Abstract
Cytocompatible, co-doped cerium oxide nanoparticles exhibited strong upconversion properties that were found to kill lung cancer cells by inducing apoptosis thereby demonstrating the potential to be used as clinical contrast agents for imaging and as therapeutic agents for treatment of cancer.
Understanding the complex interactions of nanomaterials at the cellular level is required for proper designing of the nanoparticles for therapeutics. Fluorescent labeling is commonly used to study these interactions. The fluorophores commonly suffer from auto-fluorescence interference from biological tissues, photobleaching, low signal-to-noise ratio, potential damage to DNA and cell death.1,2 Though semiconductor quantum dots exhibit tunable size-dependent emission, some of the bare quantum dots possess intrinsic limitations such as toxicity and chemical instability. Therefore, developing nanoparticles of low toxicity with imaging as well as therapeutic properties is a key challenge in nanomedicine. Upconversion phosphors (UCPs), capable of converting near-infrared (NIR) radiation into shorter wavelengths through a multi-photon process, offer an alternative with minimal photo damage and auto-fluorescence due to the noninvasive nature of light.3 Since upconversion occurs within the host crystal and is therefore less affected by the chemical and biological environments, it allows synthesis of materials without the loss of surface chemical reactivity.
Metal fluorides, oxysulfides and phosphates are the host lattices widely used to study the upconversion process3–9 while only a few reports are available on oxide matrices.10,11 Among the metal oxide, rare earth oxides, in particular cerium oxide nanoparticles or ceria (CNPs)12,13 and europium hydroxide nanorods,14,15 have been shown to be biocompatible. Beside biocompatibility CNPs are also reported to exhibit unique regenerative antioxidant properties.16–18 In this study, we synthesized rare earth co-doped CNPs with a sensitizer (Yb3+) and an emitter (primarily Er3+) having near-infrared (NIR)-to-visible upconversion fluorescence. To demonstrate the tunability of emission wavelength, the dopant chemistry was changed to Ho3+ or Tm3+. Briefly, ammonia solution was added to aqueous nitrate solutions of Ce3+ (0.1 M), Yb3+ (20%) and Er3+ (2%), to maintain the pH above 10. The resultant upconversion nano cerium oxide (referred as UNC, hereafter) precipitate was washed, annealed at 900 °C for 2 h and filtered through 100 nm membrane filter.19 A similar procedure was used to synthesize CeO2 : 20%Yb,2%Ho and CeO2 : 20Yb%,0.5%Tm. To evaluate the size and morphology high resolution transmission electron microscopy (HRTEM) studies were carried out with FEI Tecnai F30. Powder X-ray diffraction (XRD) was performed with monochromatized CuKα radiation.
UNCs have a primary particle size of about 20 nm along (see supporting information, Fig. 1S†) with a few particles in the size range of 40 nm due to the interfusion to form larger agglomerated particles. The presence of Yb and Er in cerium matrix was confirmed using Energy Dispersive X-ray analysis in TEM (see supporting information, Fig. 2S†).
