Abstract
Postsynaptic dorsal column (PSDC) neurons transmit noxious visceral information from the lower thoracic and lumbosacral spinal cord. Cuneothalamic neurons in the PSDC pathway and upper thoracic (T3–T4) spinal neurons ascending through the ventrolateral funiculus (VLF) have been shown to transmit nociceptive cardiac information. Therefore, we hypothesized that upper thoracic PSDC neurons transmit noxious cardiac information. Neuronal responses to intrapericardially injected mechanical (1.0 ml saline) and noxious chemical (0.2 ml algogenic chemicals) stimuli were recorded from antidromically activated PSDC and VLF neurons in the T3–T4 spinal cord of anesthetized Sprague-Dawley rats. Of the PSDC neurons, 43% responded to mechanical stimulation, but only one responded to noxious chemical stimuli. Fifty-eight percent of VLF neurons responded to mechanical stimulation and all responded to noxious chemical stimulation. Fluoro-Ruby (FR)-labeled PSDC neurons in the T3–T4 spinal cord of Sprague-Dawley rats were processed for c-fos immunohistochemistry following intrapericardial stimulation with mechanical, chemical, or control stimuli. Sections were viewed under epifluorescence and light microscopy to detect FR-labeled neurons containing a c-fos immunoreactive (IR) nucleus. An average of 6 PSDC neurons per rat was found in the T3 and T4 spinal segments. The average number of c-fos-IR neurons per segment varied by type of stimulus: 12 (control), 67 (chemical) and 85 (mechanical) for T3 and 8 (control), 37 (chemical) and 62 (mechanical) for T4. None of the 200 PSDC neurons examined expressed c-fos-IR regardless of stimulus. Together, these results suggest that thoracic PSDC neurons transmit mechanical cardiac information, but they play a minimal role in cardiac nociception.
Keywords: visceral pain, cardiac pain, spinal cord, angina pectoris, spinothalamic tract
1. INTRODUCTION
Angina pectoris is the chest pain following myocardial ischemia that is typically described as a crushing or burning pain of the left chest that sometimes radiates down the proximal shoulder and left arm and up to the neck and jaw. Approximately 9.8 million Americans suffer from angina pectoris (Lloyd-Jones et al., 2009). Understanding the neural mechanisms and the pathways involved in cardiac nociception can lead to better treatment of this prevalent disease.
Recently, the postsynaptic dorsal column (PSDC) pathway had been described as a major visceral pain pathway (Al-Chaer et al., 1996b, 1998). Cell bodies of PSDC neurons are found in spinal cord laminae III, IV, VII and X with axons projecting through the dorsal columns (DC) to either the gracile (GN) or cuneate (CN) nucleus (Giesler et al., 1984; Hirshberg et al., 1996; Tracey, 1995; Wang et al., 1999). Neurons in these nuclei transmit visceral nociceptive information to the ventroposterolateral thalamus (VPL) (Cliffer et al., 1989; Hirshberg et al., 1996; Al-Chaer et al., 1996b, 1997a).
Since primary afferent neurons in the DC transmit innocuous tactile information, PSDC neurons were first assumed to transmit only innocuous somatic information. However, several clinical and experimental studies indicate that PSDC neurons play a role in transmitting visceral nociceptive information. For example, midline myelotomies transecting the DC have been reported to relieve pain associated with pelvic cancer in terminal patients (Hirshberg et al., 1996; Nauta et al., 1997; Becker et al., 1999). Recordings of antidromically activated PSDC neurons in the lumbosacral spinal cord have responded to colorectal distension (CRD) in rats (Hirshberg et al., 1996; Al-Chaer et al., 1996a, 1997b) and primates (AlChaer et al., 1999). Neuronal activity in the GN also increased following noxious stimulation of the uterus, vagina, cervix (Berkley and Hubscher, 1995), colon (Hirshberg et al., 1996; Al-Chaer et al., 1996a, 1997a) and pancreas (Wang and Westlund, 2001). DC lesions significantly abolished or reduced neuronal activity in the VPL responding to CRD in rats (Hirshberg et al., 1996; Al-Chaer et al., 1996b) and primates (Al-Chaer et al., 1998) and to chemical stimulation of the pancreas (Houghton et al., 2001). In addition, severe inflammation of the colon significantly increased responses of PSDC neurons (Al-Chaer et al., 1997b) and electromyography recordings to graded CRDs (Palecek and Willis, 2003).
That PSDC neurons transmit visceral nociceptive information is also indicated by behavioral and immunohistochemical studies. Writhing-like behavior in rats, indicative of duodenal pain, was significantly reduced by DC lesions (Feng et al., 1998). Rats with pancreatitis resumed normal rearing behaviors (Houghton et al., 1997) and rats with severe colonic inflammation resumed normal exploratory activity (Palecek et al., 2002) following DC lesions. In an immunohistochemical study, Palecek et al. (2003) utilized the proto-oncogene c-fos to identify PSDC neurons activated by ureter distension. This study showed a significantly greater number of activated neurons in the PSDC pathway as compared to the spinothalamic (STT) pathway of rats.
Previous studies of the PSDC pathway have focused on the abdominal and pelvic viscera; however, no information is known about the role of PSDC neurons in transmitting nociceptive information from the heart. During cardiac ischemia, the myocardium releases inflammatory mediators that stimulate chemosensitive receptors on cardiac afferent neurons in the epicardium (Baker et al., 1980; Lombardi and Malliani, 1981, Nerdrum et al. 1986). Previous studies have shown that cardiac nociceptive information is transmitted via the STT (Blair et al., 1982; Ammons et al., 1985a, b), as well as other ascending pathways in the ventrolateral funiculus (VLF) of the spinal cord. Only two studies have compared the role of the PSDC pathway with that of the VLF in transmitting information from the heart. Zhang et al. (1997) reported that cardiopulmonary sympathetic afferent (CPSA) information is transmitted bilaterally in the VLF, but not the DC, to the C1–C3 spinal segments from the thoracic spinal cord in rats. Chandler et al. (1998) compared cardiac and somatic information transmitted directly into the VPL from the STT and cuneothalamic pathways and showed that both pathways transmitted cardiac information in the monkey. It has been suggested that the STT and PSDC may play different roles due to the significant differences in neuronal characteristics following electrical stimulation of CPSA and mechanical stimulation of the somatic fields.
Since the PSDC pathway appears to play a major role in abdominal and pelvic visceral pain and that cuneothalamic neurons seem to conduct cardiac information, we hypothesized that upper thoracic PSDC neurons transmit cardiac nociceptive information to the cuneate nucleus. The aim of this study was to determine the electrophysiological and immunohistochemical effects of mechanical and chemical cardiac stimuli on PSDC neurons in the T3–T4 spinal segments. Extracellular potentials of neurons from the PSDC pathway were recorded during mechanical and noxious chemical stimulation of the heart and compared to responses of VLF neurons to the same cardiac stimuli. C-fos expression of fluorescent-labeled PSDC neurons was used to determine the activation of PSDC neurons following mechanical and chemical cardiac stimulation. Preliminary results have been published in abstract form (Goodman and Foreman, 2007; Goodman et al., 2009).
2. RESULTS
2.1 Electrophysiology
Extracellular responses were recorded to establish the contribution of the PSDC and VLF pathways in transmitting nociceptive cardiac information. Nine PSDC neurons met all three criteria for antidromic activation. The average recording depth of these neurons was 0.28 mm (± 0.08 mm) with an average constant latency of 8.3 ±3.2 ms. Fourteen VLF neurons met all three criteria for antidromic activation. The average depth of recording for these was 0.74 mm (± 0.08 mm). The average constant latency for the VLF neuronal responses was 3.4 ± 0.6 ms.
All PSDC and VLF neurons responded similarly to mechanical stimulation of the somatic receptive fields: the left chest or upper left arm and shoulder, where most cardiac pain is referred. Neuronal responses to somatic stimulation were characterized as low threshold (LT), high threshold (HT) and wide dynamic range (WDR). All of the 9 PSDC neurons recorded were WDR neurons responding to innocuous brush and noxious pinch. The 14 VLF neurons represented all groups: 29% LT, 57% WDR and 14% HT (4 /14, 8 /14 and 2 /14, respectively). Following somatic characterization, 2 PSDC and 2 VLF neurons were lost or died, reducing the number of antidromically activated PSDC and VLF neurons to 7 and 12, respectively.
2.1.1 Response to Chemical Stimulation
Extracellular neuronal responses of PSDC and VLF neurons to 0.2 ml algogenic chemicals into the pericardial sac were recorded. Neuronal response characteristics of the PSDC and VLF neurons are compared in Table 1. Only one PSDC neuron responded to noxious chemical stimulation precluding statistical comparisons of response characteristics between PSDC and VLF neurons.
Table 1.
Neuronal response characteristics of PSDC and VLF neurons in response to chemical stimulation of the heart. No statistical analysis was done since there was only one responsive PSDC neuron.
