Abstract
The use of pharmacologic MRI (phMRI) in mouse models of brain disorders allows noninvasive in vivo assessment of drug-modulated local cerebral blood volume changes (ΔCBV) as one correlate of neuronal and neuro-vascular activities. In this report, we employed CBV weighted phMRI to compare cocaine-modulated neuronal activity in dopamine transporter (DAT) knockout (KO) and wild-type mice. Cocaine acts to block the dopamine, norepinephrine and serotonin transporters (DAT, NET and SERT) that clear their respective neurotransmitters from the synapses, helping to terminate cognate neurotransmission. Cocaine consistently reduced CBV, with a similar pattern of regional ΔCBV in brain structures involved in mediating reward in both DAT genotypes. The largest effects (−20% to −30% ΔCBV) were seen in the nucleus accumbens and several cortical regions. Decreasing response amplitudes to cocaine were noted in more posterior components of the cortico-mesolimbic circuit. DAT KO mice had significantly attenuated ΔCBV amplitudes, shortened times to peak response and reduced response duration in most regions. This study demonstrates that DAT knockout does not abolish the phMRI responses to cocaine, suggesting that adaptations to loss of DAT and/or retained cocaine activity in other monoamine neurotransmitter systems underlie these responses in DAT KO mice.
Introduction
Assessment of the effects of psychotropic substances requires an understanding of their sites, mechanisms and time-courses of action. Functional neuroimaging techniques have made it possible to study drug-induced changes in brain activity in vivo (Leslie and James, 2000). Pharmacological magnetic resonance imaging (phMRI), a variant of functional MRI, is well adapted to monitor drug action in the brain dynamically and noninvasively and to map the brain systems and networks targeted by psychoactive drugs (Chen et al., 2001). A number of important classes of medications used in the treatment of psychiatric disorders and many drugs of abuse target plasma membrane reuptake transporters for the monoamine neurotransmitters dopamine, norepinephrine, and serotonin (DAT, NET, and SERT, respectively) inhibiting the reuptake of the released neurotransmitters (Gainetdinov and Caron, 2003).
Dopamine systems, including the cortico-mesolimbic system, are crucial for normal reward-seeking behavior and are likely to make major contributions to the development of addiction (Girault and Greengard, 2004). Cocaine is a prototypical psychomotor stimulant with powerful mood altering properties (Johanson and Fischman, 1989; Uhl et al., 2002). It has dramatic effects on the reward pathway through interaction with the monoamine transporters (Gainetdinov and Caron, 2003). Moreover, chronic exposure has a multitude of effects in the brain, including modulation of signaling cascades, neurotrophic factors, monoamine receptors, and neuroplasticity (El-Ghundi et al., 2007; Thomas et al., 2008). Contrary to initial expectations, DAT knockout (KO) mice display evidence for cocaine reinforcement in conditioned place preference and even in some self-administration paradigms (Rocha et al., 1998; Sora et al., 1998). However, in one line of DAT KO mice, individual mice differ somewhat in their cocaine self-administration behavior. Most do not self-administer. Those that do self-administer fail to continue responding when challenged with more demanding schedules of reinforcement (Thomsen et al., 2009). This may be due to disruption of aspects of incentive motivation (or aversion) mediated by dopamine, since DAT KO mice do not exhibit cocaine conditioned locomotion (Hall et al., 2009). That cocaine is rewarding at all in DAT KO mice may be, in part, a consequence of neuroadaptations in the SERT and NET systems; consistent with the finding that SERT and NET blockers have reinforcing effects in DAT KO mice that are not observed in wildtype mice (Hall et al., 2002). These data suggest that although aspects of cocaine reward are maintained, at least to a certain degree, in DAT KO mice, the mechanisms underlying cocaine reward differ from those in wildtype mice.
phMRI measures the hemodynamic responses to a pharmacological challenge, and thus probes the activity of neuronal pathways targeted by the drug based on the neurovascular consequences of changed neuronal (and vascular) activities (Jenkins et al., 2004; Mandeville et al., 1998; Schwarz et al., 2007a). This technique has the potential for examining the underlying changes in regional brain activity in mouse models of disorders that display links to altered neurotransmitter activity, including attention deficit hyperactivity disorder, narcolepsy, obesity, and drug addiction. However, small brain size, rapid metabolic rates, susceptibility artifacts, and the need for anesthesia render functional MRI challenging in mice. Small animal imaging is carried out at high magnetic field strengths (>7 Tesla) to maximize signal intensity. In phMRI the sensitivity to functional changes is enhanced with exogenous magnetic resonance contrast agents such as ultrasmall superparamagnetic iron oxide particles (USPIO) that are retained in the blood pool and exhibit long circulation times (Weissleder et al., 1990). The paramagnetic signals generated by such superparamagnetic particles are so large that most of the MRI signal is determined by the cerebral blood volume (CBV), overwhelming blood oxygen level dependent (BOLD) and blood flow effects. Most current studies using this technique have used rats (Mandeville et al., 2004; Mandeville et al., 1998; Schwarz et al., 2007b) or nonhuman primates (Jenkins et al., 2004). A few research teams have successfully performed CBV weighted phMRI in mice (Luo et al., 2008; Mueggler et al., 2003; Wu et al., 2004), although low signal to noise ration (SNR) and sensitivity have precluded a broad range of investigations using genetically engineered mouse models.
In the present report, we have examined, for the first time, effects of DAT gene deletion on neurovascular responses to an acute cocaine challenge. We have used a CBV weighted phMRI technique in mice and achieved good sensitivity to the CBV change with time resolution of 1 minute and spatial resolution of < 0.2 mm. This methodology has already proved useful in larger rodent and primate systems (Chin et al., 2006; Jenkins et al., 2004; Mandeville et al., 2005; Marota et al., 2000; Sanchez-Pernaute et al., 2007; Schwarz et al., 2003). Adapting this approach for mouse brain imaging should provide a valuable means for testing hypothesizes in a variety of mouse model systems.
