SUMMARY
Fetal CD4+ lymphoid tissue inducer (LTi) cells play a critical role in the development of lymphoid-tissues. Recent studies identified that LTi cells persist in adults and are related to a heterogeneous population of innate lymphoid cells that have been implicated in inflammatory responses. However, whether LTi cells contribute to protective immunity remains poorly defined. We demonstrate that following infection with Citrobacter rodentium, CD4+ LTi cells were a dominant source of interleukin-22 (IL-22) early during infection. Infection-induced CD4+ LTi cell responses were IL-23-dependent, and ablation of IL-23 impaired innate immunity. Further, depletion of CD4+ LTi cells abrogated infection-induced expression of IL-22 and anti-microbial peptides, resulting in exacerbated host mortality. LTi cells were also found to be essential for host protective immunity in lymphocyte-replete hosts. Collectively these data demonstrate that adult CD4+ LTi cells are a critical source of IL-22 and identify a previously unrecognized function for CD4+ LTi cells in promoting innate immunity in the intestine.
INTRODUCTION
Lymphoid tissue inducer (LTi) cells are the first hematopoietic cells recruited to the sites of secondary lymphoid tissue organogenesis during fetal development (Mebius et al., 1997). Production of interleukin-7 (IL-7) and RANKL by resident stromal cells induces LTi cell expression of lymphotoxin-α1β2 that activates resident stromal cells to express adhesion molecules and chemokines that promote the formation and organization of secondary lymphoid tissues (Mebius, 2003). The development of LTi cells (defined as Lin−, c-kit+, CD4+, CD44+, CD127+, CD25+, CD90+, and CCR6+) is dependent on the transcription factors RORγt and Id-2, and IL-7 has been shown to regulate their survival and function (Eberl et al., 2004; Mebius, 2003; Mebius et al., 1997; Meier et al., 2007; Sun et al., 2000; Yokota et al., 1999).
Following birth, LTi cells persist in the secondary and mucosa-associated lymphoid tissues of adult humans and mice (Cupedo et al., 2009; Eberl and Littman; Tsuji et al., 2008). In mice, adult CD4+ LTi cells are generated in the bone marrow and appear to be related to an emerging category of innate lymphoid cells (ILCs) that are phenotypically and functionally heterogeneous (Colonna, 2009; Cua and Tato, 2010; Kim et al., 2008; Sawa et al., 2010; Schmutz et al., 2009; Veiga-Fernandes et al., 2010). Although the development, lineage relationships, and fates of adult LTi cells and other ILC populations remain to be determined, they appear to influence immunity and inflammation in multiple settings. For example, CD4+ LTi cells promote the maturation of tertiary lymphoid tissues following birth and the restoration of secondary lymphoid tissues following viral infection (Scandella et al., 2008; Tsuji et al., 2008). Additionally, an IL-23-responsive CD4− c-kit− CD90+ ILC population was recently found to express IL-17A and IFNγ and promote innate intestinal inflammation in a murine model of colitis (Buonocore et al., 2010). Another ILC-like population characterized by expression of the NK cell cytotoxicity receptor NKp46 was found to express IL-22 in response to IL-23 stimulation or following exposure to the enteric pathogen Citrobacter rodentium, and was implicated in promoting host protective immunity (Cella et al., 2009; Cupedo et al., 2009; Luci et al., 2009; Sanos et al., 2009; Satoh-Takayama et al., 2008).
Early production of IL-22 is essential for innate immunity to enteric bacterial infection with C. rodentium, a mouse-specific pathogen (Zheng et al., 2008). IL-22 induces expression of anti-microbial peptides and promotes the maintenance of the epithelial cell barrier, preventing systemic bacterial dissemination, morbidity and host mortality (Zheng et al., 2008). Analysis of intestinal tissues following C. rodentium infection revealed that CD11c+ cells expressed IL-22 (Zheng et al., 2008). More recently, an NKp46+ ILC population was found to express CD11c and IL-22 in the small intestine following C. rodentium infection (Cella et al., 2009; Satoh-Takayama et al., 2008). However, depletion of a fraction of the NKp46+ ILC population, which co-expresses NK1.1, resulted in only a modest reduction in innate immunity to C. rodentium (Cella et al., 2009). Analysis of conventional NK cells and NKp46+ ILCs revealed that both populations were absent in Rag−/−IL2rg−/− mice, whereas only conventional NK cells were absent in Rag−/−Il2rb−/− mice (Satoh-Takayama et al., 2008). Rag−/−IL2rg−/− mice, but not Rag−/−Il2rb−/− mice, had an increased susceptibility to C. rodentium infection, suggesting that NKp46+ ILCs and not conventional NK cells contribute to innate anti-bacterial immunity (Satoh-Takayama et al., 2008). However, in addition to defective NKp46+ ILC development, Rag−/−Il2rg−/− mice exhibit impaired development and cytokine responses in multiple innate cell populations, including CD4+ LTi cells and other IL-22-producing ILC populations (Kim et al., 2005; Satoh-Takayama et al., 2008; Takatori et al., 2009). Therefore, the potential influence of adult CD4+ LTi cells versus other IL-22-producing ILC populations on innate immunity to bacterial infection remains to be determined.
The data in the present report demonstrate that adult CD4+ LTi cells, a subset of innate lymphoid cells, are the critical population required for early immunity to oral infection with C. rodentium. Following infection with C. rodentium, IL-22+ innate cells outnumbered IL-22+ adaptive immune cells during the first six days of infection, and CD4+ LTi cells were the dominant source of IL-22 following infection. A substantial infection-induced population expansion of CD4+ LTi cells and an increased frequency of IL-22+ CD4+ LTi cells occurred in the colon and mesenteric lymph node (mLN). Infection-induced IL-22 production by CD4+ LTi cells was found to be IL-23-dependent, and blockade of IL-23 in Rag1−/− mice substantially impaired innate immunity to C. rodentium. Furthermore, selective depletion of CD4+ LTi cells in C. rodentium infected Rag1−/− mice resulted in reduced expression of IL-22 and anti-microbial peptides in the colon, failure to control bacterial replication and dissemination, and more rapid host mortality. Finally, utilizing reconstitution of Rag1−/− mice and adoptive transfer approaches, we demonstrate that CD4+ LTi cells are critical for the promotion of IL-22-dependent innate immunity in lymphocyte-replete hosts. Taken together, this report identifies adult CD4+ LTi cells as a critical IL-23-dependent innate source of IL-22 following enteric bacterial infection in mice and reveals a previously unrecognized function for CD4+ LTi cells in contributing to innate immunity.
RESULTS
IL-22+ innate populations outnumber IL-22+ adaptive populations following C. rodentium infection
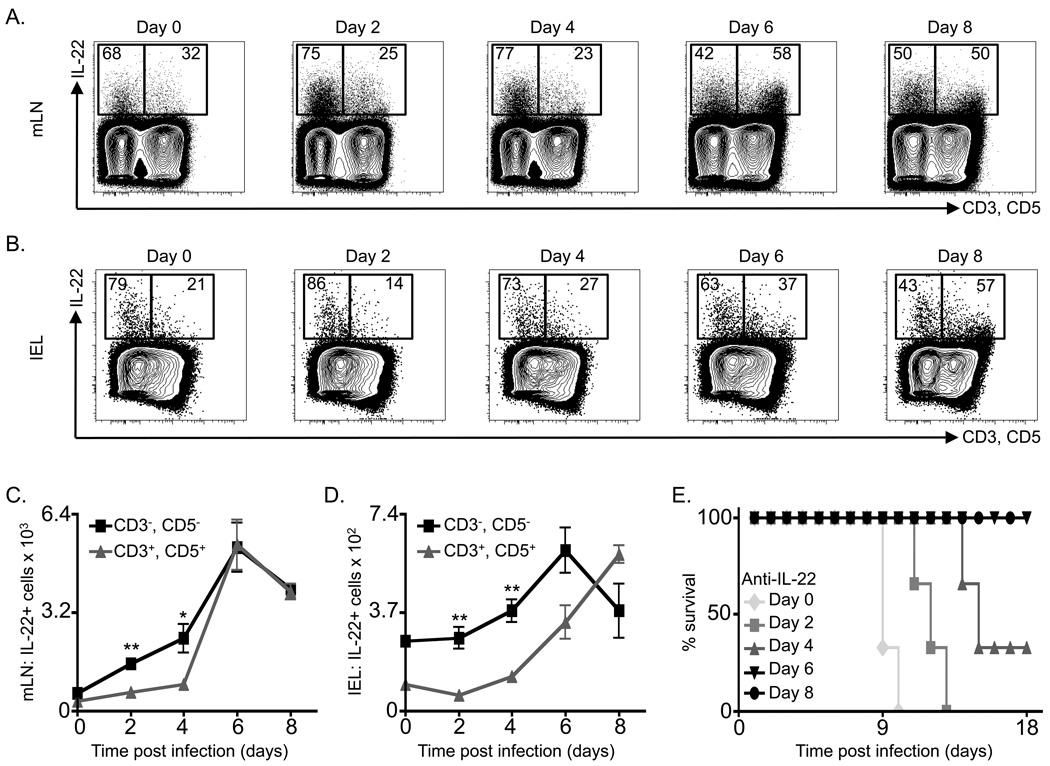
It has been previously reported that IL-22 is necessary for immunity to C. rodentium during the first 8-days of infection (Zheng et al., 2008). However, the cellular sources of IL-22 following C. rodentium infection remain unclear. As IL-22 can be expressed by a number of innate and adaptive immune cells, C57BL/6 mice were infected with C. rodentium and IL-22 production was examined in innate immune cells (CD3−, CD5−) versus adaptive immune cells (CD3+, CD5+). In naïve mice (day 0) the majority of IL-22+ cells were innate, accounting for 68% and 79% of all IL-22+ cells in the mLN and colonic intra-epithelial lymphocyte (IEL) compartment respectively (Figure 1A, B). Furthermore, innate cells were the dominant IL-22+ cell population until day 6 and day 8 post infection in the mLN and IEL compartment respectively (Figure 1A, B). Substantially increased total numbers of IL-22+ innate cells compared to IL-22+ adaptive cells were observed at days 2 and 4 post-infection in both the mLN (Figure 1C) and IEL compartment (Figure 1D). These data suggest that innate cells are the dominant source of IL-22 at early time points following infection. To more thoroughly define the temporal requirements for IL-22 in host defense to C. rodentium, anti-IL-22 mAb was administered to mice at different time points following infection. All mice that received anti-IL-22 mAb starting on day 0 or day 2 post-infection succumbed to infection by day 13 (Figure 1E). However, blockade of IL-22 starting on day 4 post-infection resulted in an intermediate degree of mortality (Figure 1E). Furthermore, mice receiving anti-IL-22 mAb beginning on day 6 or day 8 post-infection did not succumb to C. rodentium (Figure 1E), suggesting that IL-22 is only essential for immunity to C. rodentium during the first six days of infection. Collectively, these data demonstrate that following C. rodentium infection, innate immune cells are the dominant sources of IL-22 production required for early resistance to enteric bacterial infection.