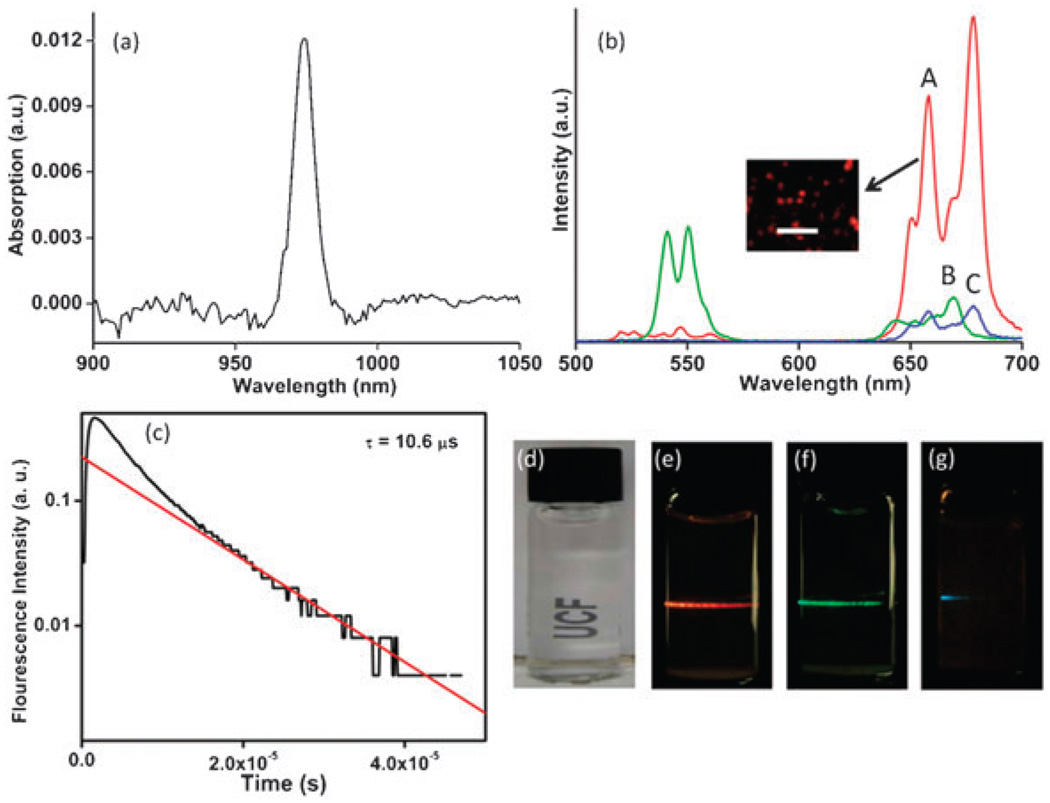
Fig. 1 shows the absorption spectrum for UNC indicating the absorption feature at 975 nm (Fig. 1a) and emission spectra of UNC along with CeO2 : 20%Yb,2%Ho and CeO2 : 20%Yb,0.5%Tm (Fig. 1b and CeO2 : 20%Yb,0.5%Tm alone shown in supporting information, Fig. 3S). The transition 4F9/2 → 4I5/2 leads to a red emission in UNC with a small amount of green light (around 540 nm 4S3/2 → 4I5/2 transition) due to Er3+. On co-doping with Ho3+ green emission appears as a result of 5F4, 5S2 → 5I8 transition along with weak red light. Tm3+ co-doping results in weak blue emission (1G4 → 3H6) due to lower probability for multi photon transitions.20 UNC powders when excited by 975 nm light from the diode laser could be easily seen to emit red light (Fig. 1b inset). In earlier studies using fluoride hosts, the decay dynamics indicate a fluorescent lifetime of about 3 ms compared to 10.6 µs for UNC (Fig. 1c).21 The very short emission time observed in the present work indicates the presence of significant fluorescence quenching. Since oxide matrices have higher phonon energy than the fluorides this strong quenching is not surprising. In spite of quenching, the upconversion emission intensity is sufficient to utilize the water dispersible UNCs as potential biomarkers (Fig. 1d–g). Thus, the emission color can be tuned from red, green or blue by varying the co-dopant from Er3+, Ho3+ and Tm3+, respectively.
Fig. 1.
(a) The optical absorption spectrum of UNC. (b) The measured emission spectra of samples of (A) UNC, (B) CeO2 : 20 Yb%,2%Ho and (C) CeO2 : 20%Yb,0.5%Tm. The inset demonstrates the red emission from UNC observed under an optical microscope upon NIR excitation. Scale bar in the inset corresponds to 10 mm. (c) The measured emission decay dynamics of the red light emitted from UNC. The solid line is an exponential decay fit with decay time of 10.6 µsec. (d) Nanoparticles readily dispersed in water under visible light. (e–g) On NIR excitation (975 nm), emission wavelengths can be tuned from red, green or blue by varying the co-dopant chemistry from Er3+, Ho3+ or Tm3+, respectively.
As mentioned earlier, CNPs can scavenge free radicals and mimic enzymatic antioxidants such as superoxide dismutase and catalase.17,18 We followed catalase mimetic activity of UNCs by monitoring peroxide levels using UV-visible spectrophotometry. In presence of 100 µM UNC, concentration of hydrogen peroxide reduced with time (see supporting information, Fig. 4S†). From this observation, it is evident that UNCs have redox activity similar to CNPs.