Material and Methods
CBV responses to cocaine were studied in 6 DAT KO mice, and 6 wildtype control mice consisting of 4 wildtype littermates and 2 C57BL/6J female mice (The Jackson Laboratory, Bar Harbor, ME.). The DAT KO mice used in these experiments have been described previously (Sora et al., 1998). Pilot experiments on isoflurane anesthetized mice of different strains and ages were carried out to establish a suitable cocaine dose. In bench top experiments that did not involve MRI, the electrocardiogram, respiration and expired CO2 (MicroCapStar CO2 Monitor, IITC Life Science Inc., CA) were continuously recorded before and after cocaine injection. Criteria for the choice of the cocaine dose were: a) no arousal from anesthesia and b) stable physiologic parameters after injection. Behavioral changes have been reported starting at a cocaine dose of 5 mg/kg intraperitoneal (IP) in conscious mice (Jamshidi et al., 2004), while cocaine induced seizures start to appear at a dose of 70 mg/kg IP. Doses of 20 to 40 mg/kg IP increase striatal and nucleus accumbens dopamine levels 3-fold in wild type mice (Mateo et al., 2004; Rocha et al., 1998). In this work, cocaine hydrochloride (Sigma-Aldrich, MO) was dissolved in normal saline solution (5 mg/ml) for IP injection at a dose of 30 mg/kg.
P904 (Guerbet Research, Aulnay-Sous-Bois, France) is a research prototype USPIO with a hydrodynamic diameter of 25 to 30 nm and a glucose derivative coating designed for macrophage and vascular imaging applications (Sigovan et al., 2009). It was provided as a 500 mM (≈ 28 mg/ml) solution and was diluted to 6 mg/ml in sterile normal saline solution for IV injection. A volume of ≈ 4.2 µl/g body weight was injected IV resulting in a dose of 25 mg/kg.
All experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the California Institute of Technology.
In vivo phMRI
In vivo MRI experiments were performed in a horizontal bore Bruker Biospec/Avance 7T/30 cm small animal MR system using a custom made 12-rung linear birdcage RF coil (length 45 mm, inner diameter 26 mm) for transmission and reception. The mice were positioned prone in a plastic cradle with an integral head mask providing 1.5 to 1.7% isoflurane in N2/O2 70%/30% and incorporated ECG electrodes for the front paws and tail. Rectal temperature (Opsens OTGM, Canada) and respiration (BIOPAC Systems, CA) were monitored continuously. Simultaneous recording of the electrocardiogram during gradient echo imaging was not always reliable, but heart rates at the start and end of acquisition were comparable. Body temperature was maintained between 36 and 38°C using heated air passed through the RF coil and a cylindrical plastic extension that covered the mouse body, preventing rapid loss of the warm air. The isoflurane level was adjusted to achieve respiratory rates of 80 to 120 breaths per minute and was not changed during image acquisition. The mice were fitted with tail vein catheters for USPIO injection and intraperitoneal catheters for drug injection. Both catheters had extension lines with total volumes of 200 µl and were preloaded with USPIO and drug in normal saline solution before positioning the mouse in the magnet.
An autoshiming routine resulted in a 1H linewidth of < 0.13 ppm (FWHM) in a cube of 4 × 4 × 4 mm3 brain tissue. After obtaining stable physiologic conditions, relative CBV maps were obtained based on the intravascular susceptibility effect of the USPIO (Berry et al., 1996; Mandeville et al., 1998).
For each brain, 14 contiguous axial 0.75 mm thick slices, with a field of view of 19 × 19 mm2, were acquired from the olfactory bulb to the cerebellum using a 2D multiple gradient echo sequence with TR = 600 ms, flip angle = 35°, 100 × 100 matrix, spectral line width 60 kHz, three echoes at TE = 2.5, 6.0 and 9.5 ms, and an acquisition time of 1 minute per repetition.
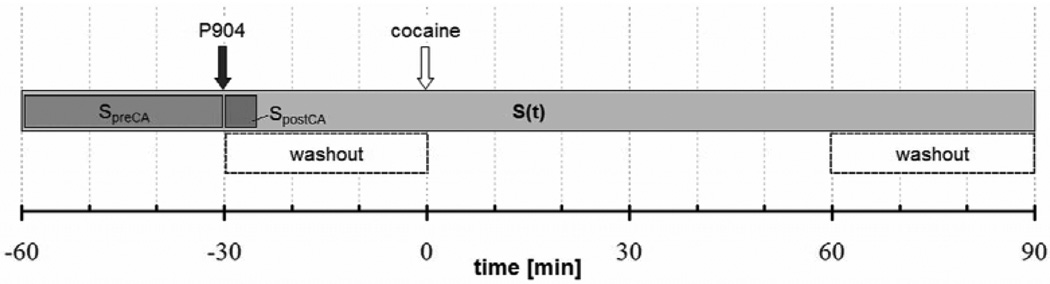
Figure 1 illustrates the phMRI protocol: R2* weighted images were acquired for 30 minutes prior to contrast agent administration followed by 30 post-contrast baseline acquisitions. After the pharmacological challenge, image acquisition continued for 90 minutes. The USPIO and cocaine were injected by flushing the IV/ IP catheter with 250 µl saline solution over a duration of 1 minute while the mouse was positioned inside the scanner.
Figure 1.
Experimental protocol: In phMRI experiments gradient echo images were acquired continuously with a time resolution of 1 minute. The baseline intervals before and after P904 injection were 30 minutes each. SpreCA. is the constant baseline signal before contrast agent injection; SpostCA is the average of five consecutive signals acquired immediately after contrast agent injection; and S(t) is the time dependent CBV-weighted signal after contrast agent injection. The acquisitions in the interval between contrast agent and cocaine injection and the acquisitions at > 60 minutes after cocaine were used to detrend the signal and to derive the contrast agent washout rate.
MRI data analysis
Pre-contrast and post-contrast images were automatically co-registered with a 2D rigid body (3 parameters) model using Automated Image Registration (AIR) software (Woods et al., 1998). Image analysis was performed with ImageJ (Abramoff et al., 2004). No spatial or temporal smoothing was applied to the data.
The relative signal decrease upon contrast agent administration is
| (1) |
where SpreCA is the mean signal intensity prior to contrast agent (CA) injection and SpostCA is the signal intensity averaged over the first five acquisitions after contrast agent injection (Figure 1). The signal decrease depends on the relaxivity of the contrast agent and its initial tissue concentration. These factors determine the transverse relaxation rate change
| (2) |
where TE is the echo time.