| n | Total neurons responded |
Excitatory change (imp/s) |
Spontaneous Activity (imp/s) |
Latency (s) |
Time to Peak (s) |
Time to recovery (s) |
|
|---|---|---|---|---|---|---|---|
| PSDC | 7 | 1 | 2.6 | 0.3 | 7.5 | 3.7 | 75.4 |
| VLF | 12 | 12 * | 12.2 ± 3.2 | 5.7 ± 1.5 | 5.3 ± 1.6 | 27.5 ± 7.0 | 85.5 ± 39.8 |
Means ± SE
p = 0.0003, Fisher’s Exact Test
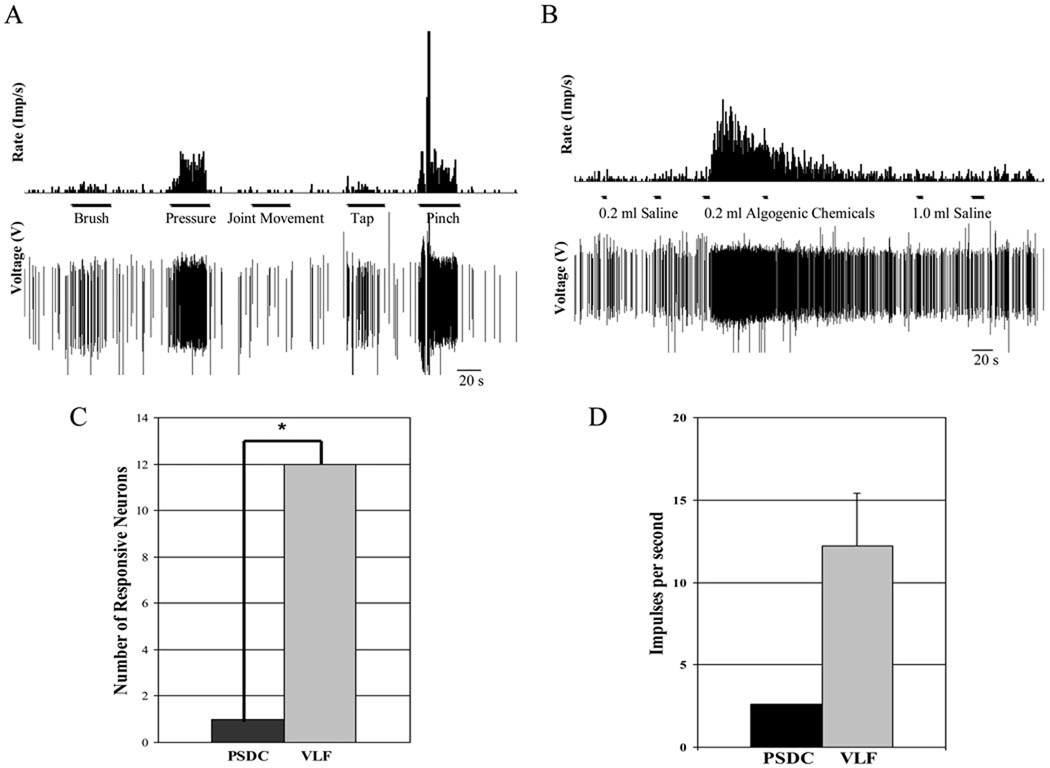
All VLF neurons were activated in response to the intrapericardial algogenic chemicals. The number of VLF and PSDC neurons that responded to chemical stimulation was significantly different (p = 0.0003, Fischer’s Exact Test, Figure 2A.). The one chemosensitive PSDC neuron was recorded at a depth of 0.43 mm and was characterized as a WDR neuron. The average recording depth of the chemosensitive VLF neurons was 0.73 mm (± 0.95 mm). These neurons were classified as 33% LT, 42% WDR, and 25% HT. The 12 VLF neurons and the PSDC neuron displayed a characteristic burst response to the chemical stimuli. The maximal excitatory response of the PSDC neuron was 2.6 imp/s; whereas, the mean maximal excitatory response of the VLF neurons was 12.2 (± 3.21 SE) imp/s (Figure 2B.).
Figure 2.
Extracellular neuronal responses to stimulation. A. An example of a neuronal response to the stimulation of the receptive field in raw tracing and histogram forms. B. An example of a neuronal response to intrapericardial stimuli in raw tracing and histogram forms. C. Comparison of the number of PSDC and VLF neurons that responded to chemical stimulation of the heart. The VLF neurons significantly outnumber the PSDC neurons (12/12 vs 1/7), *p= 0.0003 (Fisher’s Exact Test). D. Comparison of excitatory responses of the PSDC (n=1, No statistics were performed) and the VLF neurons (n=12) to the intrapericardial injection of algogenic chemicals.
2.1.2 Response to Mechanical Stimulus
Neuronal responses to the mechanical stimulus (1.0 ml saline) injected into the pericardial sac were recorded from antidromically activated PSDC and VLF neurons at the T3–T4 spinal level. Table 2 shows some characteristics of these neurons. PSDC and VLF neurons were not significantly different in any of the neuronal characteristics, such as spontaneous activity or response latency. Inhibitory neurons could not be compared statistically.
Table 2.
Neuronal response characteristics of PSDC and VLF neurons in response to mechanical stimulation of the heart. There were no statistical differences between the PSDC and VLF neuronal responses to mechanical stimulation (Student’s t-test).
| n | Total neurons activated |
Excitatory change (imp/s) |
Inhibitory change (imp/s) |
Spontaneous Activity (imp/s) |
Latency (s) |
Time to Peak (s) |
Time to recovery (s) |
|
|---|---|---|---|---|---|---|---|---|
| PSDC | 7 | 2 | 2.2 ± 0.9 | - | 2.6 ± 1.5 | 2.3 ± 1.8 | 33.6 ± 11.1 | 19.9 ± 12.4 |
| 1 | - | −2.0 | 5.4 | 0.7 | 53.4 | 43.8 | ||
| VLF | 12 | 7 | 2.2 ± 0.5 | - | 4.0 ± 1.8 | 1.7 ± 0.6 | 46.5 ± 10.5 | 26.8 ± 8.8 |
| 1 | - | −4.1 | 14.1 | 3.7 | 29.5 | 27.4 |
Means ± SE
Three of the 7 PSDC neurons (43%) showed a greater response to the mechanical stimulus than to the 0.2 ml saline control; of these, two neurons were excited and one was inhibited by the stimulus. Eight of 12 VLF neurons (67%) responded to the mechanical stimulus. Fischer’s Exact Test analysis showed that the number of VLF and PSDC neurons that responded to the mechanical stimulus was not significantly different (p = 0.3765).
The excitatory responses, differences between the number of impulses per second during maximal responses and the spontaneous activity 20 seconds prior to stimulation, were also compared. The mean excitatory response for PSDC neurons (2.2 ± 0.9 imp/s) was similar to that of VLF neurons (2.2 ± 0.5 imp/s, p= 0.9618).
2.2 Immunohistochemistry
2.2.1 FluoroRuby-labeled PSDC neurons
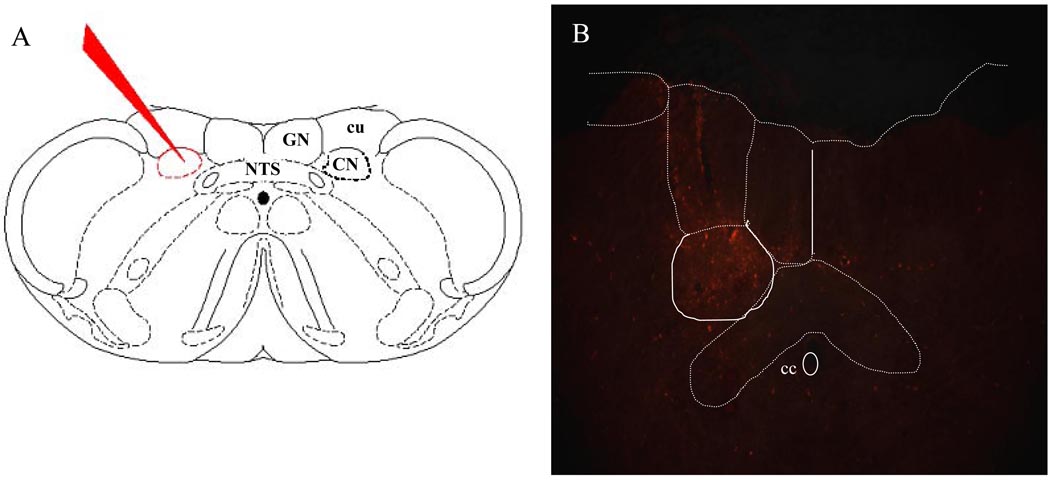
The brainstems were examined to confirm that the injections of Fluoro-Ruby (FR) were restricted to the CN in the 17 experimental animals (Figure 3.). Thus, it is likely that all FR-labeled PSDC neurons projected to the CN. Likewise, for the injection sites of the 4 control animals, the Fluoro-Emerald (FE) was restricted to the GN in 2 rats and the VPL thalamus in 2 rats.
Figure 3.
The location of injections into the dorsal column nuclei is depicted in A. (NTS, nucleus of the solitary tract; Gr, gracile nucleus; CN, cuneate nucleus; cu, external cuneate nucleus). The photomicrograph (B) shows an example of one injection site into the CN (cc, central canal).
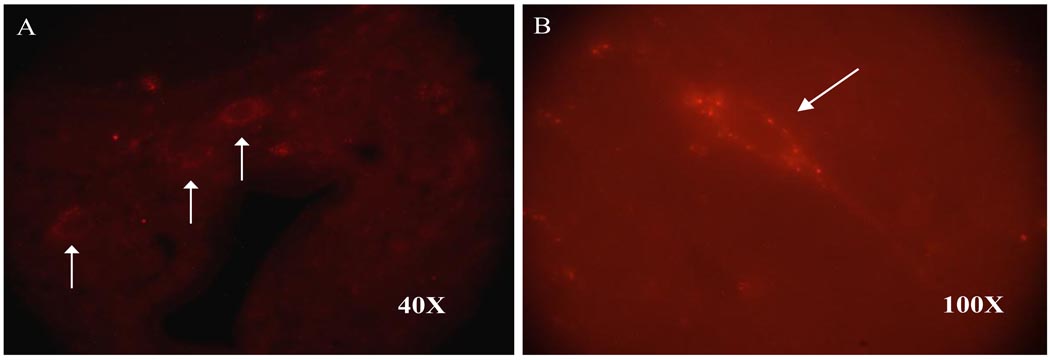
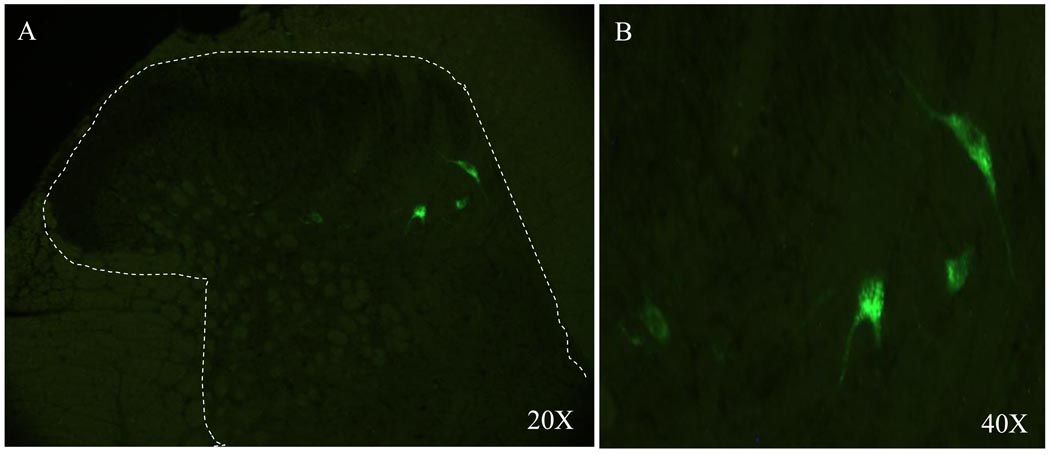
The FR-labeled PSDC neurons were counted in T3 and T4 spinal sections. The total number of FR-labeled PSDC neurons in the 17 experimental rats was 105 and 95 in the T3 and T4 spinal segments, respectively; thus, the average number of FR-labeled neurons per rat was 6 PSDC neurons in both the T3 and T4 spinal segments (Figure 4). FR-labeled PSDC neurons were scattered throughout laminae IV, V, VII and X.