Figure 1. Innate immune cells are the first population to expand with exposure to C. rodentium infection and are the dominant source of IL-22 required for immunity.
C57BL/6 mice were infected with C. rodentium on day 0 and sacrificed on days 2, 4, 6 and 8. Cells were briefly stimulated ex vivo and the frequency of IL-22+, CD3+, CD5+ cells versus IL-22+, CD3−, CD5− cells were examined in the (A) mLN and (B) colon IEL compartment. Absolute numbers of IL-22+ CD3+/CD5+ cells versus IL-22+, CD3−, CD5− cells in the (C) mLN and (D) IEL compartment. (E) Percent survival of IL-22 neutralizing mAb treated and infected C57BL/6 mice. Antibody treatment was initiated on the indicated day and continued every 3 days. All data are representative of 2 independent experiments with a minimum of 3 mice per group or time point. Data shown are the mean ± SEM. * p < 0.05 ** p < 0.01.
Adult CD4+ LTi cells proliferate and are a dominant source of IL-22 following C. rodentium infection
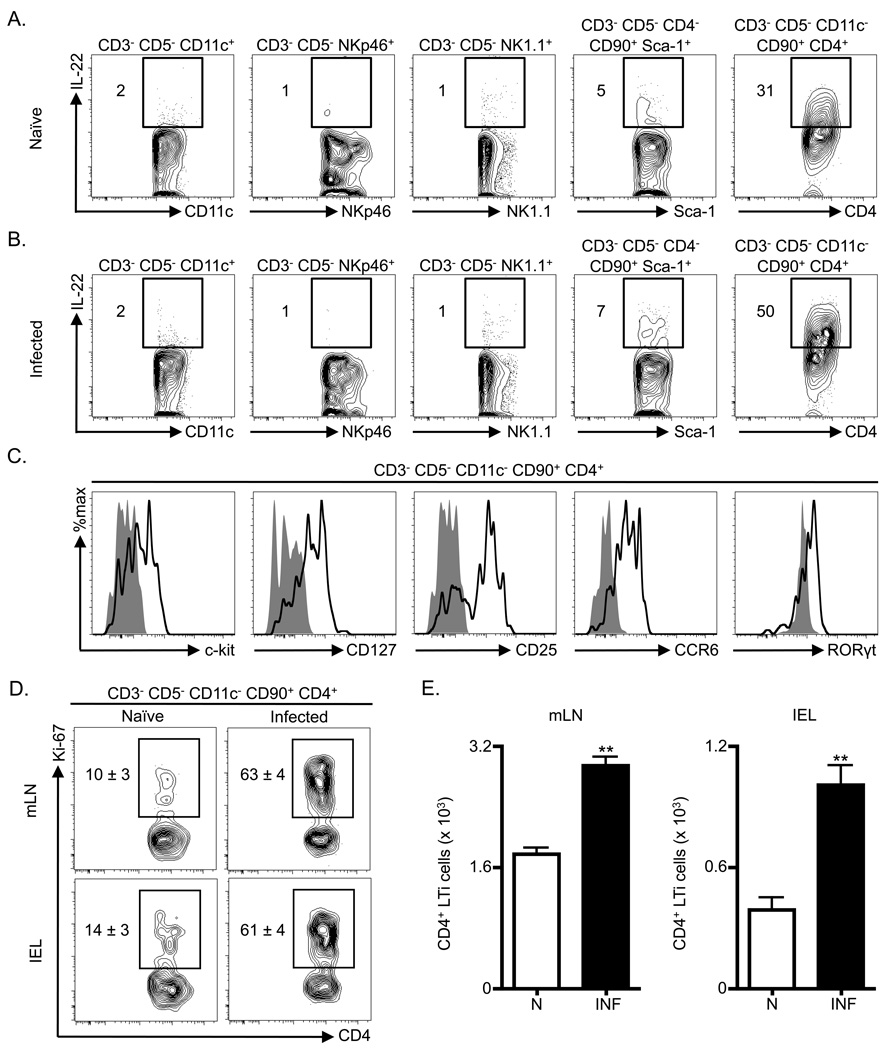
Several innate sources of IL-22 have previously been identified including dendritic cells (DCs), conventional NK cells, NKp46+ ILCs, CD4− ILCs and CD4+ LTi cells (Buonocore et al., 2010; Cella et al., 2009; Satoh-Takayama et al., 2008; Takatori et al., 2009; Zenewicz et al., 2008; Zheng et al., 2008). To investigate the innate sources of IL-22 following C. rodentium infection, the frequency of IL-22+ cells was examined in multiple cell populations in the mLN of naïve or infected mice. In comparison to naïve mice, C. rodentium-infected mice exhibited minimal changes in the frequency of IL-22+ DCs (CD3−, CD5−3 , CD11c+), NKp46-expressing cells (CD3−, CD5−, NKp46+), conventional NK cells (CD3−, CD5−, NK1.1+) or CD4− ILCs (CD3−, CD5−, CD4−, CD90+, Sca-1+) (Figure 2A, B). In contrast, analysis of the CD4+ LTi cell population (CD3−, CD5−, CD11c−, CD90+, CD4+) revealed an infection-induced increase in the frequency of IL-22+ cells that was higher than other cell populations examined (Figure 2A, B). CD4+ LTi cells also exhibited the highest frequency of IL-22+ cells in the IEL compartment of infected mice (Figure S1A). Furthermore, a comparison of IL-22-expression in CD4+ T cells versus CD4+ LTi cells in the mLN at early time points following infection with C. rodentium revealed no increase in the frequency of IL-22+ CD4+ T cells until day 6 post-infection (Figure S1B), whereas increases in the frequency of IL-22+ CD4+ LTi cells were observed at all early time points post-infection (Figure S1C). Collectively these data demonstrate that CD4+ LTi cells are a dominant source of IL-22 production early following C. rodentium infection.
Figure 2. Adult CD4+ LTi cells expand and are a dominant innate source of IL-22 following C. rodentium infection.
C57BL/6 mice were infected with C. rodentium on day 0 and sacrificed on day 4. Frequency of ex vivo stimulated IL-22+ innate cells in the mLN of (A) naïve and (B) infected mice gated as indicated for various surface markers. (C) The gated CD3− CD5− CD11c− CD90+ CD4+ population in the mLN of C. rodentium infected mice was stained with anti- c-kit, CD127, CD25, CCR6 and RORγt antibodies (bold black line) and corresponding isotype and negative control antibodies (solid grey histograms). C57BL/6 mice were infected with C. rodentium on day 0 and sacrificed on day 8. (D) Frequency of Ki-67+ CD4+ LTi cells (CD3− CD5− CD11c− CD90+ CD4+) in the mLN and IEL compartment. (E) Absolute numbers of CD4+ LTi cells in the mLN and IEL compartment of naïve (N) and infected (INF) mice. All data are representative of 2 or more independent experiments with a minimum of 3–4 mice per group. Data shown are the mean ± SEM. ** p < 0.01. See Figure S1 for additional data.