Prior to quantitative analysis, the signal intensity after contrast agent injection, S(t), was detrended (Schwarz et al., 2003) to eliminate the effect of contrast agent washout by fitting two time intervals of S(t) prior to and > 60 minutes after pharmacological challenge (Figure 1) to a two parameter model (Perles-Barbacaru et al., 2010). Assuming that the T2-weighted signal is a simple exponential function (S0(t) ∝ exp[−TE R2*(t)]), we find that:
| (3) |
where SpreCA is defined as above. The fitting parameter κ provides an estimate of the initial ΔR2* following USPIO injection (Equation 2), while τ−1 is the apparent washout rate of the contrast agent from the tissue. In this model we assume negligible T1-effects, an instantaneous USPIO bolus and mono-exponential USPIO washout in the plasma, since USPIO is confined to the intravascular compartment.
The relative CBV change (ΔCBV) is calculated for each image voxel according to Mandeville et al. (1998) by assuming a linear relationship between CBV and ΔR2*:
| (4) |
where S0(t) represents the fit to the signal time-course after contrast agent injection under resting conditions with no drug challenge (Equation 3). S(t) refers to the measured signal after contrast agent injection. We analyzed the ΔCBV using the signal from the 3rd echo at TE = 9.5 ms, while the ROIs were drawn on the images of the first two echoes.
Quantitative analyses of the parametric maps from Equation 4 describing the CBV change upon cocaine injection were performed by examining several cerebral ROIs forming the drug reward circuitry. We analyzed ROIs in the prefrontal, frontal and frontoparietal cortex (CTX), nucleus accumbens (ACB), dorsal striatum (DS), amygdala (AMY), hippocampal formation (HPF), thalamic nuclei (TH), hypothalamus (HY), midbrain (MB), ventral tegmental area (VTA), substantia nigra (SNr), pons (P), medulla (MY) and cerebellum (CBX). To rule out confounding systemic vascular changes associated with the pharmacological stimulus and independent of neuronal activity, we also analyzed the signal change in an extra-cerebral ROI located in the masseter or temporalis muscle.
To describe the regional time course of the ΔCBV response we used a simplified gamma variate model
| (5) |
The three model parameters (ΔCBVpeak = amplitude, Tpeak = time to peak, and α = shape factor) were compared between DAT KO and wildtype control groups using unpaired T-tests. In particular, for each ROI noted above, the gamma variate model (Equation 5) was fitted to the data of one animal at a time and to the combined data from all animals in one group (examples for ACB and VTA can be seen in Figure 3). An F-test was used to compare the relative difference in the sum of squares of the residuals from each fit and the relative difference in the degrees of freedom. To test for significant differences in each model parameter, an F-test was applied to the results of nonlinear regression with independent and shared parameter of interest. T-tests for each model parameter confirmed the statistically significant differences detected by the F-test. To test whether the entire response curves were different the F-test was performed on the results of the nonlinear regression to the separate data and to the combined data of both groups. P<0.01 was considered statistically significant. Curve fitting and statistical tests were performed using GraphPad Prism version 4.03 for Windows (GraphPad Software, La Jolla, CA).
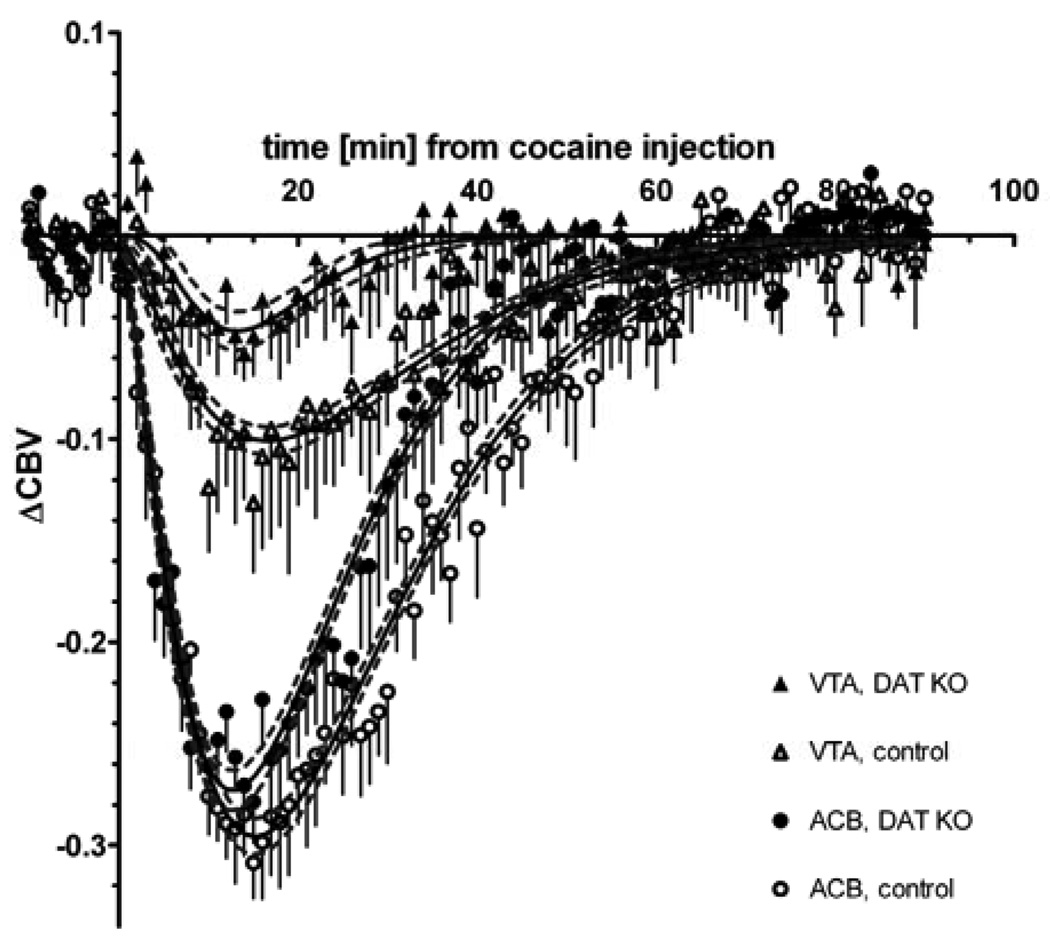
Figure 3.
