen in several population-based studies.1, 6, 7, 11, 13
Previous research has shown that children with two obese parents have a higher risk of obesity than those with one or no obese parent.2 We can now extend this conclusion to the severity of obesity, as the most severely obese 7- and 15-year-olds were more likely to have two obese parents. It has been shown earlier that the risk for childhood obesity to persist into adulthood is higher in children of obese parents2, 4, 7, 8, 14 and that the persistence of childhood obesity is increased by the severity of obesity.33, 34 Taken together, these findings offer a very poor prognosis for the obese children and adolescents of obese parents and it is logical to make an extra effort to provide early obesity treatment.
Gender was not a strong distinguishing factor with regard to the variance of severity of obesity or obesity onset. Gender was only significantly correlated with severity of obesity at age 15 in the final multivariate model (boys had a somewhat higher BMI SDS at age 15). The results of other studies are conflicting: some found different correlations in parental adiposity between boys and girls,8 whereas others did not.33 Perez-Pastor et al.35 have reported based on a study in the UK a close association between paternal and son's BMI, as well as between maternal and daughter's BMI, indicating a social and not genetic inheritance of obesity. However, in this Swedish cohort we were not able to confirm this observation as the associations between child and parental relative weight were not different for boys/girls and mothers/fathers.
Inverse associations between socio-economic status and adiposity in children are well established.36 Our results, indicating a negative correlation between parental education and severity of obesity at age 15, were in accordance with this.
We saw no relevant associations between birth weight and the dependent variables. Population-based studies show inconsistent results with regard to birth weight as a determinant of the weight status of children.4, 5, 9, 10, 15, 37 We chose to not include birth weight in the final multivariate models, as the analyses showed that this variable did not affect the correlation with parental BMI.
There were several limitations in the present study. Self-reported parental height and weight were used to calculate the BMI of parents. Self-reports are known to underestimate the BMI. There are, however, epidemiological studies showing that self-reported height and weight in young adults have been reliable for identifying relationships.2 Although the amount of explanation was high or very high in the final multivariate models for severity of obesity, the share of explained variance that could be ascribed to parental BMI was relatively small.
In conclusion, the impact of parental BMI on the severity of obesity in children is strengthened as the child grows into adolescence, whereas the age at obesity onset is of limited importance. Our results support the theory that the influence of parental relative weight primarily affects the severity of childhood obesity and not the timing. Our study confirms that obese children with two obese parents constitute a high-risk group for severe adolescent obesity. Obesity treatment, as well as intervention studies, needs to be differentiated for children with different family risk factors and different severities of obesity at different ages.
Acknowledgments
This study was funded by the Swedish Association of Local Authorities and Regions and the Swedish National Board of Health and Welfare, AFA Insurance and the Swedish Council for Working Life and Social Research.
The authors declare no conflict of interest.
References
- Maffeis C. Aetiology of overweight and obesity in children and adolescents. Eur J Pediatr. 2000;159 (Suppl 1:35–44. doi: 10.1007/pl00014361. [DOI] [PubMed] [Google Scholar]
- Lake JK, Power C, Cole TJ. Child to adult body mass index in the 1958 British birth cohort: associations with parental obesity. Arch Dis Child. 1997;77:376–381. doi: 10.1136/adc.77.5.376. [DOI] [PubMed] [Google Scholar]
- Bralic I, Vrdoljak J, Kovacic V. Associations between parental and child overweight and obesity. Coll Antropol. 2005;29:481–486. [PubMed] [Google Scholar]
- Burke V, Beilin LJ, Dunbar D. Family lifestyle and parental body mass index as predictors of body mass index in Australian children: a longitudinal study. Int J Obes Relat Metab Disord. 2001;25:147–157. doi: 10.1038/sj.ijo.0801538. [DOI] [PubMed] [Google Scholar]
- Danielzik S, Czerwinski-Mast M, Langnase K, Dilba B, Muller MJ. Parental overweight, socioeconomic status and high birth weight are the major determinants of overweight and obesity in 5–7 y-old children: baseline data of the Kiel Obesity Prevention Study (KOPS) Int J Obes Relat Metab Disord. 2004;28:1494–1502. doi: 10.1038/sj.ijo.0802756. [DOI] [PubMed] [Google Scholar]
- Danielzik S, Langnase K, Mast M, Spethmann C, Muller MJ. Impact of parental BMI on the manifestation of overweight 5–7 year old children. Eur J Nutr. 2002;41:132–138. doi: 10.1007/s00394-002-0367-1. [DOI] [PubMed] [Google Scholar]
- Maffeis C, Talamini G, Tato L. Influence of diet, physical activity and parents' obesity on children's adiposity: a four-year longitudinal study. Int J Obes Relat Metab Disord. 1998;22:758–764. doi: 10.1038/sj.ijo.0800655. [DOI] [PubMed] [Google Scholar]
- Magarey AM, Daniels LA, Boulton TJ, Cockington RA. Predicting obesity in early adulthood from childhood and parental obesity. Int J Obes Relat Metab Disord. 2003;27:505–513. doi: 10.1038/sj.ijo.0802251. [DOI] [PubMed] [Google Scholar]