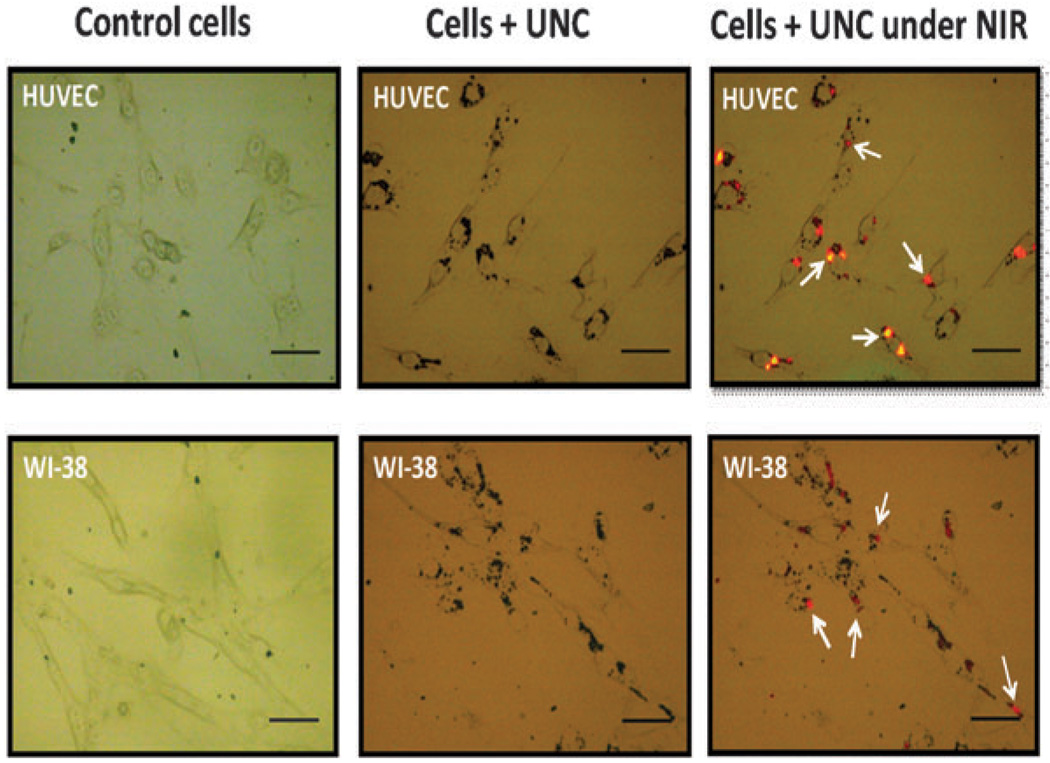
To examine the imaging capabilities of UNCs in a biological model for the first time, we exposed the cells (human umbilical vein endothelial cell; HUVEC and human lung fibroblasts cell line; WI-38) in cultured monolayers to varying doses of UNCs, and observed a concentration-dependent increase in visible light emission. Fig. 2 shows UNC treated cells upon excitation with a 975 nm light with a 4 ns pulse rate, 10 Hz pulse repeat rate and a pump power of 170 mW (detailed protocol in supplementary information†). Although UNCs have a visible emission, the important step towards the biomedical application is to assess the biocompatability. For these studies we chose primary (HUVEC) as well secondary cell line (WI-38). We assessed toxicity by the metabolic reduction of a tetrazolium dye (MTT) and by the release of lactate dehydrogenase (LDH) (see supplementary information for details†).
Fig. 2.
Optical images of HUVEC and WI-38 control cells (without nanoparticles), along with cells incubated with UNCs under ambient light and NIR excitation (scale bar corresponds to 50 µm). Arrowheads indicate the red emission from UNC when cells were excited at a wavelength of 975 nm.