Further analysis of CD4+ LTi cells (CD3−, CD5−, CD11c−, CD90+, CD4+) from infected mice revealed expression of c-kit, CD127 (IL-7Rα), CD25 (IL-2Rα), CCR6, and the intracellular transcription factor RORγt (Figure 2C). To examine whether CD4+ LTi cells were proliferating, cells from naïve or infected mice were stained for the nuclear antigen Ki-67. In comparison to naïve mice, CD4+ LTi cells from the mLN or IEL compartment of infected mice exhibited an increase in the frequency of Ki-67+ cells (Figure 2D), suggesting that C. rodentium infection induced a proliferative response of CD4+ LTi cells. Consistent with these results, significantly increased total numbers of CD4+ LTi cells were observed in the mLN and IEL compartment of infected mice in comparison to naïve mice (Figure 2E, p < 0.01). These data demonstrate that infection with C. rodentium induces a population expansion and increased production of IL-22 in CD4+ LTi cells.
Infection-induced IL-22 production by adult CD4+ LTi cells is dependent on IL-23
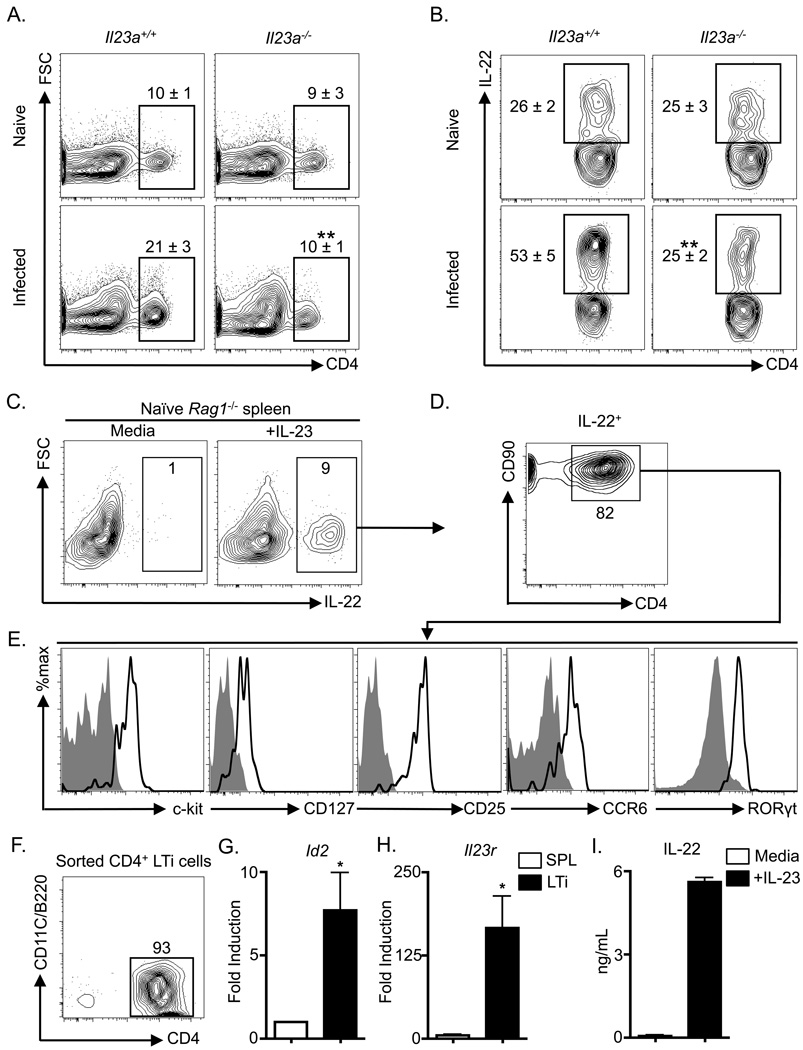
In vitro, IL-23 can induce IL-22 expression in a number of cell types including Th17 cells, LTi cells and other ILC populations (Buonocore et al., 2010; Cella et al., 2009; Liang et al., 2006; Satoh-Takayama et al., 2008; Takatori et al., 2009). To test whether in vivo infection-induced IL-22 production from CD4+ LTi cells was IL-23 dependent, Il23a+/+ and Il23a−/− mice were infected with C. rodentium and the CD4+ LTi cell response was examined by flow cytometry. Naïve Il23a+/+ versus Il23a−/− mice displayed similar basal frequencies of CD4+ LTi cells (Figure 3A, top) and IL-22+ CD4+ LTi cells in the mLN (Figure 3B, top). However, infection-induced increases in the frequency of CD4+ LTi cells in the mLN of infected Il23a+/+ mice were not observed in infected Il23a−/− mice (Figure 3A, bottom). Infection with C. rodentium resulted in an increase in the frequencies of IL-22+ CD4+ LTi cells in Il23a+/+ mice (Figure 3B, left) that was not observed in infected Il23a−/− mice (Figure 3B, right), demonstrating that infection-induced CD4+ LTi cell-derived IL-22 responses are predominantly IL-23-dependent. Consistent with IL-23-dependent IL-22 production in innate cells, further examination of the innate lymphoid compartment revealed that stimulation of splenocytes from naïve Rag1−/− mice with rIL-23 resulted in an increased frequency of IL-22+ cells compared to cells cultured in media alone (Figure 3C). Phenotypic analysis of IL-22+ cells from rIL-23-stimulated cultures revealed that the majority co-expressed CD90 and CD4 (Figure 3D). The IL-22+ innate cells were confirmed to be CD4+ LTi cells as this population co-expressed c-kit, CD127 (IL-7Rα), CD25 (IL-2Rα), CCR6, and the intra-cellular transcription factor RORγt (Figure 3E). The non-CD4+ IL-22+ cells in spleen cultures most closely resembled CD4− LTi cells as they also expressed c-kit and CD90, but lacked expression of NK1.1, NKp46 and CD11c (Figure S2A). CD4+ LTi cells were also the dominant source of IL-23-induced IL-22 in innate cells isolated from the IEL compartment (Figure S2B). Examination of sorted CD4+ LTi cells (Figure 3F) revealed a mononuclear morphology (Figure S3C) and significant elevated expression of mRNA encoding Id2 (Figure 3G, p < 0.05) and Il23r (Figure 3H, p < 0.05). Sorted CD4+ LTi cells secreted increased amounts of IL-22 protein following stimulation with rIL-23 (Figure 3I), demonstrating that IL-23 could directly induce IL-22 expression in CD4+ LTi cells. Collectively these data indicate that CD4+ LTi cells are a dominant IL-23-responsive, IL-22 producing innate cell population, and that in vivo infection-induced CD4+ LTi cell responses are regulated by IL-23.
Figure 3. Citrobacter rodentium-induced adult CD4+ LTi cell responses are dependent on IL-23.
Il23a+/+ and Il23a−/− mice were infected with C. rodentium on day 0 and sacrificed on day 8. (A) Frequency of CD4+ LTi cells in the mLN of naïve and infected mice. Populations are gated on live CD3− CD5− CD90+ CD4+ cells. (B) Frequency of ex vivo stimulated IL-22+ CD4+ LTi cells in the mLN. Populations are gated on live CD3− CD5− CD90+ CD4+ cells. All data are representative of 2 or more independent experiments with a minimum of 3–4 mice per group. Naïve Rag1−/− mouse splenocytes were cultured overnight with or without rIL-23. (C) Frequency of IL-22+ cells with gating on live cells. (D) Frequency of CD90+ CD4+ cells in the IL-22+ gated population of (C). (E) The IL-22+ CD90+ CD4+ population of (D) was stained with c-kit, CD127, CD25, CCR6 and RORγt antibodies (bold black lines) and corresponding isotype and negative control antibodies (solid grey histograms). (F) CD4+ LTi cells were purified from naïve Rag1−/− splenocytes and stained with CD4, CD11c and B220 antibodies. Fold induction of (G) Id2 and (H) Il23r mRNA in purified CD4+ LTi cells relative to total unfractionated Rag1−/− splenocytes (SPL). (I) IL-22 protein in culture supernatants from purified CD4+ LTi cells stimulated in the presence or absence of rIL-23. All in vitro data are representative of 2 or more independent experiments with a minimum of 2–3 replicate wells. Data shown are the mean ± SEM. * p < 0.05 ** p < 0.01. See Figure S2 for additional data.