ΔCBV response in two cerebral structures (nucleus accumbens and ventral tegmental area) to an IP injection of 30 mg/kg cocaine at t = 0. The error bars represent SEM; N = 6 DAT KO and N = 6 wildtype mice. The continuous black lines are the best gamma variate fit with the 95% confidence interval shown as dashed gray lines.
Results
During and within one minute after intravenous injection of P904, respiratory amplitudes and rates were increased. Pilot experiments showed that cocaine doses up to 1.5 mg/kg IV or 30 mg/kg IP did not alter these physiologic parameters for more than 3 minutes. We administered cocaine intraperitoneally during MRI experiments to avoid sudden hypervolemia and to avoid the tail and body motion that often accompanied intravenous cocaine administration. An intraperitoneal dose of 30 mg/kg cocaine transiently reduced and then increased the respiratory amplitude for 1 to 3 minutes.
Analysis of the signal from a ROI in the dorsal striatum (area 3.2 ± 0.2 mm2) acquired at TE = 9.5 ms showed that a P904 dose of 25 mg/kg yielded a relative signal decrease (Equation 1) of 0.58 ± 0.02 during the 5 minutes following contrast agent injection with an apparent tissue half-life (τ·ln2) of 156 ± 19 minutes (N = 12, average ± standard error of the mean). Although the apparent tissue half-life computed according to Equation 3 does not vary from region to region, it is greater than 2 hours in all regions. Thus, the detrending model in Equation 3 takes reasonable account of the slowly decreasing P904 concentration.
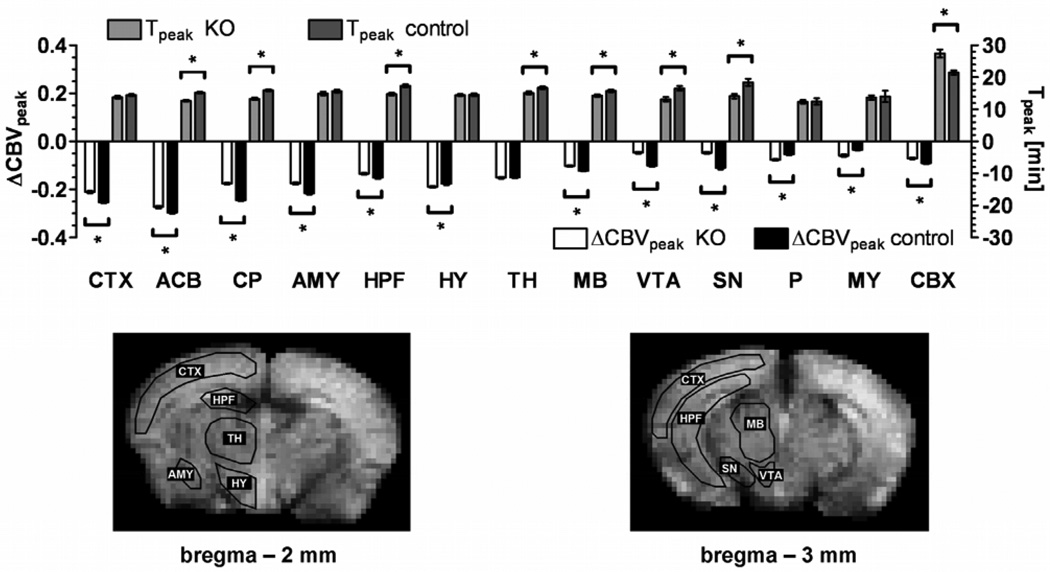
Saline injection produced no measurable effect on CBV (data not shown). Figure 2 shows that a spatially and temporally dependent CBV change was observed in cerebral tissue of mice treated with cocaine, while there was no measurable effect on extra-cerebral vasculature. Cocaine induced a marked CBV decrease (Figure 2) that lasted between 40 and 60 minutes. The ventral striatum/nucleus accumbens exhibited the strongest response to cocaine. The time course of the ΔCBV response to cocaine in two ROIs within the mesolimbic pathway is shown in Figure 3 for DAT KO and wildtype mice. The time-courses in all examined ROIs, except thalamic nuclei, differed significantly between DAT genotypes. Figure 4 shows that the DAT KO mice display an attenuated ΔCBV response in all regions except the hypothalamus, thalamus, pons and medulla oblongata. Also, in the knockout mice peak responses were reached at earlier time-points for most brain structures and the total duration of the hemodynamic response to cocaine was shorter than in wildtype mice (Figure 3 and 4).
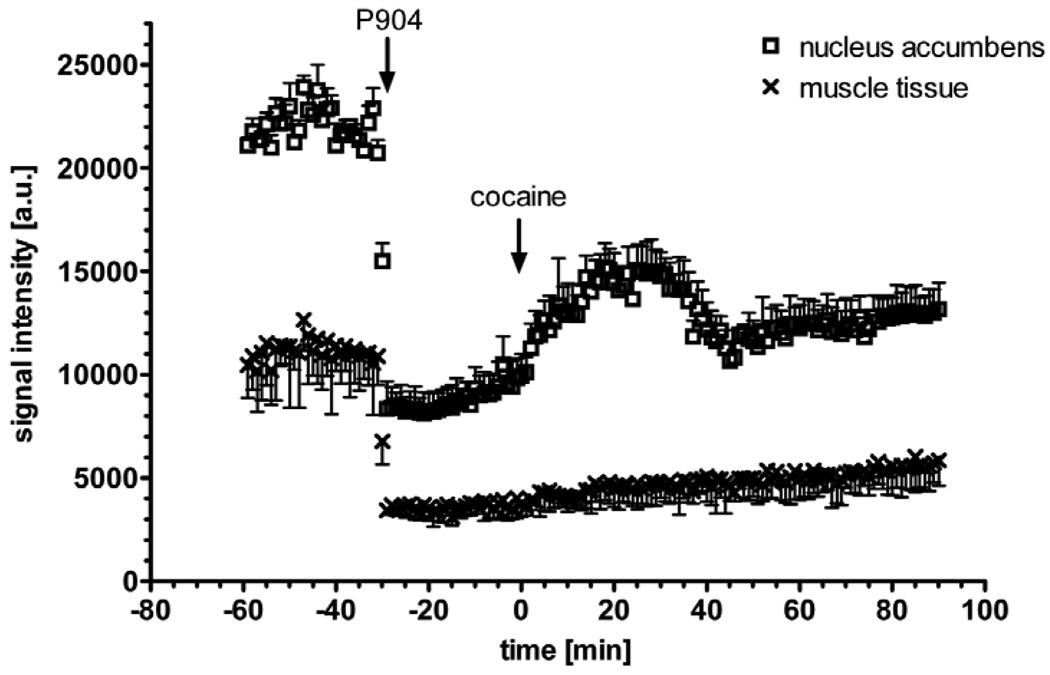
Figure 2.