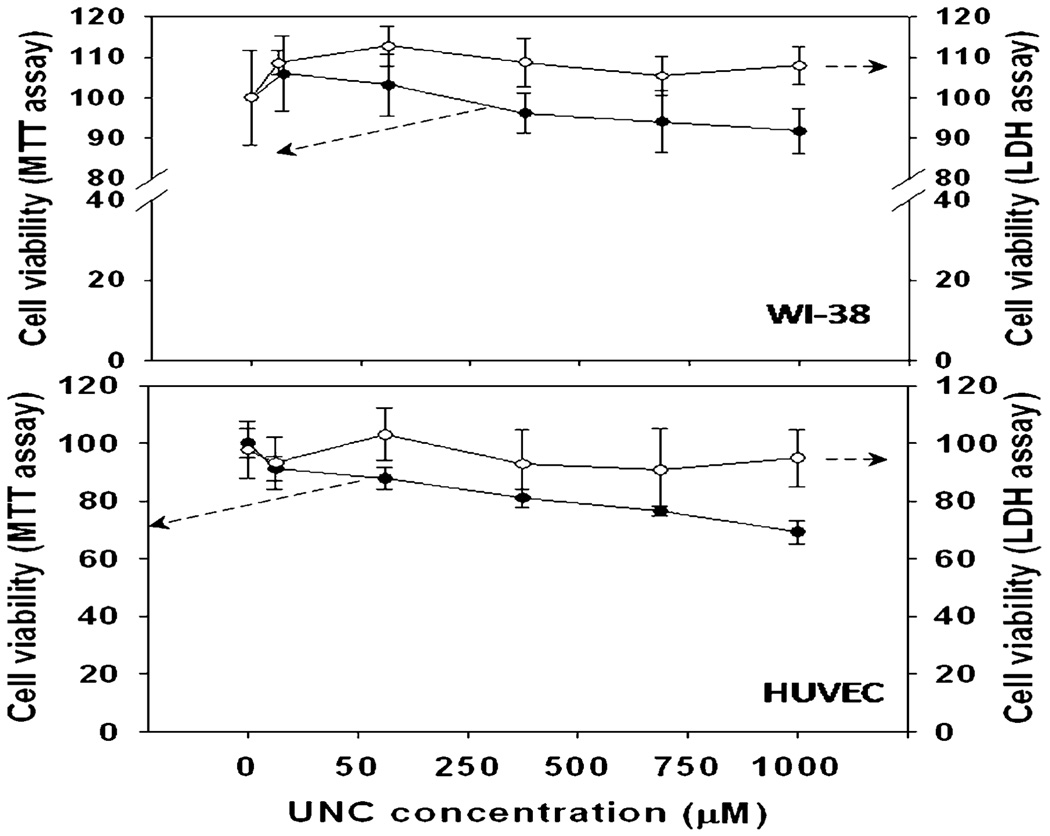
No significant toxicity was observed inWI-38 cells with UNCs (up to 1000 µM). Some toxicity was observed in HUVECs in a dose dependent manner when viability was determined by MTT assay, however no significant release of LDH occurred, suggesting the material caused a decrease in cellular metabolic rate without lysis of the cell membrane (Fig. 3).
Fig. 3.
UNCs are not toxic in cell culture models of WI-38 and HUVEC. Cells were cultured in their standard conditions. MTT dye reduction (●) and LDH release (○) was assessed after 24 h treatment with varying concentrations of UNC. Data represent the mean of at least six cultures and standard deviation is plotted as error.
In order to investigate the therapeutic application of UNC on cancer cells in vitro, lung cancer cells (CRL-5803) were cultured for 24 h and then the media was aspirated and replaced with fresh media containing various nanoparticle concentrations (0, 0.01 nM, 0.1 nM, 1 nM, 10 nM, 100 nM). Post 24 h treatment cell proliferation determined by the Cell Titer-Glo® Luminescent Cell Viability and Proliferation Assay (Promega; Madison, WI) and activation of apoptosis was determined by measuring the activation of caspase-3/7 via the Caspase-Glo® 3/7 Assay (Promega; Madison, WI).