Innate immunity to Citrobacter rodentium is dependent on IL-23
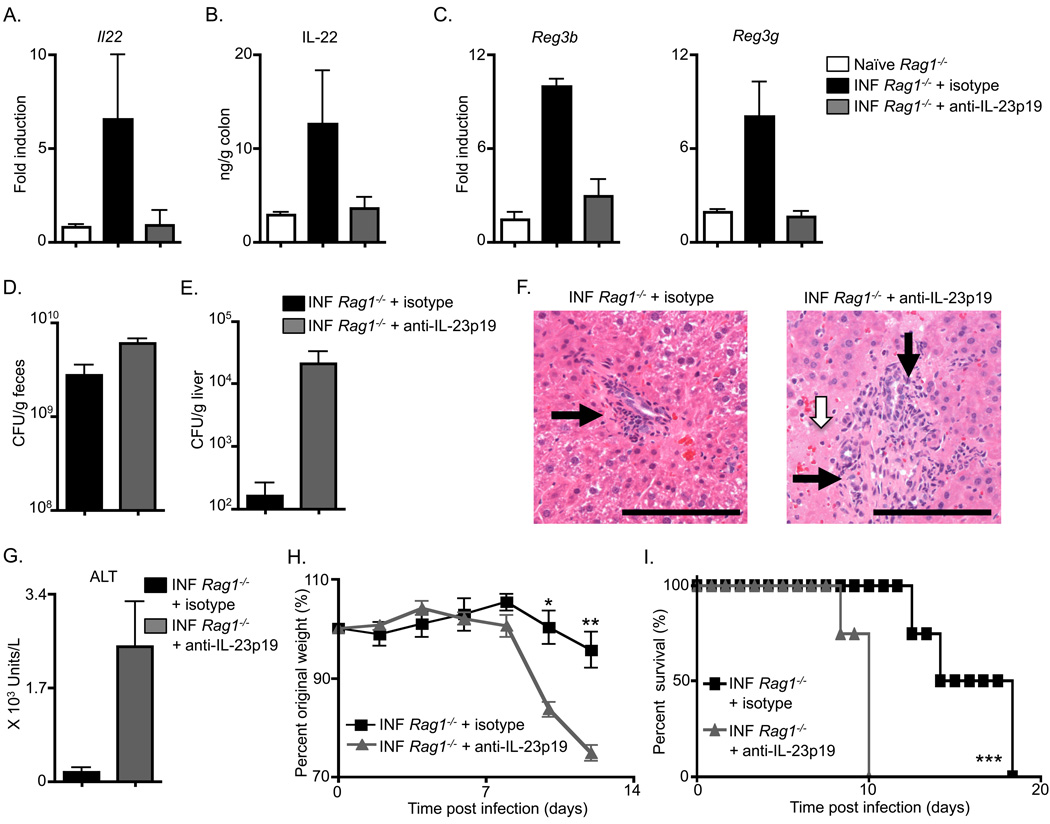
Following C. rodentium infection, Il23a−/− mice exhibit elevated bacterial loads and succumb to infection (Mangan et al., 2006), and it has been proposed that this defective immunity may be the result of impaired Th17 cell effector responses observed in the absence of IL-23 (McGeachy et al., 2009). However, whether IL-23 is required for innate immunity to C. rodentium has not been examined. To test the role of IL-23 in innate immunity to C. rodentium, Rag1−/− mice were infected and administered either isotype control or anti-IL-23p19 mAb. Examination of the colonic tissue following infection revealed that isotype mAb-treated Rag1−/− mice exhibited an increase in Il22 mRNA (Figure 4A) and IL-22 protein (Figure 4B) over naïve Rag1−/− mice. Administration of multiple doses of anti-IL-23p19 mAb to infected Rag1−/− mice reduced both intestinal Il22 transcript (Figure 4A) and IL-22 protein (Figure 4B) in comparison to isotype treated and infected Rag1−/− mice. Consistent with a decrease in IL-22 expression, infected Rag1−/− mice administered anti-IL-23p19 mAb also exhibited reduced mRNA encoding the IL-22-dependent anti-microbial peptides RegIIIβ and RegIIIγ in the colon (Figure 4C). Furthermore, anti-IL-23p19 mAb treated Rag1−/− mice displayed an increase in fecal colony forming units (CFU) at day 10 post-infection compared to isotype control mAb (Figure 4D). Reduced expression of IL-22 and lower amounts of anti-microbial peptides in the colon have previously been reported to result in systemic dissemination of C. rodentium to peripheral tissues resulting in substantial tissue inflammation, morbidity and mortality (Zheng et al., 2008). Consistent with this, in comparison to isotype mAb treatment, anti-IL-23p19 mAb treatment of Rag1−/− mice resulted in increased CFU of C. rodentium in the liver (Figure 4E), increased inflammatory cell infiltrates (black arrows, Figure 4F) and the appearance of necrotic lesions in the liver (white arrow, Figure 4F). Anti-IL-23p19 mAb treatment also resulted in elevated amounts of the liver enzyme alanine transaminase (ALT) in comparison to isotype mAb treatment (Figure 4G), indicative of increased liver damage. Finally, in comparison to Rag1−/− mice receiving an isotype mAb treatment, those receiving an anti-IL-23p19 mAb treatment exhibited an increased rate of weight loss (Figure 4H) and succumbed to infection at earlier time points (Figure 4I). These data demonstrate that CD4+ LTi cell responses and innate immunity to C. rodentium are IL-23-dependent.
Figure 4. Innate immunity to Citrobacter rodentium is dependent on IL-23.
C57BL/6 Rag1−/− were administered an isotype control mAb or an anti-IL-23p19 mAb starting on day 0, infected with C. rodentium on day 0, and sacrificed on day 10. (A) Fold induction of Il22 transcript in colonic RNA from antibody treated and infected (INF) mice compared to naïve mice. (B) IL-22 protein in the supernatant of colon homogenates and (C) Fold induction of Reg3b and Reg3g transcript in colonic RNA from antibody treated and infected mice compared to naïve mice. C. rodentium CFU in the (D) fecal pellets and (E) liver of antibody treated and infected mice. (F) H&E stained histological sections of the liver of antibody treated and infected mice; inflammatory cell infiltrates (black arrows) and necrotic lesions (white arrow). Scale bar, 100 µm. (G) Serum ALT levels, (H) Percent of original whole body weight and (I) percent survival of antibody treated and infected mice. All data are representative of 3 or more independent experiments with a minimum of 3–4 mice per group. Data shown are the mean ± SEM. * p < 0.05 ** p < 0.01 *** p < 0.001.
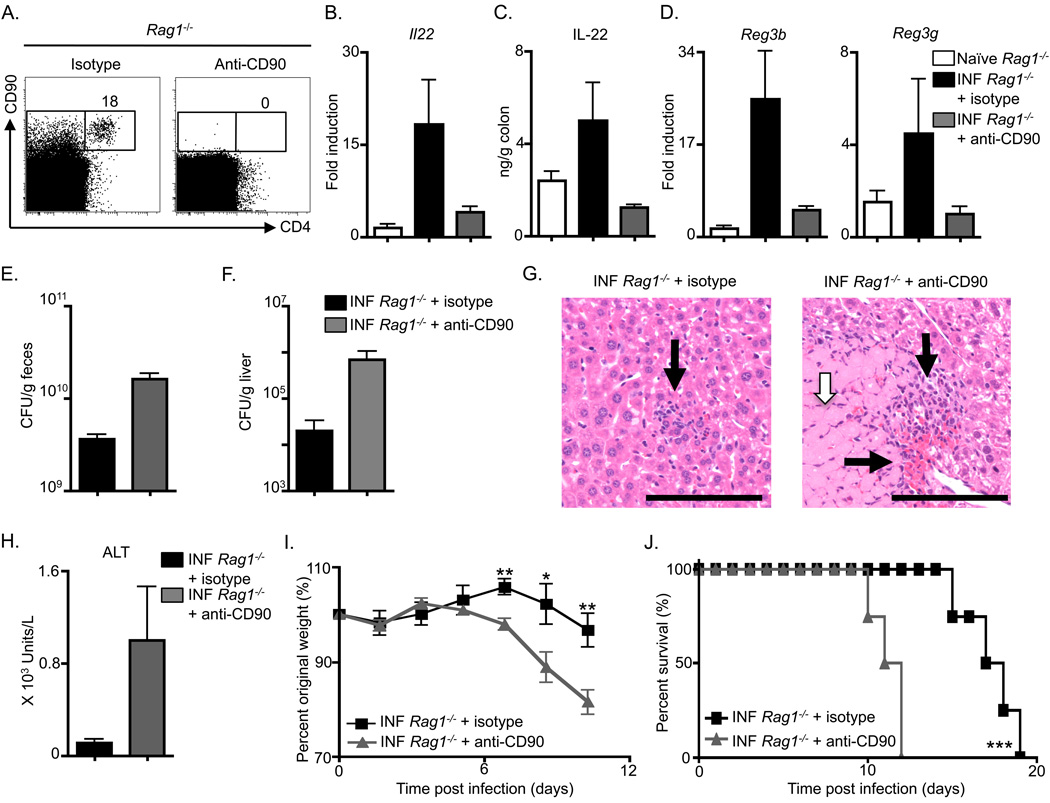
Depletion of adult CD4+ LTi cells substantially impairs innate immunity to C. rodentium
To test the contribution of adult CD4+ LTi cells to innate immunity in the gut, anti-CD90 mAb was administered to infected Rag1−/− mice. Anti-CD90 mAb treatment resulted in efficient depletion of CD4+ LTi cells in comparison to isotype mAb treatment (Figure 5A). Despite complete depletion of CD4+ LTi cells, analysis of cells expressing NK cell receptors revealed that anti-CD90 mAb administration did not result in substantial changes in the frequency of NK1.1+ cells in the spleen (Figure S3A, B) or NKp46+ cells in the IEL compartment (Figure S3C). Rag1−/− mice receiving mAb treatments were analyzed for innate immunity to C. rodentium. Infection-induced increases in both Il22 mRNA (Figure 5B) and IL-22 protein (Figure 5C) were reduced in colon tissue of Rag1−/− mice that received anti-CD90 mAb in comparison to isotype mAb treatment. The reduction of IL-22 expression in anti-CD90 mAb treated Rag1−/− mice also correlated with a reduction in colonic mRNA for the genes encoding RegIIIβ and RegIIIγ (Figure 5D). Furthermore, examination of fecal burdens revealed that anti-CD90 mAb treated Rag1−/− mice exhibited higher C. rodentium CFU compared to control treated mice (Figure 5E), increased dissemination of C. rodentium to the liver (Figure 5F), and augmented liver pathology characterized by increased inflammatory infiltrates (Figure 5G, black arrows) and liver necrosis (Figure 5G, white arrow) in comparison to isotype mAb treated mice. Finally, similar to anti-IL-23p19 mAb treated Rag1−/− mice, those treated with anti-CD90 mAb exhibited increased serum concentrations of ALT (Figure 5H), an elevated rate of weight loss (Figure 5I), and decreased survival in comparison to isotype mAb treated Rag1−/− mice (Figure 5J). Taken together, these results demonstrate that depletion of CD90+ cells in Rag1−/− mice impairs IL-22 production and substantially reduces innate resistance to C. rodentium.