Effect of IP cocaine on the signal time course in cerebral (nucleus accumbens, open symbols) and in extra-cerebral tissue (muscle, closed symbols). Injection of 25 mg/kg P904 IV at t = −30 min (left arrow) yields a 62.5% signal drop in nucleus accumbens (4.06 mm3) and 69.1% signal drop in muscle (9.58 mm3) at TE = 9.5 ms. The second arrow indicates the IP injection of cocaine (30 mg/kg), which leads to a signal increase in the cerebral tissue corresponding to a CBV decrease. The error bars show the standard deviation of the signal within the ROI in a single mouse. This is a typical signal time course prior to detrending.
Figure 4.
Quantitative analysis of the regional CBV change after cocaine injection. Peak amplitude and the time to peak of the gamma variate fit to the signal time course are shown for specific brain regions. The error bars indicate the standard error of the mean (N = 6 per group). In all ROIs there is a statistically significant difference (P < 0.01) between DAT KO and wildtype mice in at least one of the three fitting parameters (the shape factor α is not shown). The overall time course of the ΔCBV response is statistically different for KO and control mice in all ROIs except the thalamus. Typical ROI outlines are shown on two coronal brain slices: prefrontal, frontal and frontoparietal cortex (CTX), nucleus accumbens (ACB), caudate putamen (CP), amygdala (AMY), hippocampal formation (HPF), thalamic nuclei (TH), hypothalamus (HY), midbrain (MB), ventral tegmental area (VTA), substantia nigra (SN), pons (P), medulla (MY), cerebellum (CBX).
Discussion
Drug induced CBV changes
Species differences in cerebral responses to cocaine have been reported previously (Lyons et al., 1996; Zocchi et al., 2001), but have remain unexplained. In this study, a negative ΔCBV response to cocaine was observed in mice. In rats, ΔCBV increases after cocaine challenge have been observed with MRI (Ceolin et al., 2007; Marota et al., 2000; Schwarz et al., 2003; Schwarz et al., 2004), while cocaine induced ΔCBV decreases have been observed in humans (Kaufman et al., 1998). Mechanisms for the negative CBV response may be related to reduced cerebrovascular feedback and/or decreased neuronal activation (Mandeville et al., 2005; Peoples et al., 1998) accompanied by a decreased cerebral glucose metabolism (Lyons et al., 1996; Thanos et al., 2008). Cerebral microvascular constriction has also been observed in rhesus monkey brain slices upon micro-application of dopamine (Krimer et al., 1998). Direct activation of D1 dopaminergic receptors on arterioles results in vasodilation (Choi et al., 2006), although the sign of the CBV response depends on both the effective concentration of dopamine and the relative regional densities of receptors in the D1/D5 and D2/D3 and possibly even D4 receptor families (Choi et al., 2006). Cocaine-induced blockade of norepinephrine and serotonin reuptake are also likely to influence the overall direction of cardiovascular changes in both the wildtype, perhaps especially in the DAT KO mice.
Confounding vascular effects
Vascular effects not related to neuroactivity also have to be considered when interpreting phMRI results. Confounding systemic effects of cocaine have been difficult to exclude (Gozzi et al., 2007; Luo et al., 2009). A limitation of the current study is that the arterial blood pressure could not be monitored during scanning. However, the transient effect (~ 5 min) of this cocaine dose on the systemic blood pressure does not seem to provide a large determinant for the sustained (~ 1 h) hemodynamic response in the brain (Du et al., 2009; Du et al., 2006; Luo et al., 2003; Schmidt et al., 2006). Moreover, any large peripheral blood pressure change following cocaine injection would have resulted in a signal change in muscle tissue. Our findings suggest that the measured CBV change in the current study is largely independent of systemic effects of the drug.
There is evidence that the CBV response may depend on the type of anesthetic used in the experiment (Du et al., 2009; Luo et al., 2009). In previous studies, which used different anesthetics, the anesthetic used may have been confounded with apparent species differences. In a study by Du and colleagues (Du et al., 2006), a CBV decrease of −4 to −8% was observed with optical techniques in the cortex of rats under isoflurane anesthesia after an intravenous dose of 1 mg/kg cocaine whether or not a peripheral blood pressure decrease during the first minutes was prevented by phenylephrine. Consistent anesthesia conditions were employed throughout the present work. Comparisons among the different genotypes are thus credible, regardless of whether the nature of differences in CBV responses between previous studies is the result of true species differences or procedural differences (e.g. the choice of anesthetic). Nevertheless, care must be taken in comparisons with conscious animals or with results from studies that use other anesthesia regimens.
Reward circuitry and monoamine transporter knockout mice
The cortico-mesolimbic dopaminergic circuit is thought to mediate many of the rewarding effects of many drugs of abuse, including cocaine, which prolongs dopaminergic transmission. However, cocaine also elevates extracellular serotonin and norepinephrine levels (Li et al., 1996) which may account for its pronounced rewarding effects compared to more selective agents. Rodents, in general, and monoamine transporter knockout mice, in particular, have been used in efforts to identify the mechanisms of action of cocaine and other drugs of abuse. A study by Schwarz and colleagues in rats (Schwarz et al., 2004) has shown that the ΔCBV time course is not correlated with the local dopamine concentration or with the local cocaine concentration in all brain regions. A more complex mechanism for the ΔCBV response involving other neurotransmitters and downstream consequences of the effects produced by cocaine is supported by the fact that, in our study, a straightforward pharmacokinetic model did not fit the ΔCBV time-course. The ΔCBV response was, therefore, described by a gamma variate model that allowed for a gradual onset of the ΔCBV response.