Figure 4.
Photomicrographs of FR-labeled PSDC neurons (arrows) in the T3 spinal segment of a chemically stimulated rat at 40X (A) and 100X (B).
2.2.2 Quantitative Evaluation of c-fos Expression
There were few c-fos-immunoreactive (IR) T3–T4 neurons (0–5 neurons/animal) in the perfusion and anesthesia baseline controls. c-fos-IR neurons were counted and compared in the T3–T4 spinal segments for rats in the 3 experimental groups. In the T3 spinal segment, an average of 67 (538/8) c-fos-IR neurons per rat were found in the chemically stimulated group, 85 (509/6) in the mechanically stimulated group and 12 (35/3) in the saline control group. The average number of c-fos-IR neurons in the T4 spinal segment was 37, 62 and 8 (totals per group: 296, 370 and 24) in the chemically stimulated, mechanically stimulated and saline control groups, respectively.
The number of c-fos-IR neurons of the T4 chemical group was significantly greater than the number of c-fos-IR neurons in the T4 saline control group (p= 0.0269). The number of c-fos-IR neurons in the T3 segment of the chemical and mechanical groups and the T4 segment of the mechanical groups were not significantly different from the saline controls due the high variability of the number of c-fos-IR neurons in the experimental groups.
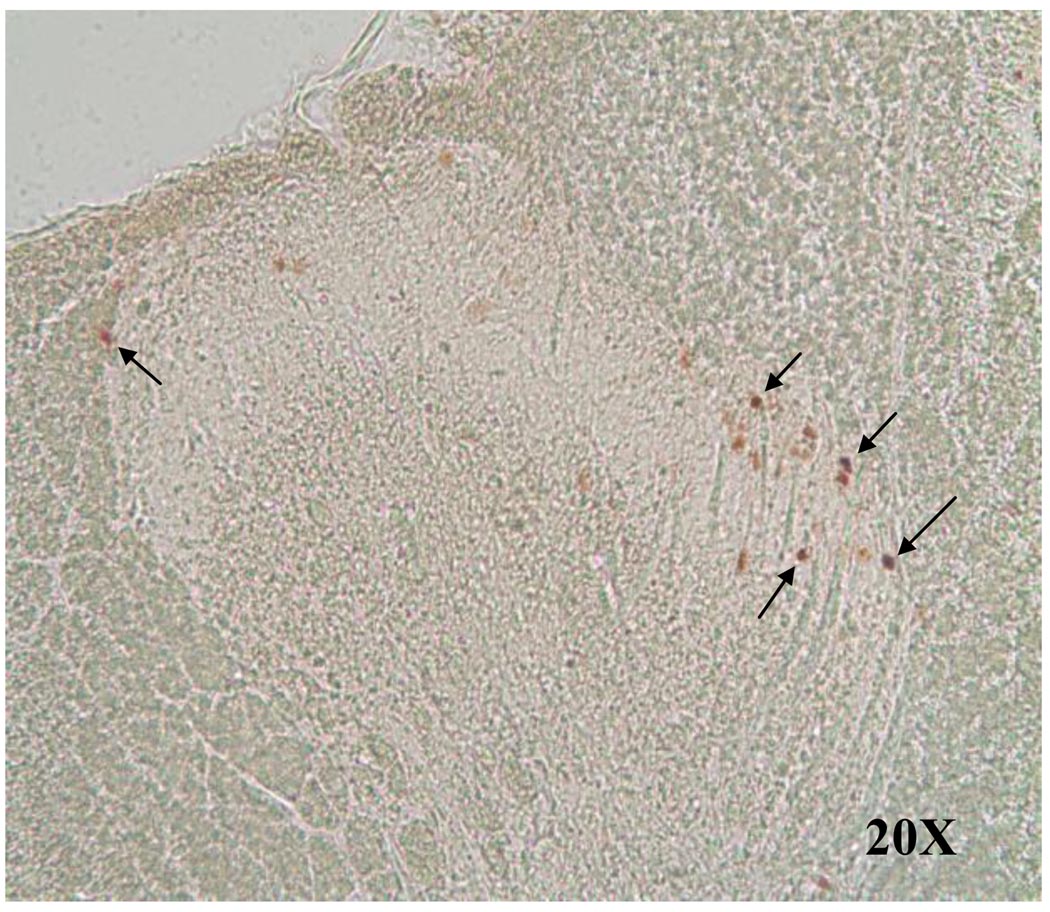
The majority of c-fos-IR neurons were located in the medial superficial dorsal horn in chemically stimulated rats (Figure 5), in the medial and lateral superficial dorsal horn in mechanically stimulated rats. Both of these stimuli produced a few c-fos-IR neurons in the deeper laminae, including the area surrounding the central canal.
Figure 5.
Photomicrograph showing c-fos-IR neurons (arrows) in the spinal gray matter of a T3 spinal segment following chemical cardiac stimulation under brightlight microscopy at 20X.
2.2.3 Colocalization of Retrograde Tracers and c-fos
We did not detect any double-labeled, e.g. FR-labeled + c-fos-IR PSDC neurons, in any of the experimental or saline control groups. There were c-fos-IR neurons located in the same sections as with FR-labeled neurons, but none of the labels were co-localized to individual neurons. Table 3 summarizes the average number of FR-labeled neurons and c-fos-IR neurons in each experimental and control group for the T3 and T4 spinal segments. An average of 4 ± 1.4 PSDC neurons in the T3 and 5± 2.5 PSDC neurons in the T4 spinal segments were found in all the rats injected. The number of FR-labeled PSDC neurons containing c-fos-IR is also reported.
Table 3.
Summary of the mean number of FR-labeled PSDC neurons, c-fos-IR neurons and double –labeled (FR-labeled + c-fos-IR) neurons in 1 series of T3 and T4 spinal cord sections. (* p= 0.0269 as compared to T4 saline control; Student’s t test, p≤ 0.05 for significance)
| T3 Spinal Segment |
Control n = 3 |
Chemical n = 8 |
Mechanical n = 6 |
FR-labeled and Fos-IR PSDC neurons |
|---|---|---|---|---|
| FR-labeled PSDC neurons (per animal) |
12 ± 7 | 3 ± 1.3 | 3 ± 2.1 | |
| Fos-IR neurons (per animal) |
6 ± 1.5 | 67 ± 14.9 | 85 ± 25.0 | |
| T4 Spinal Segment |
Control n = 3 |
Chemical n = 8 |
Mechanical n = 6 |
FR-labeled and Fos-IR PSDC neurons |
| FR-labeled PSDC neurons (per animal) |
2 ± 1.0 | 5 ± 4.1 | 7 ± 4.1 | |
| Fos-IR neurons (per animal) |
1 ± 1.0 | 37 ± 6.4* | 62 ± 16.8 |
Means ± SE
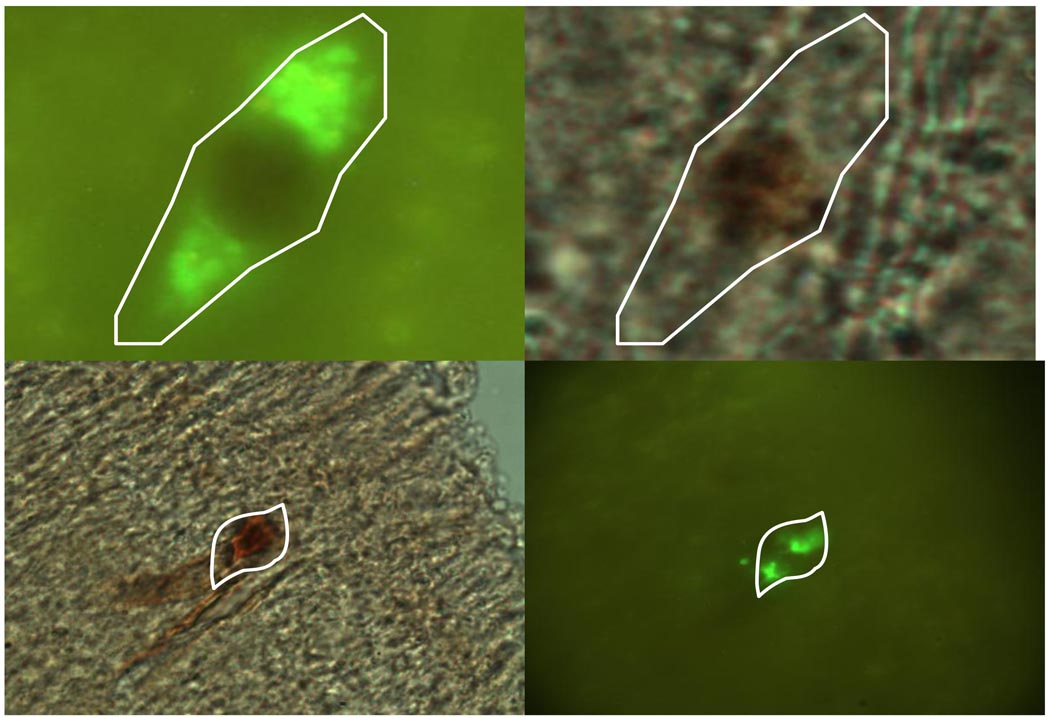
Two sets of positive controls were included to validate the results of this study. To show that PSDC neurons produce c-fos, FE-labeled PSDC neurons in the lumbosacral spinal cord (L6 – S2) were traced from the GN and activated with noxious CRD. In these controls, 16 sections were counted per rat since only 2 rats were used in each positive control. In the two gracile-injected rats, an average of 54 FE-labeled PSDC neurons (Figure 6) and 817 c-fos-IR neurons per rat were observed. To show that neurons of known cardiac nociceptive pathways of the upper thoracic spinal cord produce c-fos in response to noxious chemical stimulation, FE was injected in the VPL to label STT neurons known to transmit cardiac nociception (not quantified). Double-labeled (FE-labeled + c-fos-IR) of both the L6 – S2 PSDC and T3–T4 STT neurons are shown in Figure 7.
Figure 6.