Figure 5. Anti-CD90 mAb treatment impairs innate immunity to Citrobacter rodentium.
C57BL/6 Rag1−/− were administered an isotype control mAb or an anti-CD90 mAb starting on day 0, infected with C. rodentium on day 0, and sacrificed at day 10. (A) Frequency of CD4+ CD90+ cells in Lin− gated splenocytes from antibody treated Rag1−/− mice. (B) Fold induction of Il22 transcript in colonic RNA from antibody treated and infected (INF) mice compared to naïve mice. (C) IL-22 protein in the supernatant of colon homogenates and (D) fold induction of Reg3b and Reg3g transcript in colonic RNA from antibody treated and infected mice compared to naïve mice. C. rodentium CFU in the (E) fecal pellets and (F) liver of antibody treated and infected mice. (G) H&E stained histological sections of the liver of antibody treated and infected mice; inflammatory cell infiltrates (black arrows) and necrotic lesions (white arrow). Scale bar, 100 µm. (H) Serum ALT levels, (I) percent of original whole body weight and (J) percent survival of antibody treated and infected mice. All data are representative of 3 or more independent experiments with a minimum of 3–4 mice per group. Data shown are the mean ± SEM. * p < 0.05 ** p < 0.01 *** p < 0.001. See Figure S3 for additional data.
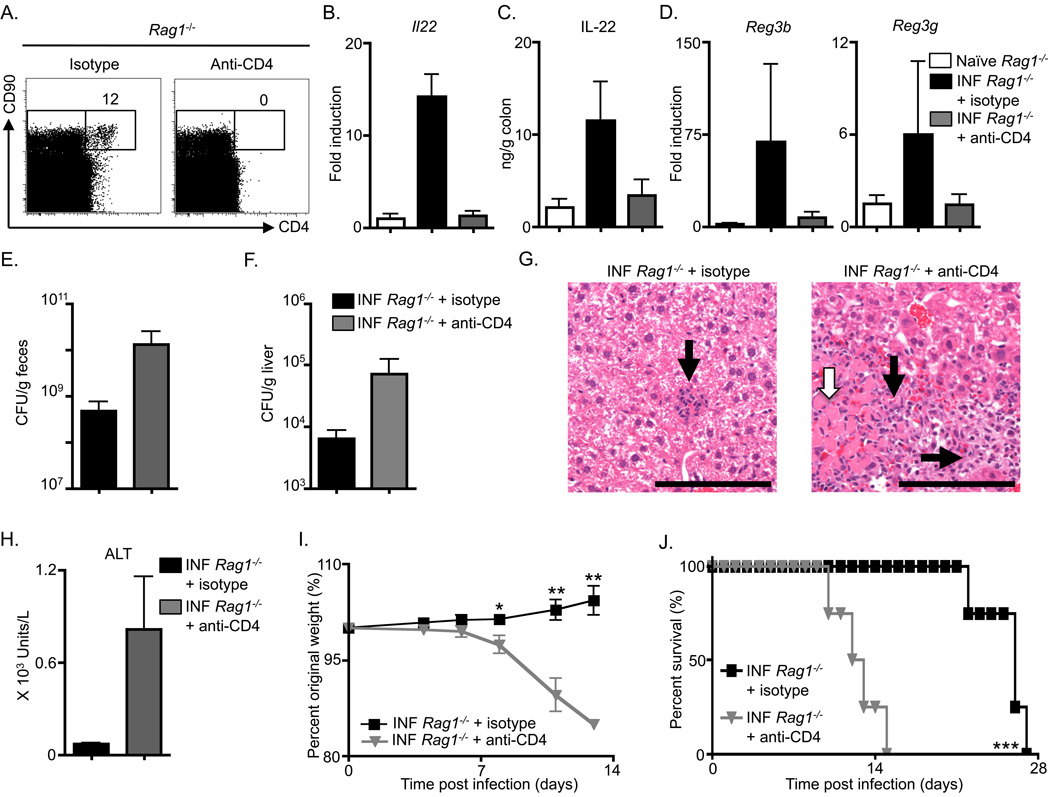
Anti-CD90 mAb administration depletes both CD4+ and CD4− innate cell populations (Figure 5A), including an IL-22-expressing c-kit− CD4− ILC population that was recently reported to contribute to intestinal inflammation (Buonocore et al., 2010). Therefore, to examine whether the anti-CD90 mAb-mediated loss in innate immunity to C. rodentium was due to depletion of the CD4+ LTi cells or CD4− ILCs, infected Rag1−/− mice were treated with either control or anti-CD4 mAb. Examination of spleens from mAb treated mice revealed that CD4+ LTi cells, but not CD4− CD90+ cells, were successfully depleted following administration of anti-CD4 mAb in comparison to isotype mAb treated mice (Figure 6A). Similar to anti-CD90 mAb administration, anti-CD4 mAb administration did not result in substantial changes in the frequency of NK1.1+ cells in the spleen (Figure S3B) or NKp46+ cells in the IEL compartment (Figure S3C). Treatment of infected Rag1−/− mice with anti-CD4 mAb also abrogated C. rodentium infection-induced increases in both colonic Il22 mRNA (Figure 6B) and IL-22 protein (Figure 6C) in comparison to isotype mAb treatment. In addition, anti-CD4 mAb treated Rag1−/− mice demonstrated a reduction in colonic mRNA for genes encoding RegIIIβ and RegIIIγ following infection (Figure 6D). Analysis of fecal burdens demonstrated that anti-CD4 mAb treated Rag1−/− mice exhibited higher C. rodentium CFU (Figure 6E). Bacterial dissemination of C. rodentium was also evident in CD4-depleted Rag1−/− mice as higher bacterial burdens were found in the liver (Figure 6F), in addition to an observed increase in inflammatory infiltrates (Figure 6G, black arrows) and necrosis (Figure 6G, white arrow). Finally, Rag1−− mice treated with anti-CD4 mAb exhibited increased serum concentrations of ALT (Figure 6H), exaggerated weight loss (Figure 6I), and increased rate of mortality in comparison to isotype mAb treated Rag1−/− mice (Figure 6J). A similar defect in innate immunity was previously observed in Rag−/−Il2rg−/− mice and attributed to defects in NKp46+ ILC populations (Satoh-Takayama et al., 2008). However, given that this population is reported to lack CD4 expression (Satoh-Takayama et al., 2008) and we do not observe a decrease in the frequency of NKp46+ populations following anti-CD4 mAb administration (Figure S3C), our collective observations from the effect of anti-CD90 or anti-CD4 mAb administration suggest that defective innate immunity to C. rodentium observed in Rag−/−IL2rg−/− mice may be attributed to defects in adult CD4+ LTi cell responses. Notwithstanding this, these data collectively indicate that CD4+ LTi cells are a critical innate source of IL-22 following infection and directly contribute to innate immunity to enteric bacterial infection.
Figure 6. Adult CD4+ LTi cells mediate innate immunity to Citrobacter rodentium.
C57BL/6 Rag1−/− were administered an isotype control mAb or anti-CD4 mAb starting on day 0, infected with C. rodentium on day 0, and sacrificed at day 10. (A) Frequency of CD4+ CD90+ cells in Lin− gated splenocytes from antibody treated Rag1−/− mice. (B) Fold induction of Il22 transcript in colonic RNA from antibody treated and infected (INF) mice compared to naïve mice. (C) IL-22 protein in the supernatant of colon homogenates and (D) fold induction of Reg3b and Reg3g transcript in colonic RNA from antibody treated and infected mice compared to naïve mice. C. rodentium CFU in the (E) fecal pellets and (F) liver of antibody treated and infected mice. (G) H&E stained histological sections of the liver of antibody treated and infected mice; inflammatory cell infiltrates (black arrows) and necrotic lesions (white arrow). Scale bar, 100 µm. (H) Serum ALT levels, (I) Percent of original whole body weight and (J) percent survival of antibody treated and infected mice. All data are representative of 3 or more independent experiments with a minimum of 3–4 mice per group. Data shown are the mean ± SEM. * p < 0.05 ** p < 0.01 *** p < 0.001.