In this study similar patterns of spatial activation were observed in both DAT KO and wildtype mice. The largest negative responses were observed in the nucleus accumbens, frontal and parietal cortex, dorsal striatum, and amygdala (in decreasing order). These structures have substantial dopaminergic innervation and constitute a substantial portion of the brain reward circuitry that mediates the rewarding effects of drugs of abuse (Koob and Volkow, 2010). The time to peak response was 12–18 minutes in all structures examined, except the cerebellum where it was 20–28 minutes. Quantitative regional analysis revealed a significant decrease in amplitude and a slight decrease in duration of the hemodynamic response in DAT KO mice compared to wildtype mice. The ΔCBV response to cocaine in these regions was attenuated but not entirely abolished in DAT KO mice. This is consistent with the maintenance of some reinforcing effects of cocaine in DAT KO mice at the cocaine dose used here as indicated by conditioned place preference (Sora et al., 2001; Sora et al., 1998) and cocaine self-administration (Rocha et al., 1998) studies. However, the reduced ΔCBV response in DAT KO mice may also be considered to be consistent with the weaker reinforcing effects that have been demonstrated in a more recent self-administration study (Thomsen et al., 2009). More importantly, it may indicate a difference in the underlying mechanism of cocaine effects in DAT KO and wildtype mice. Cocaine continues to produce a conditioned place preference in DAT KO mice, but DAT KO mice also exhibit novel rewarding effects of selective SERT and NET blockers (Hall et al., 2002). The rewarding effects of these drugs may be due to consequences of SERT and NET blockade in the VTA/SN. Along with its rewarding effects, cocaine continues to produce increases in extracellular dopamine in the striatum in this strain of DAT KO mice (Shen et al., 2004). This might be due to cocaine blockade of SERT, a hypothesis consistent with the elimination of cocaine conditioned place preference in DAT/SERT double KO mice (Sora et al., 2001). Furthermore, although fluoxetine, a selective serotonin reuptake inhibitor, has no effect in wildtype mice, it is rewarding (Hall et al, 2002) and increases striatal dopamine levels in DAT KO mice (Shen et al., 2004). The locus of this effect may be the VTA/SN, as local infusions of cocaine in either the dorsal or ventral striatum do not increase extracellular dopamine levels in DAT KO mice (Mateo et al., 2004; Shen et al., 2004), but local injections of cocaine or fluoxetine into the VTA do increase extracellular dopamine levels in the nucleus accumbens of DAT KO, but not wildtype, mice (Mateo et al., 2004). In the present study, the greatest differences in ΔCBV between KO and wildtype mice were observed in the VTA/SN. These results are consistent with the hypothesis presented above that the mechanism of cocaine-induced increases in striatal dopamine in DAT KO mice involve at least some cocaine actions in the VTA/SN rather than dopaminergic terminal fields.
DAT KO responses to cocaine are less negative than those of wildtype animals in all brain regions except the pons, hypothalamus, and medulla oblongata (and not statistically different in the thalamus). These are all regions that are not involved in drug reward. They display only modest densities of dopaminergic innervation. Thanos and colleagues (Thanos et al., 2008) used PET measured 18F-fluorodeoxyglucose (18FDG) uptake in DAT KO and wildtype mice to assay metabolic activity. They found an overall decrease in baseline 18FDG uptake in DAT KO versus wildtype mice, and increased uptake after cocaine challenge in DAT KO mice only in the thalamus and cerebellum. In that PET study 18FDG was introduced 30 minutes after cocaine injection with an 80 minute acquisition time. Thus, the 18FDG uptake reflects cocaine effects well after the CBV changes observed here have returned to baseline. In other studies with rats and primates using intravenous rather than IP injection of drugs (Jenkins et al., 2004; Marota et al., 2000), both time to maximum change in CBV and return to baseline are significantly reduced from values observed here due to different routes of administration. Regardless of how cocaine is administered, ΔCBV changes measure a fast response of the reward circuit to cocaine challenge, while 18FDG uptake evaluates longer term responses, averaged over 80 minutes, that may reflect adaptations to the initial cocaine effect. Taken together, the PET and phMRI results imply that cocaine impacts the cortico-mesolimbic reward circuit in the first minutes after challenge with the most impact in the more dopaminergically-innervated anterior structures. Cocaine subsequently exerts a more sustained influence on the more “limbic” parts of the circuit.
Conclusion
This study demonstrates the applicability of the CBV weighted phMRI technique to mapping hemodynamic effects of centrally acting drugs in mouse models of disease. The technique is minimally invasive and provides good functional sensitivity. We also note that, as with other functional MRI techniques, the mechanisms underlying the CBV changes may not be exclusively specific to neuronal activation. The negative ΔCBV response to cocaine challenge is rapid (~15 min) throughout the reward circuit and attenuated in DAT KO mice. Since knockout of DAT has a small but significant effect on the immediate response to cocaine in brain regions thought to be involved in cocaine reward, knockout of DAT appears to produce circuit-wide changes, not just changes in dopaminergic neurons. The largest responses to cocaine (−20% to −30% ΔCBV) are seen in the cortex and nucleus accumbens. There are decreasing responses in more posterior parts of the reward circuit (CP > AMY > VTA ~ SN). Nevertheless, some of the greatest differences between DAT KO and wildtype mice were observed in midbrain structures, VTA and SN, which may indicate a different mechanism of action of cocaine in DAT KO mice. The fact that there are much more modest differences in the nucleus accumbens and dorsal striatum between DAT KO and wildtype mice is consistent with the retention of some cocaine reward, under at least some conditions (Rocha et al., 1998; Sora et al., 2001; Sora et al., 1998; Thomsen et al., 2009). Thus these results emphasize the ultimate importance of the nucleus accumbens at early as well as later stages of drug exposure (Koob and Volkow, 2010).