Photomicrographs showing FE-labeled PSDC neurons in the L6 - S2 spinal segments of positive controls after the injection in the gracile nucleus. The area containing the neurons in A (20X) is enlarged in B (40X).
Figure 7.
Photomicrographs show examples of double-labeled (FE-labeled + c-fos-IR) PSDC neurons in the lumbosacral spinal cord in response to CRD (top row, 100X) and STT neurons in the T3–T4 spinal cord responding to intrapericardial algogenic chemicals (bottom row, 40X).
3. DISCUSSION
This study is the first to record and examine the responses of upper thoracic PSDC neurons to mechanical and noxious chemical cardiac stimulation electrophysiologically and immunohistochemically. The results did not support the PSDC pathway as the major cardiac pain pathway. Our hypothesis that PSDC neurons transmit cardiac nociceptive information was based on results of previous studies that PSDC neurons of the lower thoracic and lumbosacral spinal segments transmit noxious visceral information (Al-Chaer et al., 1996a, 1997b, 1999) and cuneothalamic neurons, upon which PSDC neurons synapse, transmit cardiac information (Chandler et al., 1998). Results of this study show that PSDC neurons do not transmit cardiac nociception, but they suggest that PSDC neurons may have a function in the transmission of mechanical cardiac information. Thus, the results of this project do not support our hypothesis but support the theory that the ascending pathways of the VLF, including the STT, as the major pathways that transmit nociceptive information from the heart (Foreman, 1998, 1989; Blair et al., 1981, 1982; Ammons et al. 1985a, 1985b).
3.1 Noxious Chemical Stimulation of the Heart
During myocardial ischemia, the myocardium releases bradykinin, adenosine, and other inflammatory mediators that stimulate chemoreceptors on cardiac afferent fibers. These fibers transmit this nociceptive information to neurons in the dorsal horn of the spinal cord. In experimental studies, a mixture of algogenic chemicals (bradykinin, serotonin, adensosine, histamine and prostaglandin E2) evoked greater pain-like behaviors in conscious rats and greater electrophysiological responses of thoracic neurons than bradykinin alone (Euchner-Wamser et al., 1994). As the chemical stimulus in this project, intrapericardial injections of these algogenic chemicals mimic the release of the inflammatory mediators produced during myocardial ischemia.
Chemical stimulation has been used to test the response of lower thoracic and lumbosacral PSDC neurons to abdominal and pelvic visceral stimuli in previous studies. Inflammatory mediators released following the application of mustard oil increased background activity of PSDC, GN and VPL neurons and potentiated these responses to CRD (Al-Chaer et al., 1996a, 1997b). Bradykinin applied to the surface of the pancreas also increased neuronal responses of dorsal column nuclei (DCN) and VPL neurons (Houghton et al., 2001; Wang and Westlund, 2001). Thus in the present study, the upper thoracic PSDC neurons were expected to respond to chemical cardiac stimulation, but of the PSDC neurons found, only one weakly responded to chemical stimulation. This is consistent with the immunohistochemical study, in which no c-fos-IR PSDC neurons were found in T3–T4 segments, signifying the lack of activation of these neurons following chemical stimulation. This is in sharp contrast to the responses of the neurons in the VLF, which all responded robustly to the chemical stimulation. In addition, STT neurons in the VLF expressed Fos in response to intrapericardial chemical stimulation. Previous studies have reported that STT neurons showed increased activity following intracardiac injections of bradykinin (Blair et al., 1982; Ammons et al., 1985b). Therefore, the results of our study support the ascending pathways of the VLF as the major nociceptive pathways of the heart.
3.2 Responses of Neurons to Mechanical Stimulation
Some cardiac afferents may also be activated by the stimulation of mechanoreceptors in the epicardium. This low threshold sensory information is also transmitted to dorsal horn neurons in the spinal cord. Ventricular mechanoreceptors have been shown to signal changes in cardiovascular events, such as premature ventricular contraction (Foreman, 1986). These contractions can be perceived but may not be noxious since cardiac mechanorecptors transmit information in the innocuous range and have been shown to excite dorsal horn, spinoreticular tract and STT neurons (Baker et al., 1980, Foreman, 1986). In a preliminary study of spinal neurons, there was a significant increase in neuronal activity in response to only high volumes of saline (1.0 ml), which stimulated cardiac mechanoreceptors; thus, the same volume was used as a mechanical stimulus for this project.
This is the first study to examine mechanoreceptive cardiac input onto PSDC and VLF neurons. The PSDC and VLF neuronal responses to mechanical stimulation were weak and showed no differences in response characteristics. The responses of these neurons signify that they may play a minor role in transmitting mechanical cardiac information.
Since PSDC neurons synapse onto the same neurons in the CN as the primary afferents, it is not surprising that PSDC neurons transmit mechanical information from the heart. The weakness of the response is unexpected considering that PSDC neurons vigorously respond to colorectal (Al-Cher et al. 1996a, 1997b), vaginal, uterine and cervical distension (Berkley and Hubscher, 1995). VLF responses to mechanical stimulation were also weak. These VLF neurons responded robustly to input from somatic receptive fields. Thus, upper thoracic PSDC and VLF neurons receive only a small amount of input from cardiac mechanoreceptors.
The mechanical input onto the responsive PSDC and VLF neurons were most likely from mechanoreceptors in the epicardium, not the pericardium. Mechanoreceptors stimulated by pericardial distension transmit information through the cervical vagus, thoracic vagus and phrenic nerves (Kostreva and Pontus, 1993). Only cardiac sympathetic afferents project into the upper thoracic spinal segments; thus, neuronal responses can only be attributed to the stimulation of the heart.
3.3 Input of Somatic Receptive Fields
During angina pectoris, pain is referred to the deep muscle of the left chest and shoulder. Dorsal horn neurons are classified by the mechanical input from the somatic receptive fields. The PSDC neurons in this study were classified as WDR neurons; whereas, those in the VLF contained LT, WDR and HT neurons. This is consistent with Al-Chaer et al. (1997b), who reported all PSDC neurons responded to both low and high threshold input of the somatic receptive fields. No nociceptive-specific PSDC neurons were found in the rat in either study. Although PSDC neurons respond vigorously to noxious pinch, these neurons do not respond to thermal stimulation, which is an important function of C fibers (Giesler et al., 1985). All three classifications have been reported in the tracts that project through the VLF. Studies of these tracts found that the majority of STT, spinoreticular, spinomesenphalic and spinohypothalamic tract (SHT) were HT or WDR, but the STT and SHT had a few neurons classified as LT (Chandler et al., 1991, 1998; Hobbs et al., 1989; Chen and Pan, 2002; Foreman et al., 1984; Katter et al., 1996). Therefore, PSDC neurons received input primarily from Aδ fibers that are related to mechanosensitivity of the innocuous ventricular distension; whereas, VLF neurons have input from Aδ and C fibers. These lightly myelinated and unmyelinated fibers are commonly associated with nociceptive chemoreceptors (Foreman, 1989; Cervero, 1994).
3.4 Fos
Fos, the nuclear protein of the immediate-early gene c-fos, has been shown to be produced following neuronal activation by a variety of noxious visceral stimuli (Palecek et al., 2003). In the present study, a significant increase in c-fos expression was observed in the medial superficial lamina of the T3 spinal segment following chemical stimulation of the heart. Previous studies (Albutaihi et al., 2004; Hua et al., 2004) have shown a similar increase in rats, although Hua et al. (2004) reported this increase following chemical cardiac stimulation in the lateral superficial dorsal horn. The expression of c-fos was also increased in the deeper lamina, including lamina V, VII and X, in all of the studies.
Since the mechanical stimulus was innocuous, observed increases in c-fos expression were unexpected. This increase in the number of c-fos-immunoreactive neurons was not significant as compared to the saline control (p = 0.1322 for T3 and p = 0.959 for T4). Possible explanations for the observed increase may be that the mechanical stimulus activated nociceptive afferents in the chest wall or the increased the pressure on the lungs which activated the chest wall afferents (Cervero, 1994). Either of these explanations would account for the increase in c-fos-IR following the mechanical stimulus.
The location of neurons expressing c-fos differed following the mechanical stimulus and the saline control. Neurons expressing c-fos were located in laminae I and II of the medial and lateral superficial dorsal horn. Albutaihi et al. (2004) reported a similar expression pattern in a volume control of intrapericardial injection.
3.5 VLF as Major Pathway of Cardiac Nociception
Chandler et al. (1998) noticed distinct differences between the input of cuneothalamic and STT neurons to the VPL thalamus. Electrical stimulation of the CPSA activated 50% of cuneothalamic and 100% of STT neurons. Since electrical stimulation does not differentiate between modalities, the percentage of PSDC and STT neurons activated in our study generally agrees with these findings, except that the PSDC neurons responded to mechanical stimulation and the STT neurons responded to chemical stimulation. Other characteristics of neuronal responses differed between the two pathways, including response pattern, duration of response and average latency. These differences in light of the present study support the argument that the ascending pathways in the VLF of the spinal cord are the major route for transmitting nociceptive information from the heart.
Two series of lesioning experiments provide additional evidence about the importance of the VLF pathways; Zhang et al. (1997) and Ness (2000) determined that visceral nociceptive information does not travel in the dorsal column, but bilaterally via the VLF. Zhang et al. (1997) recorded the responses of C1–C3 spinal neurons to electrical CPSA stimulation before and after lesioning the DC, ipsilateral VLF, then the contralateral VLF. Ness (2000) measured pseudaffective visceromotor and cardiovascular reflexes, signals of visceral pain, before following the same series of lesions. Both studies suggest that the ascending pathways in the VLF are responsible for the transmission of nociceptive information from the heart to the thalamus.
The findings of this study from the heart do not support the results of previous studies that stimulated the abdominal and pelvic viscera to determine the function of the PSDC. Possible explanations for this disparity are the distinct differences in the nervous system that innervate the heart and the abdominal and pelvic viscera: the modality of pain and the neuroanatomy of the spinal cord.
3.6 Modality of Visceral Pain
The preferred modality of pain differs between the heart and all of the abdominal and pelvic viscera. Angina pectoris is caused by the release of chemicals that activate chemosensitive cardiac afferents (Crea and Gaspardone, 2002); whereas mechanoreceptors, sensitive to the distension of hollow organs, are more pertinent in transmitting noxious abdominal and pelvic information (Camilleri et al., 1996).