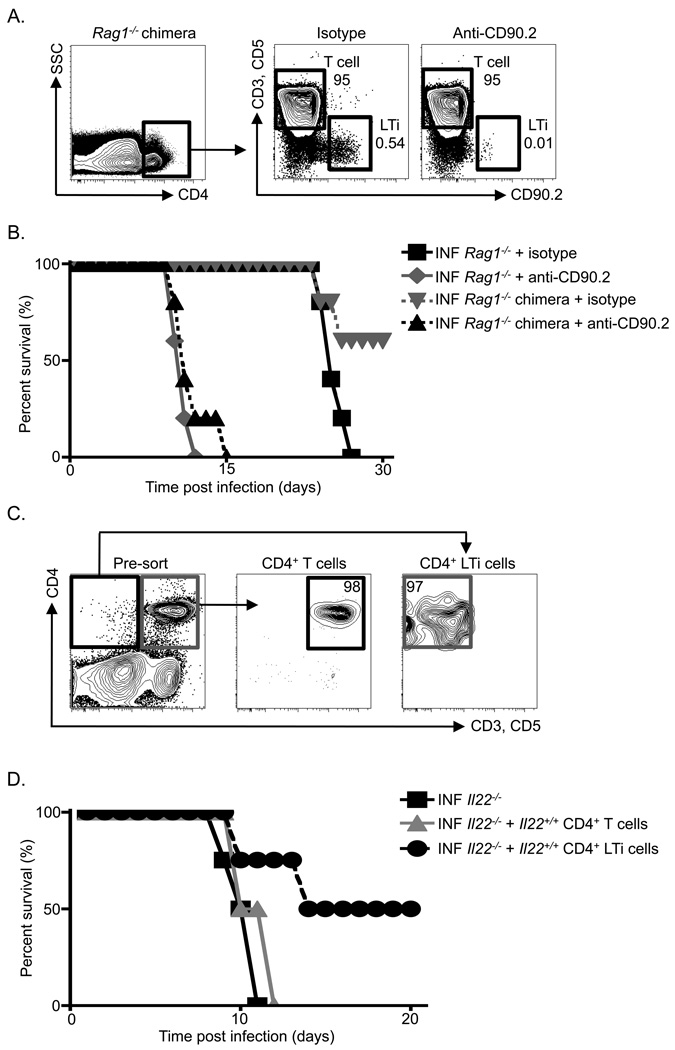
CD4+ LTi cells promote essential innate immunity in lymphocyte-replete hosts
To test whether CD4+ LTi cells can promote innate immunity in a lymphocyte-replete host, two models systems were utilized. First, Rag1−/− mice expressing the CD90.2 allele were reconstituted with purified T cells (CD3+) and B cells (CD19+) from a C57BL/6 mouse expressing the CD90.1 allele (Figure 7A, top). Gating on splenic CD4+ cells in an isotype mAb-treated CD90-disparate chimera revealed a population of donor CD90.2−, CD3+, CD5+, CD4+ T cells and a population of recipient CD90.2+ CD3−, CD5−, CD4+ LTi cells (Figure 7A). Administration of anti-CD90.2 mAb to the chimeras revealed a selective depletion of recipient CD4+ LTi cells but not donor CD4+ T cells (Figure 7A), thus providing an in vivo model to interrogate LTi cell function in lymphocyte-replete hosts. CD90-disparate chimeras and unreconstituted Rag1−/− mice were infected with C. rodentium and treated with an isotype mAb or anti-CD90.2 mAb. As observed previously, Rag1−/− mice receiving an isotype mAb survived for approximately 26 days post-infection, while Rag1−/− mice receiving an anti-CD90.2 mAb succumbed to infection around day 12 (Figure 7B). In contrast, while 66% of isotype mAb treated Rag1−/− chimeras did not succumb to infection and survived longer than isotype mAb treated Rag1−/− mice, 100% of LTi cell-depleted anti-CD90.2 mAb treated Rag1−/− chimeras succumbed to infection at early time points despite the presence of donor lymphocytes (Figure 7A, B). These data suggest that adaptive immunity is required for protection to C. rodentium at late time points, however, LTi cells are required for innate immunity at early time points in a lymphocyte-replete host.
Figure 7. Adult CD4+ LTi cells are necessary and sufficient to promote innate immunity to C. rodentium infected lymphocyte-replete hosts.
(A) Diagram of the generation of CD90-disparate chimeras with representative flow cytometry plots demonstrating depletion of recipient CD4+ LTi cells but not donor CD4+ T cells in anti-CD90.2 mAb treated chimeras in comparison to isotype mAb treated chimeras. (B) Percent survival of isotype and anti-CD90.2 mAb treated and infected (INF) mice. Displayed data are from two independent experiments with 3 mice per group per experiment. (C) Diagram of innate and adaptive immune cell adoptive transfer approaches utilizing Il22+/+ donors and Il22−/− recipients with representative flow cytometry plots demonstrating purity of transferred populations. (D) Percent survival of antibody treated and infected (INF) mice. Displayed data are from two independent experiments with 2 mice per group per experiment.
We next investigated whether CD4+ LTi cells are sufficient to provide innate immunity to C. rodentium in a lymphocyte-replete host. Il22+/+ and Il22−/− mice were infected with C. rodentium and on days 2, 3 and 4 post-infection 1 × 106 CD4+ T cells or 1.5 × 103 CD4+ LTi cells from Il22+/+ hosts were purified and adoptively transferred into infected Il22−/− hosts (Figure 7C). Analysis of host mortality revealed that consistent with a previously published study (Zheng et al., 2008), 100% of C. rodentium infected Il22−/− mice succumbed to infection at early time points (Figure 7D). All infected Il22−/− mice receiving Il22+/+ CD4+ T cells also succumbed to infection at early time points. However, 50% of Il22−/− mice receiving Il22+/+ CD4+ LTi cells survived infection beyond day 20 (Figure 7D). Collectively, these studies indicate that CD4+ LTi cells play a critical role in innate immunity in the intestine of lymphocyte-replete hosts.
DISCUSSION
In this study we demonstrate that following infection with the enteric rodent pathogen C. rodentium, adult CD4+ LTi cells proliferate and upregulate IL-22 production within the first six days post-infection. CD4+ LTi cells were found to be the dominant source of IL-22 and infection-induced CD4+ LTi cell responses were predominantly IL-23-dependent. Furthermore, this report demonstrates that IL-23 is critical in mediating innate immunity to C. rodentium in mice. Consistent with being a dominant source of infection-induced IL-22, depletion of CD4+ LTi cells in C. rodentium infected Rag1−/− mice resulted in an abrogation of infection-induced IL-22 and anti-microbial peptide expression, enhanced bacterial replication and dissemination, and exacerbated host morbidity and mortality. Furthermore, in lymphocyte-replete hosts, CD4+ LTi cells were found to be critical in promoting innate immunity in the intestine.
Previous studies examining the function of adult CD4+ LTi cells revealed that they have the capacity to promote lymphoid-tissue organogenesis and the maintenance of lymphoid tissue architecture following CD8+ T cell-mediated lysis of virally-infected resident stromal organizer cells (Scandella et al., 2008; Schmutz et al.). These studies provoke the hypothesis that the function of CD4+ LTi cells in adults might be to promote lymphoid tissue maintenance and tertiary lymphoid-tissue formation. Other studies have demonstrated that LTi cells in adult mice and humans constitutively express RORγt and have the capacity to express IL-22 in vitro (Eberl and Littman, 2004; Takatori et al., 2009). As IL-22 has been found to play important roles in promoting inflammation, tissue protection and/or protective immunity (Sonnenberg et al., 2010a; Sonnenberg et al., 2010b; Zenewicz et al., 2008; Zheng et al., 2007; Zheng et al., 2008), this provokes the hypothesis that the role of CD4+ LTi cells in adults may extend beyond lymphoid-tissue generation and maintenance. Consistent with this, LTi cells are now being classified in an emerging category of ILCs, in which all members share a developmental requirement for RORγt, and several ILC populations have been implicated in promoting innate immunity and intestinal inflammation via production of host protective and/or inflammatory cytokines (Buonocore et al., 2010; Cella et al., 2009; Colonna, 2009; Cua and Tato, 2010; Eberl et al., 2004; Satoh-Takayama et al., 2008; Sawa et al., 2010; Spits and Di Santo, 2010). For example, a population of c-kit− CD4− ILCs has recently been suggested to promote intestinal inflammation through production of IL-17A and IFNγ (Buonocore et al., 2010). Further, a population of NKp46+ ILCs was implicated in promoting innate immunity to C. rodentium (Cella et al., 2009; Satoh-Takayama et al., 2008), however loss-of-function and gain- of-function experiments were difficult to interpret due to the lack of specific reagents to deplete this cell population. In contrast to these studies, we now demonstrate that CD4+ LTi cells proliferate and are the dominant source of IL-22 during C. rodentium infection. Through loss-of-function and gain-of-function approaches we provide the first evidence that CD4+ LTi cells are critical for promoting infection-induced increases in anti-microbial peptide expression, controlling bacterial replication and dissemination, and protecting from host morbidity and mortality. Collectively these data identify a previously unrecognized role for CD4+ LTi cells in innate immunity to enteric bacterial infection.