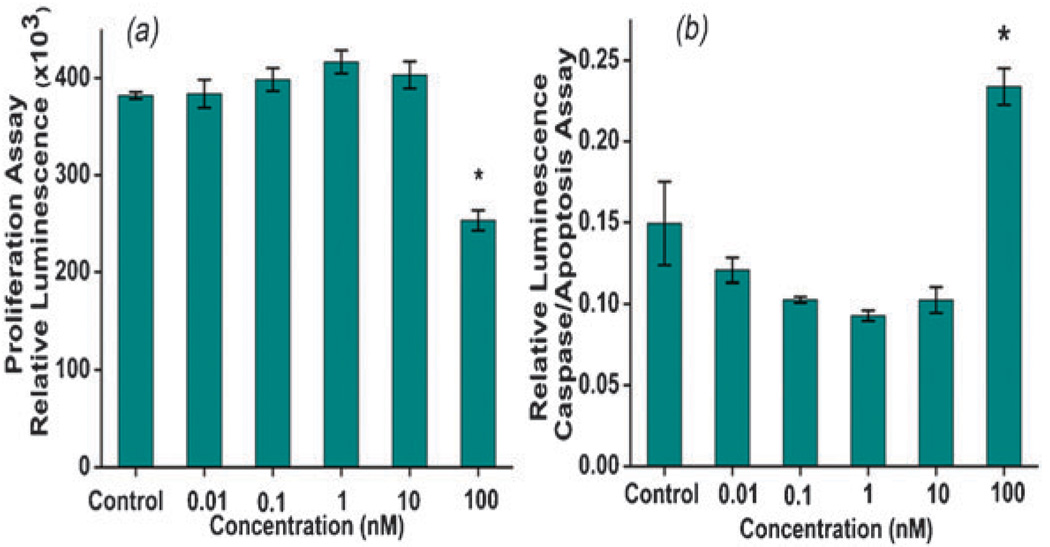
In the case of CRL-5803, proliferation of cancerous lung cells decreased by 33.7% (p < 0.0001) when exposed to 100 nM of UNC (Fig. 4a). To determine if this decrease in cell proliferation and viability was due to an increase in the activation of apoptosis, caspase-3/7 activity was measured. Caspase-3/7 activity increased by 56.6% (p = 0.012) when exposed to 100 nM UNC (Fig. 4b). Therefore, under in vitro cell culture conditions, the cellular interactions of UNCs differ between normal versus tumor cells and can be attributed to the differences in cell metabolism as well as cell structure.22 The mechanism of decrease in cell proliferation and induction of apoptosis in cancerous cells by UNCs is currently under investigation however, redox active CNPs16 may induce apoptosis by producing reactive oxygen species and oxidative stress. Similar studies on TiO2 nanoparticles have shown that they demonstrate cancer killing activity through ROS generation or surface interaction with cells.23,24
Fig. 4.
(a) Cell viability of cancerous lung cells (CRL-5803) and (b) caspase 3/7 activation in cancer cells with respect to UNC concentration, at 100 nM the viability of cancer cells decreased and the data shown are mean ± SD. Significant differences indicated by * with a p value of <0.0001 and 0.012 for a and b, respectively.
In this study, we have demonstrated that co-doped cerium oxide nanoparticles exhibit a unique promising platform for in vivo optical-based diagnostic imaging and therapeutic agents. Nanoparticles are shown to be biocompatible using MTT and LDH assays with both primary and secondary cell lines. UNCs interact in a cell specific manner preventing cancer cell proliferation by inducing apoptosis, which can have a far reaching impact in future cancer research. Use of non toxic upconverter oxide nanoparticles for imaging and therapeutic application is a novel platform technology. Tailoring the size, shape and surface can help in optimizing the clinical dosage and material property and will be discussed in future communications.
Supplementary Material
Acknowledgments
This work was supported by NSF NIRT (0708172 CBET) and NIH R01 (1R01AG031529-01).