Although the most important modality of nociception from the heart is chemical, the epicardium is innervated by both, mechanoreceptors and chemosensitive nociceptors. Mechanosensitive myelinated Aδ and unmyelinated C fibers respond vigorously to low threshold stimulation, including increases in blood pressure and weak responses to bradykinin (Baker et al., 1980). Because these neurons respond to stimuli below the noxious level, they are not considered to be involved with cardiac pain. Alternatively, chemosensitive fibers respond vigorously to the release of algogenic chemicals during myocardial ischemia, but are not stimulated by mechanical stimulation (Nerdrum et al., 1986). Chemosensitive fibers are nociceptors because they respond to stimuli outside the physiological range and evoke pseudaffective cardiovascular reflexes (Zahner et al., 2003; Nerdrum et al., 1986).
For the abdominal and pelvic viscera, stimulation of mechanoreceptors by over distending the hollow organ produces pain, i.e. noxious CRD or obstruction of pancreatic ducts or the ureter. These mechanoreceptors are polymodal fibers that also respond to chemical stimulation and can be sensitized by noxious chemicals and inflammatory mediators, i.e. mustard oil in the colon (Al-Chaer et al., 1996b, 1997b) or the application of bradykinin on the pancreas (Cervero, 1994; Houghton et al 2001). All chemosensitive fibers also are mechanoreceptive in the colon (Cervero, 1994); thus, the two groups are combined and discussed as chemosensitive mechanoreceptors in the literature.
Regardless of the innervated tissue, PSDC neurons received input from mechanoreceptors in the periphery and responded according to the modality preference of the tissue. Although PSDC responded electrophysiologically to mechanical stimulation, mechanoreception is not a noxious modality from the heart and PSDC neurons did not express c-fos following mechanical cardiac stimulation. Alternatively, distension is the main evoked sensation from the pelvic viscera (Camilleri et al., 1996). PSDC neurons from the pelvic viscera responded vigorously to noxious distension from the colon (Hirshberg et al., 1996; Al-Chaer et al., 1996a, 1997b, 1999) and expressed c-fos following colon and ureter distension (Palecek et al., 2003).
VLF neurons receive chemical and mechanical input from the heart and the pelvic organs (Palecek and Willis, 2003, Camilleri et al., 1996). The heart produces chemicals which stimulate primary afferents that activate VLF neurons to cause pain as seen by electrophysiological responses and the production of Fos in STT neurons in response to intrapericardial algogenic chemicals. In the pelvic viscera, STT neurons respond vigorously to CRD and these responses have been shown to be similar to those of PSDC neurons (Al-Chaer et al., 1999). Lesions of the VLF abolish electromyographic responses to chemical sensitivity to noxious distension (Palecek and Willis, 2003). PSDC neurons transmit mainly mechanoreceptive information, which can be either innocuous or noxious, depending on the organ system; whereas, the VLF transmits nociceptive information from all of the organ systems.
3.7 Neuroanatomy
The neuroanatomy differs between the upper thoracic, lower thoracic and lumbosacral spinal cord levels that receive inputs from the heart, abdominal and pelvic viscera, respectively. Al-Chaer et al. (1999) suggested that the difference between the PSDC and STT pathways was quantitative since the neuronal characteristics of these two pathways were similar in primates. In the present study, dextran conjugate retrograde tracers injected into the CN labeled an average of 4 neurons per animal in the T3 spinal segment and 5 in the T4 spinal segment when counting every 4th section of rats. The labeled neurons were found scattered throughout laminae IV, V, VII and X. The location of PSDC neurons were consistent with Giesler et al., (1984), who labeled an average of 14 PSDC neurons in alternate sections of the T4 spinal segment with horseradish peroxidase (HRP) in rats. In the lumbosacral spinal cord, an average of 54 PSDC neurons were labeled with dextran conjugates and 110 PSDC neurons were traced in T12 and 286 in the lumbar enlargement (L4–L6) with HRP (Giesler et al., 1984). Lumbosacral PSDC neurons were also found mainly in lamina IV with a few scattered in laminae V, VII and X (Giesler et al., 1984). From the C6–8 and L4–6 spinal segments, less than 10 neurons around the central canal project to the GN or CN (Nahin et al., 1983). In comparison, an average of 67, 108, 247 and 450 STT neurons were found in alternate sections of the T4, T12, L4–5 and L6-S2, respectively (Burstein et al., 1990). There are considerably fewer PSDC neurons in the upper thoracic spinal cord as compared to the number of PSDC neurons in the lower thoracic and lumbosacral spinal cord. More STT neurons were also found in the upper thoracic spinal cord (Burstein et al., 1990). The number of PSDC neurons in L4–5 was similar to that of the STT neurons. Therefore, the relatively small number of PSDC neurons in the upper thoracic spinal cord may also indicate the lack of importance of this pathway in cardiac nociception.
3.8 Alternative Explanation of the PSDC pathway
There are several theories that support alternative theories for the PSDC pathway. The first theory is that the PSDC pathway transmits the sensation of hollow organ distension as suggested by Amassian (1951). It was noted by White (1943) that the sensation of distension remains following bilateral high cordotomies, which eliminates all ascending pathways except for those in the dorsal columns. Support for this theory comes from more recent studies that have recorded the responses of PSDC neurons from the lumbosacral spinal cord in response to CRD (Hirshberg et al., 1996; Al-Chaer et al., 1996a, 1997b, 1999). Some studies have also shown that neurons in the GN and VPL, components of the PSDC pathway, are also responsive to distension of the colon, cervix, uterus, vagina and pancreas (Berkley and Hubscher et al., 1995; Hirshberg et al., 1996; Al-Chaer et al., 1996a, b, 1997a, 1998, Houghton et al., 2001; Wang and Westlund, 2001). Thus, it is possible that the function of the PSDC pathway may be to transmit the feeling of distension to the DCN.
3.9 Limitations of this study
Locating PSDC neurons limited both the electrophysiological and immunohistochemical studies. The first obstacle was spinal cord quiescence following stimulation of the CN and VLF for antidromic activation. Spinal cord quiescence limited the time allowed to locate the neurons. This obstacle was overcome by applying ibotenic acid on propriospinal neurons of the C1–C2 spinal cord, which greatly increased the time allowed for locating the neurons and performing the experiments.
The next obstacle was the low number of PSDC neurons that could be located and tested. In the electrophysiological studies, 24 PSDC neurons were found and 9 of these met all three criteria for antidromic activation. Since few neurons were found in the neuroanatomical studies, it seems that the number of neurons reported in this study is relative to the number of PSDC neurons present in the upper thoracic spinal cord. Low numbers of lumbosacral PSDC neurons were reported in the previous studies from the pelvic viscera (Hirshberg et al., 1996, Al-Chaer et al., 1997b).
In the immunohistochemical studies, locating PSDC neurons also had limitations. If only a few PSDC neurons were found, the fluorescent tracer may not have been absorbed by the axons of every PSDC neuron. The average number of PSDC neurons found in this study is similar to those reported in previous studies of PSDC neurons in the upper thoracic spinal cord using related techniques (Giesler et al., 1984).
The other limitation of fluorescent tracers is leakage outside the target injection site in cases where higher than average numbers of PSDC neurons were found as in the case of one animal. The restriction of fluorescent tracer to the CN was visually confirmed in this animal, but it is acknowledged that the tracer could have been absorbed by axons of solitary and reticular neurons bordering the CN (Nahin et al., 1983).
Overall, the number of PSDC neurons was limiting factor rather than the techniques used in this study.
3.10 Conclusion
Although the electrophysiological and immunohistochemical studies of the PSDC pathway indicated that it does not contribute significantly as a major pathway in cardiac nociception, this pathway could function in the transmission of mechanical cardiac information.
4. EXPERIMENTAL PROCEDURES
All experimental protocols were approved by the University of Oklahoma Health Sciences Center Institutional Animal Care and Usage Committee and were consistent with the guidelines of the International Association for the Study of Pain and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
4.1 Electrophysiology Experiments
Male Sprague-Dawley rats (250–500g, Charles River, Boston, MA) were anesthetized with sodium pentobarbital (65 mg/kg i.p.). Body temperature was maintained at 37 ± 1°C using a thermostatically controlled heating pad with a rectal thermocouple probe feedback sensor. A tracheotomy was performed and the animal was artificially ventilated with room air at a tidal volume of 2.5–5.0 ml/ stroke at 55–60 strokes/ min.
The left jugular vein was catheterized for the infusion of sodium pentobarbital (15–25 mg/kg/hr) via a syringe pump to maintain the appropriate level of anesthesia. Mean arterial blood pressure was monitored from the right carotid artery via a polyethylene catheter containing 0.1 ml heparin/ 20 ml saline connected to a blood pressure monitor. The mean blood pressure signals were directly transmitted to a 1401 plus Data Acquisition system (Cambridge Electronic Design, CED) and then to Spike 2 Analysis software (CED, Cambridge, England). To determine the anesthesia level, a noxious stimulus (e.g. tail pinch) was applied periodically to show that the arterial pressure remained constant throughout the experiment. .
A midline thoracotomy was performed to insert a silicone catheter (2 mm OD, 1 mm ID, 13–14 cm long with 6–8 small holes cut into the last centimeter) into the pericardial sac to administer experimental stimuli. The catheter was secured with silk suture and cotton pellets placed around it before the incision was closed and the animal rotated to the prone position.
To record neuronal activity, a laminectomy was performed to expose the T3–T4 thoracic spinal segments. The DCN and upper cervical spinal segments were exposed to access the ascending VLF at the C1 level, as well as the CN. A saline-moistened cotton ball was placed over these areas until placement of the electrodes for antidromic activation of axons in the VLF and PSDC pathway.
The rat’s head was then placed in a stereotaxic headholder (Kopf) and ventroflexed to allow simultaneous placement of electrodes on the left CN and into the right VLF. The dura and pia mater of the T3–T4 spinal segments were carefully removed and the spinal cord was covered with warm agar for stabilization during the neuronal recordings.
To prevent spinal cord quiescence, ibotenic acid (0.3 mg/1 ml saline, pH 5.0, Sigma) was applied to the upper cervical spinal cord. For this, a piece of Whatman filter paper (1 mm × 2 mm) was soaked with the ibotenic acid solution and placed on the dorsal surface of the C1 spinal cord approximately 1mm below the obex. Applying ibotenic acid in this manner lesioned cell bodies of propriospinal neurons in this region that have inhibitory effects on neurons in the T3–T4 region (Foreman et al. 2002, Qin et al. 2004) without disrupting axons of passage (Schwarcz et al. 1979, Contestabile et al. 1984). The filter paper was removed after 1 hour or when the mean blood pressure decreased to 85 mmHg. After the filter paper was removed and the area washed with saline, gel foam was positioned around the circumference to limit excess fluids from entering the site. The rat was then paralyzed with pancuronium (0.2 ml/hr, i.p.).