The regulatory signals that influence LTi cell and ILC function are poorly defined. Recently it has been shown that IL-23 can promote innate cell-driven colitis, and that IL-23 can promote ILC production of IL-22, IL-17A and IFNγ in vitro (Buonocore et al., 2010; Cella et al., 2009; Satoh-Takayama et al., 2008; Takatori et al., 2009; Uhlig et al., 2006). IL-23 has previously been found to be necessary for immunity to C. rodentium in wild-type mice (Mangan et al., 2006; Zheng et al., 2008), however the impaired resistance to infection was thought to be due to defective generation and/or maintenance of CD4+ Th17 cells. Here, we demonstrate that IL-23 promotes infection-induced expression of IL-22 in CD4+ LTi cells and is essential for innate immunity to enteric bacterial infection in vivo.
These data provide evidence that CD4+ LTi cells are critical for innate immunity in the lymphopenic environment of a Rag1−/− mouse, and these findings may have important clinical implications. For example, several clinical conditions in patients are characterized by the development of severe lymphopenia, including AIDS, cancer and tissue transplantation, and a leading cause of mortality in these patients is secondary bacterial infections at mucosal sites (Bonnet et al., 2007; Douek et al., 2003; Lewden et al., 2008; Segel et al., 2003; Storek et al., 1997; Wallace et al., 1993; Zitvogel et al., 2008). The ability to specifically target and promote LTi cell-dependent innate immunity with novel immunotherapies could be beneficial in managing these conditions. Thus, these studies and future investigations on the development and regulation of both CD4+ LTi cells and innate immunity to enteric bacterial infections in lymphocyte-deficient mice could be of substantial value in the design of new intervention strategies for these humans conditions.
Notwithstanding this, it is important to consider the potential functions of CD4+ LTi cells in promoting innate immunity in lymphocyte-replete hosts. Employing CD90-disparate chimeras and adoptive transfer approaches we provide the first report that CD4+ LTi cells are necessary and sufficient to partially restore innate immunity and prolong survival following enteric bacterial infections in lymphocyte-replete hosts. This was surprising given that CD4+ T cells outnumber CD4+ LTi cells in vivo. However, it is consistent with data that CD4+ LTi cells are the dominant source of IL-22 early during infection, while infection-induced T cell-derived IL-22 is not detectable until time points when IL-22 is no longer required for immunity to infection. Therefore, CD4+ LTi cells appear to be sentinel innate cells at mucosal sites, which represent an essential early source of IL-22. CD4+ T cells and B cells are also required for immunity to Citrobacter, yet appear to be critical at later time points as mice deficient in these cells survive for several weeks post infection (Simmons et al., 2003). Collectively, these data provoke a model in which CD4+ LTi cells expand and produce IL-22 to control early enteric bacterial infection until a sufficient adaptive immune response can be initiated to effectively clear the pathogen.
All ILC populations require RORγt for their development (Buonocore et al., 2010; Eberl et al., 2004; Sanos et al., 2009), and RORγt has been suggested to regulate expression of IL-23R in CD4+ T cells (Ivanov et al., 2006; Zhou et al., 2007). The development of ILC populations may render them constitutively responsive to IL-23-mediated signals, suggesting that IL-23 may represent a conserved pathway that stimulates innate cell cytokine responses to promote either intestinal inflammation (Buonocore et al., 2010) or innate host defense following enteric bacterial infection. Therefore, in addition to influencing adaptive immune responses through the induction and maintenance of secondary lymphoid tissues, CD4+ LTi cells also influence innate immune responses through the production of host-protective cytokines, increasing IL-22-dependent expression of anti-microbial peptides and promoting innate immunity. Cellular immunity evolved prior to organized secondary lymphoid tissues (Burnet, 1968), and it has been proposed that LTi cells may represent a primitive form of cellular immunity (Kim et al., 2009; Lane et al., 2008). Given that these data demonstrate that CD4+ LTi cells promote innate immunity, it is likely that host-protective functions of CD4+ LTi cells evolutionarily preceded the acquisition of lymphoid tissue-inducing functions and that CD4+ LTi cells represent an ancient precursor to more elaborate forms of cellular anti-bacterial immunity. Collectively these data provide insights into the evolution of the IL-23-Th17 cell axis in the context of inflammation and immunity at mucosal sites. Moreover, the identification of a role for CD4+ LTi cells in lymphopenic and lymphocyte-replete hosts highlights the potential to target this cell population in promoting innate immunity and efficacy of vaccination while limiting debilitating chronic inflammation at mucosal sites.
EXPERIMENTAL PROCEDURES
Mice and use of monoclonal antibodies in vivo
C57BL/6 mice, C57BL/6 Rag1−/−, and C57BL/6 CD90.1 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Il23a−/− mice on a C57BL/6 background were generated by Lexicon Pharmaceuticals, Inc. (The Woodlands, TX) and were provided by M. Elloso (Centocor, Radnor, PA). 129 Il22−/− mice were generated at Lexicon Genetics in collaboration with Pfizer and subsequently backcrossed to Balb/cBy at the Jackson Laboratory with colony mates used for all groups. All mice were maintained in specific pathogen-free facilities at the University of Pennsylvania. All protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC), and all experiments were performed according to the guidelines of the University of Pennsylvania IACUC. Generation of the anti-IL-23p19 mAb (CNTO 6163) was previously described (Fitch et al., 2009). Anti-CD4 mAb (GK1.5) was purified from ascites and anti-CD90.2 mAb (30H12) was purchased from BioXCell (West Lebanon, NH). Anti-IL-22 mAb (IL22-01) was provided by Pfizer. All mAb treatments were administered i.p. every 3 days at a dose of 250 µg/mouse starting on day 0 of infection or the designated day post-infection.
Citrobacter rodentium infection and assessment of CFU
Citrobacter rodentium (formerly Citrobacter freundii, biotype 4280) strain DBS100 (provided by Bruce Vallance, University of British Columbia, Vancouver, British Columbia, Canada) was prepared by selecting a single colony and culturing in LB broth overnight. Mice were inoculated with approximately 1 × 1010 CFU in 200 µL via oral gavage. Analysis of CFU from overnight cultures, mechanically homogenized fecal pellets, or mechanically homogenized livers were determined via serial dilutions on MacConkey’s agar.
Isolation of cells and flow cytometric analysis
Spleens and mLNs were harvested and single-cell suspensions were prepared at necropsy. For IEL isolation, colons were isolated, attached fat removed, and tissues cut open longitudinally. Luminal contents were removed by shaking in cold PBS and tissue was cut into 1–2 cm pieces and washed in RPMI containing 5% FCS. Epithelial cells were removed by incubating tissue in stripping buffer (1 mM EDTA, 1 mM DTT, and 5% FCS) for 10 minutes at 37°C. The remaining tissue was then incubated for an additional 20 minutes in stripping buffer to collect leukocytes from the IEL compartment.
For flow cytometric analysis, cells were stained with antibodies to the following markers TCRβ, CD5, CD3 (conjugated to PE-Cy7, eBioscience), CD19, Sca-1 (conjugated to PerCP-Cy5.5, eBioscience), CD4 (conjugated to PE-Texas Red, Invitrogen), CD90.2 (conjugated to Alexa Fluor-700, Biolegend), CD11c, B220 (conjugated to APC-eFluor780, eBioscience) c-kit, CD127, CD25, NKp46 (conjugated to FITC, eBioscience), NK1.1 (conjugated to PE, eBioscience) and CCR6 (conjugated to PE, R&D Systems). For intracellular staining of transcription factors, cells were fixed and permeabilized utilizing a commercially available kit (eBioscience) and stained with an antibody to RORγt (conjugated to PE, eBioscience). For cytokine production, cells were stimulated directly ex vivo by incubation for 4 h with 50 ng/mL PMA, 750 ng/mL ionomycin, 10 µg/mL Brefeldin A (all obtained from Sigma-Aldrich) and 10 ng/mL rIL-23 (eBioscience). Cells were fixed and permeabilized as indicated above and stained with IL22-02 (Pfizer) conjugated to Alexa Fluor 647 according to manufacturer’s instructions (Molecular Probes). Dead cells were excluded from analysis using a violet viability stain (Invitrogen). Flow cytometry data collection was performed on a FACSCanto II (BD Biosciences). Files were analyzed using FlowJo software (Tree Star Inc.). CD4+ LTi cells were sorted from naïve Rag1−/− splenocytes by excluding cells positive for the lineage markers CD11c, B220 (conjugated to APC-eFluor-780, eBioscience), Gr-1, NK1.1, and NKp46 (conjugated to FITC, eBioscience) and gating for CD90+ CD4+ cells on a FACSAria (BD Bioscience). Cytocentrifuge preparations of sorted cells were stained with H&E (Thermo Fisher Scientific).