Acknowledgments
The authors thank Hargun Sohi and Thomas Ng for their technical assistance, Davit Janvelyan for help with the AIR software, Guerbet Research (Aulnay-Sous-Bois, France) for providing the P904 contrast agent. This project was funded in part by the Beckman Institute, NIDA R01DA18184, and NCRR U24 RR021760 Mouse BIRN and by funding from the National Institute on Drug Abuse, Intramural Research Program (GRU, FSH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Berry I, Benderbous S, Ranjeva JP, Gracia-Meavilla D, Manelfe C, Le Bihan D. Contribution of Sinerem used as blood-pool contrast agent: detection of cerebral blood volume changes during apnea in the rabbit. Magn Reson Med. 1996;36:415–419. doi: 10.1002/mrm.1910360313. [DOI] [PubMed] [Google Scholar]
- Ceolin L, Schwarz AJ, Gozzi A, Reese T, Bifone A. Effects of cocaine on blood flow and oxygen metabolism in the rat brain: implications for phMRI. Magn Reson Imaging. 2007;25:795–800. doi: 10.1016/j.mri.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Chen YC, Mandeville JB, Nguyen TV, Talele A, Cavagna F, Jenkins BG. Improved mapping of pharmacologically induced neuronal activation using the IRON technique with superparamagnetic blood pool agents. J Magn Reson Imaging. 2001;14:517–524. doi: 10.1002/jmri.1215. [DOI] [PubMed] [Google Scholar]
- Chin CL, Fox GB, Hradil VP, Osinski MA, McGaraughty SP, Skoubis PD, Cox BF, Luo Y. Pharmacological MRI in awake rats reveals neural activity in area postrema and nucleus tractus solitarius: relevance as a potential biomarker for detecting drug-induced emesis. Neuroimage. 2006;33:1152–1160. doi: 10.1016/j.neuroimage.2006.06.059. [DOI] [PubMed] [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Du C, Tully M, Volkow ND, Schiffer WK, Yu M, Luo Z, Koretsky AP, Benveniste H. Differential effects of anesthetics on cocaine's pharmacokinetic and pharmacodynamic effects in brain. European Journal of Neuroscience. 2009;30:1565–1575. doi: 10.1111/j.1460-9568.2009.06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Yu M, Volkow ND, Koretsky AP, Fowler JS, Benveniste H. Cocaine increases the intracellular calcium concentration in brain independently of its cerebrovascular effects. J Neurosci. 2006;26:11522–11531. doi: 10.1523/JNEUROSCI.3612-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghundi M, O'Dowd BF, George SR. Insights into the role of dopamine receptor systems in learning and memory. Reviews in the Neurosciences. 2007;18:37–66. doi: 10.1515/revneuro.2007.18.1.37. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Caron MG. Monoamine transporters: From genes to behavior. Annual Review of Pharmacology and Toxicology. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- Girault JA, Greengard P. The neurobiology of dopamine signaling. Archives of Neurology. 2004;61:641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Ceolin L, Schwarz A, Reese T, Bertani S, Crestan V, Bifone A. A multimodality investigation of cerebral hemodynamics and autoregulation in pharmacological MRI. Magn Reson Imaging. 2007;25:826–833. doi: 10.1016/j.mri.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Hall FS, Li XF, Randall-Thompson J, Sora I, Murphy DL, Lesch KP, Caron M, Uhl GR. Cocaine-conditioned locomotion in dopamine transporter, norepinephrine transporter and 5-HT transporter knockout mice. Neuroscience. 2009;162:870–880. doi: 10.1016/j.neuroscience.2009.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Li XF, Sora I, Xu F, Caron M, Lesch KP, Murphy DL, Uhl GR. Cocaine mechanisms: enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience. 2002;115:153–161. doi: 10.1016/s0306-4522(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Jamshidi HR, Rezayat M, Zarrindast MR. Effect of apmin on tolerance to cocaine-induced locomotor activity in mice. Acta Medica Iranica. 2004;42:78–82. [Google Scholar]
- Jenkins BG, Sanchez-Pernaute R, Brownell A-L, Chen Y-CI, Isacson O. Mapping Dopamine Function in Primates Using Pharmacologic Magnetic Resonance Imaging. J. Neurosci. 2004;24:9553–9560. doi: 10.1523/JNEUROSCI.1558-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacological Reviews. 1989;41:3–52. [PubMed] [Google Scholar]
- Kaufman MJ, Levin JM, Maas LC, Rose SL, Lukas SE, Mendelson JH, Cohen BM, Renshaw PF. Cocaine decreases relative cerebral blood volume in humans: a dynamic susceptibility contrast magnetic resonance imaging study. Psychopharmacology. 1998;138:76–81. doi: 10.1007/s002130050647. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer LS, Muly EC, 3rd, Williams GV, Goldman-Rakic PS. Dopaminergic regulation of cerebral cortical microcirculation. Nat Neurosci. 1998;1:286–289. doi: 10.1038/1099. [DOI] [PubMed] [Google Scholar]
- Leslie RA, James MF. Pharmacological magnetic resonance imaging: a new application for functional MRI. Trends Pharmacol Sci. 2000;21:314–318. doi: 10.1016/s0165-6147(00)01507-8. [DOI] [PubMed] [Google Scholar]
- Li MY, Yan QS, Coffey LL, Reith MEA. Extracellular dopamine, norepinephrine, and serotonin in the nucleus accumbens of freely moving rats during intracerebral dialysis with cocaine and other monoamine uptake blockers. Journal of Neurochemistry. 1996;66:559–568. doi: 10.1046/j.1471-4159.1996.66020559.x. [DOI] [PubMed] [Google Scholar]
- Luo F, Seifert TR, Edalji R, Loebbert RW, Hradil VP, Harlan J, Schmidt M, Nimmrich V, Cox BF, Fox GB. Non-invasive characterization of beta-amyloid(1–40) vasoactivity by functional magnetic resonance imaging in mice. Neuroscience. 2008;155:263–269. doi: 10.1016/j.neuroscience.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Luo F, Wu G, Li Z, Li SJ. Characterization of effects of mean arterial blood pressure induced by cocaine and cocaine methiodide on BOLD signals in rat brain. Magn Reson Med. 2003;49:264–270. doi: 10.1002/mrm.10366. [DOI] [PubMed] [Google Scholar]