A carbon-filament glass microelectrode was used to record extracellular action potentials of single neurons at the T3–T4 level of the spinal cord. An electronic microstepper (Kopf, Tujunga, CA) guided the microelectrodes into position at a 70–80° angle to avoid dimpling the cord. The spinal cord was searched from the midline to approximately 2.5 mm lateral and from the surface to a 1.7 mm depth. Extracellular action potentials were amplified (Dagan, Minneapolis MN) and displayed on an oscilloscope (Tektronix Inc. Beaverton, OR). Each action potential was isolated in a window discriminator (Frederick Haer & Co. Brunswick, ME), which contained a low frequency filter to reduce background field potentials. The signal was then transmitted to the CED 1401 plus Data Acquisition system.
Each neuron was located and identified by antidromically activating axons from the VLF (Ness and Gebhart, 1987) or the CN (Al-Chaer et al. 1997). A bipolar electrode was inserted into the lateral medulla until the electrode reached the anterior section of the vertebra, then was retracted 1 mm to stimulate the VLF contralateral to the heart; whereas a ball electrode was placed on the dorsal surface of the ipsilateral CN for antidromic activation of thoracic PSDC neurons.
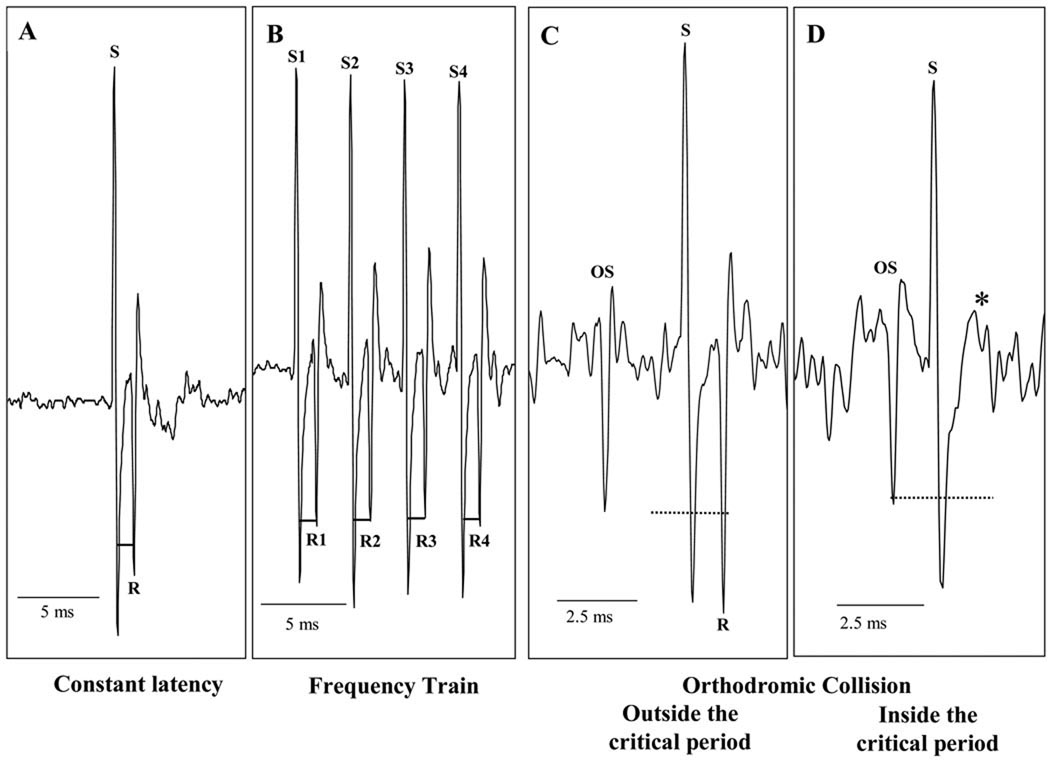
To locate antidromically activated neurons, electrical pulses (0.05–2 mA, 5–10 Hz, 0.1 ms) from the stimulator (WP Instruments, Longmont, CO) were sent to the antidromic electrodes in the VLF and CN simultaneously. The gray matter of the T3–T4 spinal segments was searched with a microelectrode until an extracellular action potential following either of the antidromic artifacts was seen on the oscilloscope. Once an antidromically activated neuron was found, the electrical pulse to the corresponding electrode remained on, while the signal to the other electrode was turned off. To verify that a neuron was antidromically activated, each neuron had to meet three criteria (Lipski, 1981): constant latency following the antidromic artifact, follow a high-frequency (250–333 Hz) train of stimuli and orthodromic-antidromic collision. (Figure 1.)
Figure 1.
Examples of Antidromic Activation Criteria. Each antidromic stimulus (S) must be followed by a neuronal response (R) at a constant latency (‒), whether it is a single stimulus (A) or a train of stimuli (B) (1.35 ms for this particular neuron). The critical interval is two times the constant latency plus the refractory period (- - - - - -). If the orthodromic stimulus (OS) falls outside the critical interval, the antidromic response is seen (C), but if the OS falls inside the critical interval, the response is not seen (D, *).
Neurons that were identified as antidromically activated were classified as LT, WDR, or HT by recording their responses to somatic receptive field manipulations. LT neurons responded to innocuous brushing of the somatic field, but had no response to noxious pinch. WDR neurons responded to innocuous stimuli, but were maximally excited by noxious pinch of the skin and underlying muscle. Neurons that only responded to noxious pinch were classified as HT neurons (Ammons et al. 1985).
To activate the cardiac afferent fibers on the surface of the heart, either mechanical or chemical intrapericardial stimuli were used. Each stimulus solution was injected into the pericardial sac for 2 minutes. Intrapericardial administration of 1.0 ml saline was used as the mechanical stimulus; whereas, the noxious chemical stimulus consisted of an intrapericardial injection of 0.2 ml of algogenic chemicals (10−3 M adenosine, 10−5 M each of bradykinin, histamine, serotonin, prostaglandin E2; Euchner-Wamser et al., 1994). The control stimulus was an intrapericardial injection of 0.2 ml saline.
All recordings were analyzed with the Spike 2 data acquisition system. The criteria to determine if a neuron responded to a stimulus were as follows: If spontaneous activity was greater than or equal to 5.0 Hz, then the maximal change in activity had to be at least 20% greater than the spontaneous activity to be considered a response. If the spontaneous activity was less than 5.0 Hz, then the maximal change in activity had to be 1.0 Hz greater than spontaneous activity to be considered a response. The rate of responses (impulses/second) was calculated by subtracting the mean of 10 s of spontaneous activity recorded 20 s before the beginning of the stimulus from the mean of 10 s at the point of greatest neuronal activity during stimulation. Latency, time to peak and time to recovery were also measured and reported. Latency is the time between the injection of stimulus and the onset of response. The time to peak is the time from the onset of the response to the maximal response; while the time to recovery is the time from the maximal response until the neuronal activity returns to baseline. Data are presented as mean ± standard error (SE). Statistical comparisons were made using the Fisher’s exact test or Student’s t test. Differences were considered statistically significant at p< 0.05.
4.2 Immunohistochemical Experiments
C-fos expression in the T3–T4 spinal segments of rats following noxious cardiac stimulation were compared with naïve rats. Previous studies have shown that PSDC neurons in the lumbosacral spinal cord retrogradely labeled with a fluorescent tracer express c-fos after noxious pelvic visceral stimulation (Palecek, 2003). Thus, similarly retrogradely labeled PSDC neurons in the lumbosacral spinal cord of rats stimulated by CRD served as positive controls.
Male Sprague Dawley rats (250–500g, Charles Rivers) were anesthetized with sodium pentobarbital (65 mg/kg i.p.) and the head attached to a Kopf stereotaxic frame with the head ventroflexed. The surgical site was sterilized and the body temperature was maintained at 37 ± 1°C with a thermostatically controlled heating pad and a rectal thermocouple probe feedback sensor.
A craniotomy was done to directly visualize the DCN. A glass micropipette (25 µm tip diameter) affixed to a 1 µl Hamilton syringe was inserted into a micromanipulator, connected to the stereotaxic frame and positioned over the DCN. In 17 animals, FluoroRuby (FR) fluorescent tracer (dextran tetramethylrhodamine, 10,000MW, Molecular Probes, Eugene, OR) was injected into the CN. FluoroEmerald (FE) fluorescent tracer (dextran fluorescein, 10,000MW, Molecular Probes, Eugene, OR) was injected into the GN to trace PSDC neurons below T6 in 2 positive controls and into the VPL to trace T3–T4 STT neurons in 2 additional controls. Approximately 6–8 separate injections of 0.25 µl fluorescent tracer were made into the nuclei from the caudal to the rostral extent of the nucleus. Five minutes after each injection, the syringe was moved to the next injection site. Histological verification of the injection sites was done after the experiments.
Antibacterial cream was applied over the brainstem and the incision was closed with suture. After the rat recovered from anesthesia, it was returned to its home cage in the animal facility. Acetaminophen (1–2mg/ ml) was added to the drinking water for 7 days for post-surgical pain relief. The rats were allowed to recover for approximately 14 days to allow transport of the tracer.
After the recovery period, the rat was anesthetized with sodium pentobarbital (65mg/kg i.p.). Pupil diameter and tail pinch response was monitored to determine anesthesia level. During the experiment, the rat was given additional injections of sodium pentobarbital as needed to maintain the appropriate level of anesthesia. A thermostatically controlled heating pad with a rectal thermocouple probe feedback sensor was used to maintain rectal temperature at 37± 1°C. A tracheotomy was performed and the animal was artificially ventilated using a positive pressure pump with room air at a tidal volume of 2.5–5.0 ml/ stroke at 55–60 strokes/ min.
A midline thoracotomy was performed to administer the cardiac stimuli as described in the above section. The rats were randomly divided into three groups: control, mechanical stimulus or chemical stimulus of the heart. Saline (0.2 ml as an innocuous control or 1 ml as the mechanical stimulus) or algesic chemicals (0.2 ml as the noxious chemical stimulus: 10−3 M adenosine, 10−5 M each of bradykinin, histamine, serotonin, prostaglandin E2, Euchner-Wamser et al., 1994) was injected into the pericardial sac for 2 min then alternated with an innocuous 0.2 ml saline injection for 2 min, three times before a 10 minute rest. This series was repeated twice more before the animal rested for an additional hour to allow for optimal activation of the c-Fos protein (Luo et al., 1998). Lastly, the animal was euthanized by giving it an overdose of sodium pentobarbitol (200mg/kg). The rats were transcardially perfused using a pump which flushed 100 ml saline followed by 400 ml of 4% paraformaldehyde (in 0.1M Sorenson’s phosphate buffer, pH 7.4).