In vitro cell stimulations
Total Rag1−/− splenocytes or total Rag1−/− IELs were cultured overnight in the presence or absence of 10 ng/mL rIL-23 (eBioscience). Cells were then stimulated for 4 h with 50 ng/mL PMA, 750 ng/mL ionomycin, and 10 µg/mL Brefeldin A for analysis of intracellular cytokine production as described above. Sorted CD4+ LTi cells from Rag1−/− splenocytes were cultured for 72 h in complete media containing 10 ng/mL rIL-7 (Peprotech) to enhance survival in the presence or absence of rIL-23 10 ng/mL for analysis of IL-22 secretion from cell-free supernatants.
ELISA
Tissue from the terminal colon was mechanically homogenized in PBS, centrifuged and the resultant supernatant measured for IL-22 protein by sandwich ELISA using IL22-01 (Pfizer) as a capture antibody and biotin-conjugated IL22-03 (Pfizer) as a detection antibody.
Serum ALT measurement
Blood from infected animals was obtained, allowed to clot, and centrifuged to obtain serum. Serum ALT was measured with a commercially available kit according to manufacturers directions (Biotron Diagnostics Inc, Hemet, CA) adapted to a 96-well plate format.
Quantitative real-time PCR
RNA was isolated from colon tissue using mechanical homogenization and TRIzol (Invitrogen). RNA from sorted populations was isolated using RNeasy mini kits (QIAGEN). cDNA was generated using Superscript reverse transcription (Invitrogen). Real-time PCR (RT-PCR) was performed on cDNA using SYBR green chemistry (Applied Biosystems) and commercially available primer sets (QIAGEN). Reactions were run on a real-time PCR system (ABI7500; Applied Biosystems). Samples were normalized to β-actin and displayed as a fold induction over naïve controls unless otherwise stated.
Histological sections
Livers were fixed with 4% paraformaldehyde, embedded in paraffin, and 5 µm sections were used for staining with H&E.
CD90-disperate Rag1−/− chimeras and Il22−/− adoptive transfers
Cells from mLN and spleen of CD90.1 C57BL/6 mice were obtained and approximately 60–80 × 106 sort purified B (CD19+) and T cells (CD3+, CD5+) were transferred i.v. to CD90.2 Rag1−/− mice. Reconstitution was permitted for 8–10 weeks post-transfer and subsequently confirmed by examination of peripheral blood lymphocytes.
C. rodentium infected Il22+/+ mice were sacrificed on days 2, 3 and 4 post-infection and approximately 1 × 106 CD4+ T cells (CD4+, CD3+, CD5+) or 1.5 × 103 CD4+ LTi cells (CD4+, CD3−, CD5−) from the spleen and mLN were sort purified and briefly stimulated for 1–2 hours in vitro with 10 ng/mL rIL-23 and 10 ng/mL IL-1β (eBioscience). Cells were then transferred i.v. into co-infected Il22−/− mice.
Statistical analysis
Results represent the mean ± SEM. Statistical significance was determined by the Student’s t test unless otherwise stated. For survival curves significance was determined using the log rank (Mantel-Cox) test. (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
HIGHLIGHTS
-
-
CD4+ LTi cells are a critical source of IL-22 following enteric bacterial infection.
-
-
Infection-induced CD4+ LTi cell responses and innate immunity are dependent on IL-23.
-
-
Depletion of CD4+ LTi cells abrogates innate immunity to enteric bacterial infection.
-
-
CD4+ LTi cells promote innate immunity in lymphopenic and lymphocyte-replete hosts.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge members of the Artis laboratory for helpful discussions and critical reading of the manuscript. We thank the Morphology Core and Pilot Feasibility Program of the National Institute of Diabetes and Digestive and Kidney Disease Center (DK50306) and Vet School Pathology Service for technical expertise, Khetemenee Lam and Adam Root (Pfizer) for purification of IL-22 diagnostic antibodies, and R. Askew (Pfizer) for discussion regarding the design of the IL-22-deficient mice. Research in the Artis lab is supported by National Institute of Health (AI61570, AI74878, AI087990 and AI083480 to D.A.; T32AI007532-08 to G.F.S), the Burroughs Wellcome Fund (Investigator in Pathogenesis of Infectious Disease Award to D.A.), and pilot grants from the University of Pennsylvania (PGFI, VCID and University Research Fund to D.A.).
M. Merle Elloso is employed by Centocor.
L.A. Fouser is employed by Pfizer.
Abbreviations used
- ILC
innate lymphoid cell
- LTi
lymphoid tissue inducer
- mRNA
messenger RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no further conflicting financial interests.
REFERENCES
- Bonnet F, Chene G, Thiebaut R, Dupon M, Lawson-Ayayi S, Pellegrin JL, Dabis F, Morlat P. Trends and determinants of severe morbidity in HIV-infected patients: the ANRS CO3 Aquitaine Cohort, 2000–2004. HIV Med. 2007;8:547–554. doi: 10.1111/j.1468-1293.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet FM. Evolution of the immune process in vertebrates. Nature. 1968;218:426–430. doi: 10.1038/218426a0. [DOI] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Fitch EL, Rizzo HL, Kurtz SE, Wegmann KW, Gao W, Benson JM, Hinrichs DJ, Blauvelt A. Inflammatory skin disease in K5.hTGF-beta1 transgenic mice is not dependent on the IL-23/Th17 inflammatory pathway. J Invest Dermatol. 2009;129:2443–2450. doi: 10.1038/jid.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kim MY, Anderson G, White A, Jenkinson E, Arlt W, Martensson IL, Erlandsson L, Lane PJ. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3− inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J Immunol. 2005;174:6686–6691. doi: 10.4049/jimmunol.174.11.6686. [DOI] [PubMed] [Google Scholar]
- Kim MY, Kim KS, McConnell F, Lane P. Lymphoid tissue inducer cells: architects of CD4 immune responses in mice and men. Clin Exp Immunol. 2009;157:20–26. doi: 10.1111/j.1365-2249.2009.03932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Rossi S, Withers D, McConnell F, Toellner KM, Gaspal F, Jenkinson E, Anderson G, Lane PJ. Heterogeneity of lymphoid tissue inducer cell populations present in embryonic and adult mouse lymphoid tissues. Immunology. 2008;124:166–174. doi: 10.1111/j.1365-2567.2007.02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane P, Kim MY, Withers D, Gaspal F, Bekiaris V, Desanti G, Khan M, McConnell F, Anderson G. Lymphoid tissue inducer cells in adaptive CD4 T cell dependent responses. Semin Immunol. 2008;20:159–163. doi: 10.1016/j.smim.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Lewden C, May T, Rosenthal E, Burty C, Bonnet F, Costagliola D, Jougla E, Semaille C, Morlat P, Salmon D, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: The "Mortalite 2000 and 2005" surveys (ANRS EN19 and Mortavic) J Acquir Immune Defic Syndr. 2008;48:590–598. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R, Acha-Orbea H, Finke D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26:643–654. doi: 10.1016/j.immuni.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage Relationship Analysis of ROR{gamma}t+ Innate Lymphoid Cells. Science. science. 2010:1194597. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T, Ludewig B. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- Schmutz S, Bosco N, Chappaz S, Boyman O, Acha-Orbea H, Ceredig R, Rolink AG, Finke D. Cutting edge: IL-7 regulates the peripheral pool of adult ROR gamma+ lymphoid tissue inducer cells. J Immunol. 2009;183:2217–2221. doi: 10.4049/jimmunol.0802911. [DOI] [PubMed] [Google Scholar]
- Segel MJ, Izbicki G, Cohen PY, Or R, Christensen TG, Wallach- Dayan SB, Breuer R. Role of interferon-gamma in the evolution of murine bleomycin lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1255–L1262. doi: 10.1152/ajplung.00303.2002. [DOI] [PubMed] [Google Scholar]
- Simmons CP, Clare S, Ghaem-Maghami M, Uren TK, Rankin J, Huett A, Goldin R, Lewis DJ, MacDonald TT, Strugnell RA, et al. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun. 2003;71:5077–5086. doi: 10.1128/IAI.71.9.5077-5086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol. 2010a;107:1–29. doi: 10.1016/B978-0-12-381300-8.00001-0. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010b;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2010 doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- Storek J, Gooley T, Witherspoon RP, Sullivan KM, Storb R. Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4 T cell counts. Am J Hematol. 1997;54:131–138. doi: 10.1002/(sici)1096-8652(199702)54:2<131::aid-ajh6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Kioussis D, Coles M. Natural killer receptors: the burden of a name. J Exp Med. 2010;207:269–272. doi: 10.1084/jem.20100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JM, Rao AV, Glassroth J, Hansen NI, Rosen MJ, Arakaki C, Kvale PA, Reichman LB, Hopewell PC. Respiratory illness in persons with human immunodeficiency virus infection. The Pulmonary Complications of HIV Infection Study Group. Am Rev Respir Dis. 1993;148:1523–1529. doi: 10.1164/ajrccm/148.6_Pt_1.1523. [DOI] [PubMed] [Google Scholar]
- Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.