- Luo Z, Yuan Z, Tully M, Pan Y, Du C. Quantification of cocaine-induced cortical blood flow changes using laser speckle contrast imaging and Doppler optical coherence tomography. Appl Opt. 2009;48:D247–D255. doi: 10.1364/ao.48.00d247. [DOI] [PubMed] [Google Scholar]
- Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16:1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville JB, Ekstrom L, Marota JJA, Jenkins BG, Vanduffel W. Contrast-enhanced fMRI of cocaine action in awake, non-human primate. Proceedings of the International Society for Magnetic Resonance in Medicine. 2005 [Google Scholar]
- Mandeville JB, Jenkins BG, Chen YC, Choi JK, Kim YR, Belen D, Liu C, Kosofsky BE, Marota JJ. Exogenous contrast agent improves sensitivity of gradient-echo functional magnetic resonance imaging at 9.4 T. Magn Reson Med. 2004;52:1272–1281. doi: 10.1002/mrm.20278. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR, Weisskoff RM. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med. 1998;39:615–624. doi: 10.1002/mrm.1910390415. [DOI] [PubMed] [Google Scholar]
- Marota JJ, Mandeville JB, Weisskoff RM, Moskowitz MA, Rosen BR, Kosofsky BE. Cocaine activation discriminates dopaminergic projections by temporal response: an fMRI study in Rat. Neuroimage. 2000;11:13–23. doi: 10.1006/nimg.1999.0520. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Budygin EA, John CE, Jones SR. Role of serotonin in cocaine effects in mice with reduced dopamine transporter function. Proc Natl Acad Sci U S A. 2004;101:372–377. doi: 10.1073/pnas.0207805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueggler T, Baumann D, Rausch M, Staufenbiel M, Rudin M. Age-dependent impairment of somatosensory response in the amyloid precursor protein 23 transgenic mouse model of Alzheimer's disease. J Neurosci. 2003;23:8231–8236. doi: 10.1523/JNEUROSCI.23-23-08231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples LL, Gee F, Bibi R, West MO. Phasic firing time locked to cocaine self-infusion and locomotion: dissociable firing patterns of single nucleus accumbens neurons in the rat. J Neurosci. 1998;18:7588–7598. doi: 10.1523/JNEUROSCI.18-18-07588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perles-Barbacaru AT, Procissi D, Demyanenko AV, Jacobs RE. Nonlinear model for preprocessing of cerebral blood volume weighted functional MRI data and for evaluating pharmacokinetic properties of USPIO. Proc. Intl. Soc. Mag. Reson. Med; 18th Annual Scientific Meeting; Stockholm (Sweden). 2010. p. 3550. [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Jenkins BG, Choi JK, Iris Chen YC, Isacson O. In vivo evidence of D3 dopamine receptor sensitization in parkinsonian primates and rodents with l-DOPA-induced dyskinesias. Neurobiol Dis. 2007;27:220–227. doi: 10.1016/j.nbd.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KF, Febo M, Shen Q, Luo F, Sicard KM, Ferris CF, Stein EA, Duong TQ. Hemodynamic and metabolic changes induced by cocaine in anesthetized rat observed with multimodal functional MRI. Psychopharmacology (Berl) 2006;185:479–486. doi: 10.1007/s00213-006-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz AJ, Gozzi A, Reese T, Bifone A. In vivo mapping of functional connectivity in neurotransmitter systems using pharmacological MRI. Neuroimage. 2007a;34:1627–1636. doi: 10.1016/j.neuroimage.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Gozzi A, Reese T, Heidbreder CA, Bifone A. Pharmacological modulation of functional connectivity: the correlation structure underlying the phMRI response to d-amphetamine modified by selective dopamine D3 receptor antagonist SB277011A. Magn Reson Imaging. 2007b;25:811–820. doi: 10.1016/j.mri.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Reese T, Gozzi A, Bifone A. Functional MRI using intravascular contrast agents: detrending of the relative cerebrovascular (rCBV) time course. Magn Reson Imaging. 2003;21:1191–1200. doi: 10.1016/j.mri.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Zocchi A, Reese T, Gozzi A, Garzotti M, Varnier G, Curcuruto O, Sartori I, Girlanda E, Biscaro B, Crestan V, Bertani S, Heidbreder C, Bifone A. Concurrent pharmacological MRI and in situ microdialysis of cocaine reveal a complex relationship between the central hemodynamic response and local dopamine concentration. Neuroimage. 2004;23:296–304. doi: 10.1016/j.neuroimage.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Sigovan M, Boussel L, Sulaiman A, Sappey-Marinier D, Alsaid H, Desbleds-Mansard C, Ibarrola D, Gamondes D, Corot C, Lancelot E, Raynaud JS, Vives V, Lacledere C, Violas X, Douek PC, Canet-Soulas E. Rapid-clearance iron nanoparticles for inflammation imaging of atherosclerotic plaque: initial experience in animal model. Radiology. 2009;252:401–409. doi: 10.1148/radiol.2522081484. [DOI] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci U S A. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. The effects of cocaine on regional brain glucose metabolism is attenuated in dopamine transporter knockout mice. Synapse. 2008;62:319–324. doi: 10.1002/syn.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. British Journal of Pharmacology. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Molecular Psychiatry. 2002;7:21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- Weissleder R, Elizondo G, Wittenberg J, Rabito CA, Bengele HH, Josephson L. Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology. 1990;175:489–493. doi: 10.1148/radiology.175.2.2326474. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Wu EX, Tang H, Asai T, Yan SD. Regional cerebral blood volume reduction in transgenic mutant APP (V717F, K670N/M671L) mice. Neurosci Lett. 2004;365:223–227. doi: 10.1016/j.neulet.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Zocchi A, Conti G, Orzi F. Differential effects of cocaine on local cerebral glucose utilization in the mouse and in the rat. Neurosci Lett. 2001;306:177–180. doi: 10.1016/s0304-3940(01)01898-5. [DOI] [PubMed] [Google Scholar]