- Mamun AA, Lawlor DA, O'Callaghan MJ, Williams GM, Najman JM. Family and early life factors associated with changes in overweight status between ages 5 and 14 years: findings from the Mater University Study of Pregnancy and its outcomes. Int J Obes. 2005;29:475–482. doi: 10.1038/sj.ijo.0802922. [DOI] [PubMed] [Google Scholar]
- Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer DL, Agras WS, Bryson S, Hammer LD. Early body mass index and other anthropometric relationships between parents and children. Int J Obes Relat Metab Disord. 2001;25:1532–1536. doi: 10.1038/sj.ijo.0801786. [DOI] [PubMed] [Google Scholar]
- Toschke AM, Beyerlein A, von Kries R. Children at high risk for overweight: a classification and regression trees analysis approach. Obes Res. 2005;13:1270–1274. doi: 10.1038/oby.2005.151. [DOI] [PubMed] [Google Scholar]
- Valerio G, D'Amico O, Adinolfi M, Munciguerra A, D′Amico R, Franzese A. Determinants of weight gain in children from 7 to 10 years. Nutr Metab Cardiovasc Dis. 2006;16:272–278. doi: 10.1016/j.numecd.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Dubois L, Girard M. Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes. 2006;30:610–617. doi: 10.1038/sj.ijo.0803141. [DOI] [PubMed] [Google Scholar]
- Gibson LY, Byrne SM, Davis EA, Blair E, Jacoby P, Zubrick SR. The role of family and maternal factors in childhood obesity. Med J Aust. 2007;186:591–595. doi: 10.5694/j.1326-5377.2007.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, O'Rahilly S. New advances in the genetics of early onset obesity. Int J Obes. 2005;29:1149–1152. doi: 10.1038/sj.ijo.0803056. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland-Cachera MF, Sempe M, Guilloud-Bataille M, Patois E, Pequignot-Guggenbuhl F, Fautrad V. Adiposity indices in children. Am J Clin Nutr. 1982;36:178–184. doi: 10.1093/ajcn/36.1.178. [DOI] [PubMed] [Google Scholar]
- WHO . Preventing and Managing the Global Epidemic of Obesity. Report of the World Health Organization Consultation of Obesity. WHO: Geneva; 1997. [Google Scholar]
- Neovius M, Janson A, Rössner S. Prevalence of Obesity in Sweden. Obes Rev. 2006;7:1–3. doi: 10.1111/j.1467-789x.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59:955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Berkowitz RI, Stallings VA, Cater JR. Weights of parents and infants: is there a relationship. Int J Obes Relat Metab Disord. 1999;23:159–162. doi: 10.1038/sj.ijo.0800785. [DOI] [PubMed] [Google Scholar]
- Pietilainen KH, Kaprio J, Rasanen M, Rissanen A, Rose RJ. Genetic and environmental influences on the tracking of body size from birth to early adulthood. Obes Res. 2002;10:875–884. doi: 10.1038/oby.2002.120. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Pietilainen KH, Tynelius P, Sorensen TI, Kaprio J, Rasmussen F. Genetic and environmental factors in relative weight from birth to age 18: the Swedish young male twins study. Int J Obes. 2007;31:615–621. doi: 10.1038/sj.ijo.0803577. [DOI] [PubMed] [Google Scholar]
- Haworth CM, Carnell S, Meaburn EL, Davis OS, Plomin R, Wardle J. Increasing heritability of BMI and stronger associations with the FTO gene over childhood. Obesity (Silver Spring) 2008;16:2663–2668. doi: 10.1038/oby.2008.434. [DOI] [PubMed] [Google Scholar]
- Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr. 2008;87:398–404. doi: 10.1093/ajcn/87.2.398. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics. 2001;108:712–718. doi: 10.1542/peds.108.3.712. [DOI] [PubMed] [Google Scholar]
- Berkowitz RI, Stallings VA, Maislin G, Stunkard AJ. Growth of children at high risk of obesity during the first 6 y of life: implications for prevention. Am J Clin Nutr. 2005;81:140–146. doi: 10.1093/ajcn/81.1.140. [DOI] [PubMed] [Google Scholar]
- Quattrin T, Liu E, Shaw N, Shine B, Chiang E. Obese children who are referred to the pediatric endocrinologist: characteristics and outcome. Pediatrics. 2005;115:348–351. doi: 10.1542/peds.2004-1452. [DOI] [PubMed] [Google Scholar]
- Unger R, Kreeger L, Christoffel KK. Childhood obesity. Medical and familial correlates and age of onset. Clin Pediatr (Phila) 1990;29:368–373. doi: 10.1177/000992289002900701. [DOI] [PubMed] [Google Scholar]
- Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S, et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118:1644–1649. doi: 10.1542/peds.2006-1379. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Shear CL, Burke GL, Srinivasan SR, Webber LS, Harsha DW, et al. Persistence of juvenile-onset obesity over eight years: the Bogalusa Heart Study. Am J Publ Health. 1987;77:588–592. doi: 10.2105/ajph.77.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garn SM, Sullivan TV, Hawthorne VM. Fatness and obesity of the parents of obese individuals. Am J Clin Nutr. 1989;50:1308–1313. doi: 10.1093/ajcn/50.6.1308. [DOI] [PubMed] [Google Scholar]
- Perez-Pastor EM, Metcalf BS, Hosking J, Jeffery AN, Voss LD, Wilkin TJ. Assortative weight gain in mother-daughter and father-son pairs: an emerging source of childhood obesity. Longitudinal study of trios (EarlyBird 43) Int J Obes. 2009;33:727–735. doi: 10.1038/ijo.2009.76. [DOI] [PubMed] [Google Scholar]
- Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross-sectional studies 1990-2005. Obesity. 2008;16:275–284. doi: 10.1038/oby.2007.35. [DOI] [PubMed] [Google Scholar]
- Ong KK. Size at birth, postnatal growth and risk of obesity. Horm Res. 2006;65:65–69. doi: 10.1159/000091508. [DOI] [PubMed] [Google Scholar]