In 2 of the positive control rats, the noxious visceral stimulation was CRD. The rats were anesthetized with sodium pentobarbital (65 mg/kg i.p.) and a tracheotomy was performed as described in the experimental rats. A 4–5 cm latex balloon was inserted intraanally into the rectum and descending colon of the rat. The balloon was inflated to a constant pressure 80 mmHg for CRD and monitored continuously via an inline pressure transducer and sphygmomanometer. The colon was distended to 80 mmHg (30 sec on, 90 sec off) for 1 h then the rat rested for 1 h, for a total of 2 h before perfusion of the animal.
The T3–T4 spinal segments of the CN experimental and the STT positive control animals and the L6-S2 segments of the GN positive control animals and the brain were removed and postfixed in the same fixative overnight at 4°C. The spinal segments were then placed into 20% sucrose (in 0.2 M Sorenson’s phosphate buffer, pH 7.2) at 4°C (2–3 nights). Frozen sections (40 µm thickness) were cut on a sliding microtome and collected in centrifuge tubes of phosphate buffered saline (PBS, pH 7.4). Sections were divided into 4 parallel series and stored at 4°C. One series (every 4th section) was stained free-floating for the immunohistochemical detection of the c-fos. The sections were rinsed in PBS (3 times, 5 min) at room temperature (RT) before being treated with hydrogen peroxide (3% H2O2 in PBS) for 5 min. Non specific staining was blocked with Avidin blocking solution (1 hour RT, Vector Laboratories) in PBS with Trition X-100 (PBS-TX, 0.3%) plus milk (3%) and normal goat serum (NGS) and then with Biotin blocking solution (1 hour RT, Vector Laboratories) in PBS-TX. The sections were incubated for 2 nights at 4°C in rabbit anti-c-fos primary antibody (1:6,000, K-25 rabbit polyclonal IgG, Santa Cruz Biotechnology, Inc.) in PBS-TX, NGS and sodium azide. Following 3 rinses in PBS-TX (10 min, RT), the sections were incubated in biotinylated goat anti-rabbit secondary antibody (1:800; BA1000, Vector Laboratories) in PBS-TX plus milk and NGS for 1 h RT. The tissue was rinsed (3 times, 10 min RT), once in PBS, and then twice in 0.1M phosphate buffer (PB, pH 7.4), before it was incubated in Avidin-Biotin Complex (Vectastain ABC kit, Peroxidase Std, Vector Laboratories, per kit instructions) for 1 h RT. The sections were rinsed in PB (3 times, 10 min RT). The sections were incubated in the chromogen, DAB (0.025%, 3, 3’ diaminobenzidine tetrahydrochloride in PB and 50 µl 0.03% H2O2) for 15 min RT, followed by 1 rinse in PBS for 5 min. Finally, the sections were mounted onto microscope slides with Vectashield (Vector Laboratories) and coverslipped for analysis.
Each section was examined under epifluorescence (Olympic microsope, Model #CX41 and #CX-RFL-2, Olympus America Inc., and X-Cite 120 Fluorescence Illumination System, EXFO Bruleigh Products Group) for retrogradely labeled PSDC neurons, using the DMG-2 cube (#31002, Chroma Technology Corp) for neurons projecting from the CN and DMB-2 cube (#31001, Chroma Technology Corp) for the neurons from the GN. The fluoresced neurons were then evaluated for c-fos immunoreactivity under brightlight microscopy. Neurons were considered double-labeled if they fluoresced red (FluroRuby) or green (FluroEmerald) and contained a brown DAB-stained nucleus (c-fos). The number of PSDC, c-fos-IR and PSDC-c-fos IR neurons were counted in 8 sections per spinal segment of each animal and averaged. Data are presented as the mean number of neurons per animal. Photomicrographs were digitized with the Insight Firewire camera (Model #11.2 Color Mosaic, Diagnostic Instruments, Inc) and saved as jpeg files using SPOT Software (4.1 Diagnostic Instruments, Inc.).
The expression of c-fos in the T3–T4 spinal segments of rats following mechanical and noxious chemical cardiac stimulation were compared with the expression observed in the same spinal segments of saline control rats. Since STT neurons are known to transmit cardiac pain, T3–T4 STT neurons from rats intrapericardially injected with algogenic chemicals were also used as positive controls.
Acknowledgements
We thank D. Holston for her expert technical assistance. This research was supported by NIH grant NS35471.
Abbreviations
- c-fos-IR
c-fos immunoreactive
- CN
cuneate nucleus
- CPSA
cardiopulmonary sympathetic afferents
- CRD
colorectal distension
- DC
dorsal column
- DCN
dorsal column nuclei
- FE
fluoro-emerald
- FR
fluoro-ruby
- GN
gracile nucleus
- HT
high threshold
- LT
low threshold
- PSDC
postsynaptic dorsal column
- STT
spinothalamic tract
- VLF
ventrolateral funiculus
- VPL
ventroposterolateral thalamus
- WDR
wide dynamic range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Albutaihi IA, DeJongste MJ, Ter Horst GJ. An integrated study of heart pain and behavior in freely moving rats (using fos as a marker for neuronal activation) Neurosignals. 2004;13:207–226. doi: 10.1159/000079336. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Pelvic visceral input into the nucleus gracilis is largely mediated by the postsynaptic dorsal column pathway. J Neurophysiol. 1996a;76:2675–2690. doi: 10.1152/jn.1996.76.4.2675. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J Neurophysiol. 1996b;76:2661–2674. doi: 10.1152/jn.1996.76.4.2661. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Westlund KN, Willis WD. Nucleus gracilis: an integrator for visceral and somatic information. J Neurophysiol. 1997a;78:521–527. doi: 10.1152/jn.1997.78.1.521. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Westlund KN, Willis WD. Sensitization of postsynaptic dorsal column neuronal responses by colon inflammation. Neuroreport. 1997b;8:3267–3273. doi: 10.1097/00001756-199710200-00016. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Feng Y, Willis WD. A role for the dorsal column in nociceptive visceral input into the thalamus of primates. J Neurophysiol. 1998;79:3143–3150. doi: 10.1152/jn.1998.79.6.3143. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Feng Y, Willis WD. Comparative study of viscerosomatic input onto postsynaptic dorsal column and spinothalamic tract neurons in the primate. J Neurophysiol. 1999;82:1876–1882. doi: 10.1152/jn.1999.82.4.1876. [DOI] [PubMed] [Google Scholar]
- Amassian VE. Fiber groups and spinal pathways of cortically represented visceral afferents. J Neurophysiol. 1951;14:445–460. doi: 10.1152/jn.1951.14.6.445. [DOI] [PubMed] [Google Scholar]
- Ammons WS, Girardot MN, Foreman RD. Effects of intracardiac bradykinin on T2–T5 medial spinothalamic cells. Am J Physiol. 1985a;249:R147–R152. doi: 10.1152/ajpregu.1985.249.2.R147. [DOI] [PubMed] [Google Scholar]
- Ammons WS, Girardot MN, Foreman RD. T2–T5 spinothalamic neurons projecting to medial thalamus with viscerosomatic input. J Neurophysiol. 1985b;54:73–89. doi: 10.1152/jn.1985.54.1.73. [DOI] [PubMed] [Google Scholar]
- Baker DG, Coleridge HM, Coleridge JC, Nerdrum T. Search for a cardiac nociceptor: stimulation by bradykinin of sympathetic afferent nerve endings in the heart of the cat. J Physiol. 1980;306:519–536. doi: 10.1113/jphysiol.1980.sp013412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R, Sure U, Bertalanffy H. Punctate midline myelotomy. A new approach in the management of visceral pain. Acta Neurochir (Wien) 1999;141:881–883. doi: 10.1007/s007010050390. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Hubscher CH. Are there separate central nervous system pathways for touch and pain? Nature Medicine. 1995;1:766–773. doi: 10.1038/nm0895-766. [DOI] [PubMed] [Google Scholar]
- Blair RW, Weber RN, Foreman RD. Characteristics of primate spinothalamic tract neurons receiving viscerosomatic convergent inputs in T3–T5 segments. J Neurophysiol. 1981;46:797–811. doi: 10.1152/jn.1981.46.4.797. [DOI] [PubMed] [Google Scholar]
- Blair RW, Weber RN, Foreman RD. Responses of thoracic spinothalamic neurons to intracardiac injection of bradykinin in the monkey. Circ Res. 1982;51:83–94. doi: 10.1161/01.res.51.1.83. [DOI] [PubMed] [Google Scholar]
- Brown AG, Fyffe RE, Noble R, Rose PK, Snow PJ. The density, distribution and topographical organization of spinocervical tract neurones in the cat. J Physiol. 1980;300:409–428. doi: 10.1113/jphysiol.1980.sp013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Dado RJ, Giesler GJ., Jr The cells of origin of the spinothalamic tract of the rat: a quantitative reexamination. Brain Res. 1990;511:329–337. doi: 10.1016/0006-8993(90)90179-f. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Saslow SB, Bharucha AE. Gastrointestinal sensation. Mechanisms and relation to functional gastrointestinal disorders. Gastroenterol Clin North Am. 1996;25:247–258. doi: 10.1016/s0889-8553(05)70374-5. [DOI] [PubMed] [Google Scholar]
- Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev. 1994;74:95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- Chandler MJ, Hobbs SF, Bolser DC, Foreman RD. Effects of vagal afferent stimulation on cervical spinothalamic tract neurons in monkeys. Pain. 1991;44:81–87. doi: 10.1016/0304-3959(91)90152-N. [DOI] [PubMed] [Google Scholar]