Footnotes
Electronic supplementary information (ESI) available: X-ray diffraction of UNCs and Experimental details. See DOI: 10.1039/c0cc01832e
Notes and references
- 1.Green M, Howman E. Chem. Commun. 2005:121–123. doi: 10.1039/b413175d. [DOI] [PubMed] [Google Scholar]
- 2.Riegler J, Nann T. Anal. Bioanal. Chem. 2004:379. doi: 10.1007/s00216-004-2706-y. [DOI] [PubMed] [Google Scholar]
- 3.Auzel F. Chem. Rev. 2004;104:139–173. doi: 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]
- 4.Chen F, Liu Y, Zhang Y, Somesfalean G, Zhang Z, Sun Q, Wang F. Appl. Phys. Lett. 2007;91:133103. [Google Scholar]
- 5.Ehlert O, Thomann R, Darbandi M, Nann T. ACS Nano. 2008;2:120–124. doi: 10.1021/nn7002458. [DOI] [PubMed] [Google Scholar]
- 6.Zijlmans H, Bonnet J, Burton J, Kardos K, Vail T, Niedbala RS, Tanke HJ. Anal. Biochem. 1999;267:30–36. doi: 10.1006/abio.1998.2965. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee DK, Rufaihah AJ, Zhang Y. Biomaterials. 2008;29:937–943. doi: 10.1016/j.biomaterials.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 8.Jalil RA, Zhang Y. Biomaterials. 2008;29:4122–4128. doi: 10.1016/j.biomaterials.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Shan J, Chen J, Meng J, Collins J, Soboyejo W, Friedberg JS, Ju J. J. Appl. Phys. 2008;104:094308. [Google Scholar]
- 10.Kamimura M, Miyamoto D, Saito Y, Soga K, Nagasaki Y. Langmuir. 2008;24:8864–8870. doi: 10.1021/la801056c. [DOI] [PubMed] [Google Scholar]
- 11.Liu YX, Xu CF, Yang QB. J. Appl. Phys. 2009;105:084701. [Google Scholar]
- 12.Thill A, Zeyons O, Spalla O, Chauvat F, Rose J, Auffan M, Flank AM. Environ. Sci. Technol. 2006;40:6151–6156. doi: 10.1021/es060999b. [DOI] [PubMed] [Google Scholar]
- 13.Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H, Yeh J, Zink J, Nel A. ACS Nano. 2008;2:2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patra CR, Abdel Moneim SS, Wang E, Dutta S, Patra S, Eshed M, Mukherjee P, Gedanken A, Shah VH, Mukhopadhyay D. Toxicol. Appl. Pharmacol. 2009;240:88–98. doi: 10.1016/j.taap.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patra CR, Bhattacharya R, Patra S, Vlahakis NE, Gabashvili A, Koltypin Y, Gedanken A, Mukherjee P, Mukhopadhyay D. Adv. Mater. 2008;20:753–756. [Google Scholar]
- 16.Heckert EG, Karakoti AS, Seal S, Self WT. Biomaterials. 2008;29:2705–2709. doi: 10.1016/j.biomaterials.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korsvik C, Patil S, Seal S, Self WT. Chem. Commun. 2007:1056–1058. doi: 10.1039/b615134e. [DOI] [PubMed] [Google Scholar]
- 18.Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS, King JES, Seal S, Self WT. Chem. Commun. 2010;46:2736–2738. doi: 10.1039/b922024k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babu S, Velez A, Wozniak K, Szydlowska J, Seal S. Chem. Phys. Lett. 2007;442:405–408. [Google Scholar]
- 20.Rapaport A, Milliez J, Szipőocs F, Bass M, Cassanho A, Jenssen H. Appl. Opt. 2004;43:6477–6480. doi: 10.1364/ao.43.006477. [DOI] [PubMed] [Google Scholar]
- 21.Rapaport A, Milliez J, Bass M, Cassanho A, Jenssen H. J. Disp. Technol. 2006;2:68–78. [Google Scholar]
- 22.Vincent A, Babu S, Heckert E, Dowding J, Hirst SM, Inerbaev TM, Self WT, Reilly CM, Masunov AE, Rahman TS, Seal S. ACS Nano. 2009;3:1203–1211. doi: 10.1021/nn9000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanley C, Layne J, Punnoose A, Reddy K, Coombs I, Coombs A, Feris K, Wingett D. Nanotechnology. 2008;19:295103–295110. doi: 10.1088/0957-4484/19/29/295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thevenot P, Cho J, Wavhal D, Timmons R, Tang L. Nanomed.: Nanotechnol., Biol. Med. 2008;4:226–236. doi: 10.1016/j.nano.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.