- Chandler MJ, Zhang J, Foreman RD. Cardiopulmonary sympathetic input excites primate cuneothalamic neurons: comparison with spinothalamic tract neurons. J Neurophysiol. 1998;80:628–637. doi: 10.1152/jn.1998.80.2.628. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Hypersensitivity of spinothalamic tract neurons associated with diabetic neuropathic pain in rats. J Neurophysiol. 2002;87:2726–2733. doi: 10.1152/jn.2002.87.6.2726. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Giesler GJ., Jr Postsynaptic dorsal column pathway of the rat. III. Distribution of ascending afferent fibers. J Neurosci. 1989;9:3146–3168. doi: 10.1523/JNEUROSCI.09-09-03146.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A, Migani P, Poli A, Villani L. Recent advances in the use of selective neuron-destroying agents for neurobiological research. Experientia. 1984;40:524–534. doi: 10.1007/BF01982314. [DOI] [PubMed] [Google Scholar]
- Craig AD., Jr Spinal and medullary input to the lateral cervical nucleus. J Comp Neurol. 1978;181:729–743. doi: 10.1002/cne.901810404. [DOI] [PubMed] [Google Scholar]
- Crea F, Gaspardone A. Cardiac ischemic pain. Heart and Metabolism. 2002;16:5–8. [Google Scholar]
- Euchner-Wamser I, Meller ST, Gebhart GF. A model of cardiac nociception in chronically instrumented rats: behavioral and electrophysiological effects of pericardial administration of algogenic substances. Pain. 1994;58:117–128. doi: 10.1016/0304-3959(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Feng Y, Cui M, Al-Chaer ED, Willis WD. Epigastric antinociception by cervical dorsal column lesions in rats. Anesthesiology. 1998;89(2):411–420. doi: 10.1097/00000542-199808000-00018. [DOI] [PubMed] [Google Scholar]
- Foreman RD, Blair RW, Weber RN. Viscerosomatic convergence onto T2–T4 spinoreticular, spinoreticular-spinothalamic, and spinothalamic tract neurons in the cat. Exp Neurol. 1984;85:597–619. doi: 10.1016/0014-4886(84)90034-7. [DOI] [PubMed] [Google Scholar]
- Foreman RD. Spinal substrates of visceral pain. In: T.L. Yaksh TL, editor. Spinal Afferent Processing. New York, NY: 1986. pp. 217–242. [Google Scholar]
- Foreman RD. Organization of the Spinothalamic Tract as a Relay for Cardiopulmonary Sympathetic Afferent Fiber Activity. In: Ottoson D, editor. Progress in Sensory Physiology. Vol 9. Springer, Berlin: 1989. pp. 1–51. [Google Scholar]
- Foreman RD. Visceral Pain: Keeping the Pathways in Equilibrium. Pain Forum. 1998;7:137–140. [Google Scholar]
- Foreman RD. The physiology of pain perception in angina pectoris. Heart and Metabolism. 2002;16:30–35. [Google Scholar]
- Giesler GJ, Jr, Nahin RL, Madsen AM. Postsynaptic dorsal column pathway of the rat. I. Anatomical studies. J Neurophysiol. 1984;51:260–275. doi: 10.1152/jn.1984.51.2.260. [DOI] [PubMed] [Google Scholar]
- Giesler GJ, Jr, Cliffer KD. Postsynaptic dorsal column pathway of the rat. II. Evidence against an important role in nociception. Brain Res. 1985;326:347–356. doi: 10.1016/0006-8993(85)90044-7. [DOI] [PubMed] [Google Scholar]
- Goodman MD, Foreman RD. Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2007; 2007. Lack of involvement of the postsynaptic dorsal column pathway in cardiac nociception. Program No. 724.23/LL18. 2007. Online. [Google Scholar]
- Goodman MD, Qin C, Thompson AM, Foreman RD. Postsynaptic dorsal column neurons and cFos colocalization following noxious cardiac stimulation. Pain Practice. 2009;9:22. [Google Scholar]
- Hirshberg RM, Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Is there a pathway in the posterior funiculus that signals visceral pain? Pain. 1996;67:291–305. doi: 10.1016/0304-3959(96)03127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs SF, Oh UT, Chandler MJ, Foreman RD. Cardiac and abdominal vagal afferent inhibition of primate T9-S1 spinothalamic cells. Am J Physiol. 1989;257:R889–R895. doi: 10.1152/ajpregu.1989.257.4.R889. [DOI] [PubMed] [Google Scholar]
- Houghton AK, Kadura S, Westlund KN. Dorsal column lesions reverse the reduction of homecage activity in rats with pancreatitis. Neuroreport. 1997;8:3795–3800. doi: 10.1097/00001756-199712010-00028. [DOI] [PubMed] [Google Scholar]
- Houghton AK, Wang CC, Westlund KN. Do nociceptive signals from the pancreas travel in the dorsal column? Pain. 2001;89:207–220. doi: 10.1016/s0304-3959(00)00364-x. [DOI] [PubMed] [Google Scholar]
- Hua F, Harrison T, Qin C, Reifsteck A, Ricketts B, Carnel C, Williams CA. c-fos expression in rat brain stem and spinal cord in response to activation of cardiac ischemia-sensitive afferent neurons and electrostimulatory modulation. Am J Physiol Heart Circ Physiol. 2004;287:H2728–H2738. doi: 10.1152/ajpheart.00180.2004. [DOI] [PubMed] [Google Scholar]
- Katter JT, Dado RJ, Kostarczyk E, Giesler GJ., Jr Spinothalamic and spinohypothalamic tract neurons in the sacral spinal cord of rats. II. Responses to cutaneous and visceral stimuli. J Neurophysiol. 1996;75:2606–2628. doi: 10.1152/jn.1996.75.6.2606. [DOI] [PubMed] [Google Scholar]
- Kostreva DR, Pontus SP. Pericardial mechanoreceptors with phrenic afferents. Am J Physiol. 1993;264:H1836–H1846. doi: 10.1152/ajpheart.1993.264.6.H1836. [DOI] [PubMed] [Google Scholar]
- Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods. 1981;4:1–32. doi: 10.1016/0165-0270(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Lombardi F, Bella Della P, Casati R, Malliani A. Effects of intracoronary administration of bradykinin on the impulse activity of afferent sympathetic unmyelinated fibers with left ventricular endings in the cat. Circ Res. 1981;48:69–75. doi: 10.1161/01.res.48.1.69. [DOI] [PubMed] [Google Scholar]
- Lu GW, Bennett GJ, Nishikawa N, Hoffert MJ, Dubner R. Extra- and intracellular recordings from dorsal column postsynaptic spinomedullary neurons in the cat. Exp Neurol. 1983;82:456–477. doi: 10.1016/0014-4886(83)90417-x. [DOI] [PubMed] [Google Scholar]
- Lu GW, Yang CT. The morphology of cat spinal neurons projecting to both the lateral cervical nucleus and the dorsal column nuclei. Neurosci Lett. 1989;101:29–34. doi: 10.1016/0304-3940(89)90435-7. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Madsen AM, Giesler GJ., Jr Anatomical and physiological studies of the gray matter surrounding the spinal cord central canal. J Comp Neurol 1;2. 1983;220:321–335. doi: 10.1002/cne.902200306. [DOI] [PubMed] [Google Scholar]
- Nauta HJ, Hewitt E, Westlund KN, Willis WD., Jr Surgical interruption of a midline dorsal column visceral pain pathway. Case report and review of the literature. J Neurosurg. 1997;86:538–542. doi: 10.3171/jns.1997.86.3.0538. [DOI] [PubMed] [Google Scholar]
- Nerdrum T, Baker DG, Coleridge HM, Coleridge JC. Interaction of bradykinin and prostaglandin E1 on cardiac pressor reflex and sympathetic afferents. Am J Physiol. 1986;250:R815–R822. doi: 10.1152/ajpregu.1986.250.5.R815. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat. J Neurophysiol. 1987;57:1867–1892. doi: 10.1152/jn.1987.57.6.1867. [DOI] [PubMed] [Google Scholar]
- Ness TJ. Evidence for ascending visceral nociceptive information in the dorsal midline and lateral spinal cord. Pain. 2000;87:83–88. doi: 10.1016/S0304-3959(00)00272-4. [DOI] [PubMed] [Google Scholar]
- Palecek J, Paleckova V, Willis WD. The roles of pathways in the spinal cord lateral and dorsal funiculi in signaling nociceptive somatic and visceral stimuli in rats. Pain. 2002;96:297–307. doi: 10.1016/S0304-3959(01)00459-6. [DOI] [PubMed] [Google Scholar]
- Palecek J, Paleckova V, Willis WD. Fos expression in spinothalamic and postsynaptic dorsal column neurons following noxious visceral and cutaneous stimuli. Pain. 2003;104:249–257. doi: 10.1016/s0304-3959(03)00013-7. [DOI] [PubMed] [Google Scholar]
- Palecek J, Willis WD. The dorsal column pathway facilitates visceromotor responses to colorectal distention after colon inflammation in rats. Pain. 2003;104:501–507. doi: 10.1016/S0304-3959(03)00075-7. [DOI] [PubMed] [Google Scholar]
- Qin C, Kranenburg A, Foreman RD. Descending modulation of thoracic visceroreceptive transmission by C1–C2 spinal neurons. Auton Neurosci. 2004;114:11–16. doi: 10.1016/j.autneu.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Hokfelt T, Fuxe K, Jonsson G, Goldstein M, Terenius L. Ibotenic acid-induced neuronal degeneration: a morphological and neurochemical study. Exp Brain Res. 1979;37:199–216. doi: 10.1007/BF00237708. [DOI] [PubMed] [Google Scholar]
- Tracey DJ. Ascending and Descending Pathways in the Spinal Cord. 2nd Edition. 1995. pp. 67–75. [Google Scholar]
- Wang CC, Willis WD, Westlund KN. Ascending projections from the area around the spinal cord central canal: A Phaseolus vulgaris leucoagglutinin study in rats. J Comp Neurol. 1999;415:341–367. doi: 10.1002/(sici)1096-9861(19991220)415:3<341::aid-cne3>3.0.co;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Westlund KN. Responses of rat dorsal column neurons to pancreatic nociceptive stimulation. Neuroreport. 2001;12:2527–2530. doi: 10.1097/00001756-200108080-00047. [DOI] [PubMed] [Google Scholar]
- White JC. Sensory Innervation of the Viscera: Studies on Visceral Afferent Neurones in Man Based on Neurosurgical Procedures for the Relief of Intractable Pain. Res. Publ. Assoc. Nerv. Ment. Dis. 1943;23:373–390. [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner MR, Li DP, Chen SR, Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol. 2003;551:515–523. doi: 10.1113/jphysiol.2003.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chandler MJ, Miller KE, Foreman RD. Cardiopulmonary sympathetic afferent input does not require dorsal column pathways to excite C1–C3 spinal cells in rats. Brain Res. 1997;771:25–30. doi: 10.1016/s0006-8993(97)00607-0. [DOI] [PubMed] [Google Scholar]