Abstract
Cerium (Ce) is a rare earth metal that is not known to have any biological role. Cerium oxide materials of several sizes and shapes have been developed in recent years as a scaffold for catalysts. Indeed even cerium oxide nanoparticles themselves have displayed catalytic activities and antioxidant properties in tissue culture and animal models. Because of ceria's ability to cycle between the +3 and +4 states at oxygen vacancy sites, we investigated whether cerium metal would catalyze a Fenton-like reaction with hydrogen peroxide. Indeed, cerium chloride did exhibit radical production in the presence of hydrogen peroxide, as assessed by relaxation of supercoiled plasmid DNA. Radical production in this reaction was also followed by production of radical cation of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS). Radical scavengers and spin traps were capable of competing with ABTS for radicals produced in this cerium dependent Fenton-like reaction. Electron paramagnetic resonance experiments reveal both hydroxyl radical and superoxide anion in a reaction containing cerium and hydrogen peroxide. Based on these results we propose that cerium is capable of redox-cycling with peroxide to generate damaging oxygen radicals.
Introduction
Cerium is a rare earth element of the lanthanide series that is being used as a molecular scaffold to build several kinds of nanostructures (1). It is well established that cerium can redox cycle between the 3+ and 4+ states, and this property is currently being manipulated to create efficient catalysts (1–5). Cerium oxide based materials have promise as industrial catalysts removing toxic gases from automobile exhaust among other potential uses (6, 7). Oxygen vacancies located at the surface of ceria nanoparticles are presumed to be the site of catalysis, and allow for redox mechanisms for catalysis as well as reaction with oxygen containing compounds. At this oxygen vacancy site cerium is present as Ce3+, and these nanoparticles are described as CeO(2–x) to emphasize the presence of these oxygen vacancies (7, 8). Indeed, the oxygen storage capabilities of cerium oxide has been shown to be tied directly to the level of oxygen vacancies at the surface of these particles (7). Thus it is clear that cerium oxide nanomaterials are emerging as a key scaffold for novel catalysts with real promise for industrial usage.
Beyond its use as an industrial catalyst, cerium oxide based materials have also shown promise as an antioxidant and radical scavenger in cell culture models as well as animal studies (9–14). Recent work from our laboratory has shown that these particles exhibit superoxide dismutase activity, possibly uncovering the molecular mechanism for the antioxidant properties of cerium oxide observed in culture and animal studies (10). The size of cerium oxide nanoparticles has been shown to affect its uptake and toxicity (15, 16), and that toxicity was at least in part due to oxidative stress (15, 17). Thus a redox active catalyst that might impart a positive radical scavenging property in one system apparently can also be shown to result in oxidative stress when present at higher doses. This catalytic behavior has been suggested to relate directly to the presence of redox active Ce3+ sites at the surface. The balance between benefit or protection and toxicity is likely to be critical to rigorous assessment of the safe use of nanomaterials in the clinic or industry alike.
Iron is a potent redox cycling metal that is well established to react with hydrogen peroxide to generate hydroxyl radicals in a reaction known as the Fenton reaction (18). In addition, copper, cadmium, chromium, mercury, nickel, and vanadium have also been shown to produce reactive radicals in the presence of hydrogen peroxide (19). Chromium has been shown to damage DNA in vitro in a Fenton-like reaction and also to promote mutagenesis in a Salmonella reversion test (20). Vanadate has also been shown to mediate production of hydroxyl radical formation in the presence of peroxides as well (21). Copper, iron, and nickel all have important biological roles in metabolism as catalysts within enzymes, and as such each biological cell must transport and sequester each of these reactive transition metals (19). Although most biological cells can effectively store metals such as iron and copper, any free metal poses an immediate threat to produce oxidative damage to DNA, RNA, and proteins (19). Emerging evidence supports the hypothesis that metal catalyzed oxidative damage is one of the keys to degenerative diseases such as Alzheimer's and Amyotrophic Lateral Sclerosis (22, 23).
Cerium, as a rare earth transition metal, may not be as well managed by cells since it is not known to be required for any biological process. Exposure of humans to materials made from cerium could indeed lead to exposure of cells and tissues to free cerium metal. Given the growing interest in developing ceria-based materials, both as industrial catalysts and as biomedical tools, we probed the ability of cerium as a free metal to react with peroxide in a Fenton like chemical reaction in vitro.
Experimental Section
Materials
Cerium (III) chloride, 4,5-dihydroxy-m-benzene-disulfonic (Tiron) and ethylenediamine tetraacetic acid (EDTA) were from Acros Organics (Geel, Belgium). 6-hydroxy-2,5,7,8-tetramethyl-chroman-2 carboxylic acid (Trolox) was from EMD Biosciences (Darmstadt, Germany). 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid), or ABTS was from Southern Biotech (Birmingham, Alabama). Tris was obtained form MP Biomedicals (Solon, Ohio). The radical spin trap 5-(Diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide (DEP-MPO) was from Axxora, LLC (San Diego, California). All chemicals were the highest grade available.
Plasmid Relaxation Experiments
To detect DNA damage, a supercoiled plasmid (pBR322) was used as a sensitive probe for Fenton-like production of hydroxyl radical. This plasmid was produced in Escherichia coli strain RK4353 and purified by alkaline lysis method using Strataprep EF plasmid kit (Stratagene, La Jolla, California). Reactions with cerium chloride were buffered by Tris (100 μM) at a pH of 7.0. Hydrogen peroxide was added to a final concentration of 88 mM and 1 μg of plasmid was incubated at 37 °C for varying times up to 60 min. To observe changes in DNA mobility due to covalent modifications introduced by radicals, reactions were stopped by addition of excess EDTA (10 mM) and the nicking of supercoiled DNA was resolved by electrophoresis on 0.7% agarose gels in Tris-Acetate-EDTA (TAE) buffer as previously described (20). Relaxation of the supercoiled plasmid to a nicked form results in slower migration of DNA and this direct damage can be assessed by agarose gel electrophoresis. DNA was visualized by soaking in TAE buffer containing 1 mg/100 mL ethidium bromide, followed by UV transillumination and imaging using an Alphaimager 2200 (Alpha Innotech Corporation, San Leandro, California).
Radical Production and Scavenging Experiments
ABTS was used to follow production of hydroxyl radical as previously described (23). ABTS is converted to a radical cation (ABTS+·) upon reaction with hydroxyl radical, and thus represents a useful tool to follow the kinetics of radical production conveniently by UV-visible spectroscopy (24). A previous study using pulse radiolysis has established this chromagen as a sensitive probe and the kinetics of its reaction with hydroxyl radical (24). The production of ABTS+· was followed at 420 nm in a 1 cm path length quartz cuvette at 25 °C using a Agilent 8453 UV-visible spectrophotometer (Santa Clara, California). Reactions were buffered by Tris (100 μM), pH 7.0, and UV-visible spectra (200-900 nm) were recorded once every second for 10 min. The conditions were identical to those used for the plasmid relaxation experiments. Various concentrations of cerium chloride were tested in the presence of 88 mM H2O2 and 100 μM ABTS.
Radical scavenging activity of several antioxidants was determined using a competition assay with ABTS as previously described (25). Each of these scavengers (Tiron, Trolox) or the spin trapping agent 5-(Diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide (DEPMPO), was added prior to addition of cerium chloride. Reactions were begun by the addition of cerium chloride. No ABTS+· was formed by addition of hydrogen peroxide, cerium chloride or scavengers alone. ABTS was added to a final concentration of 100 μM and UV-visible spectra were recorded each second for 10 minutes at 25 °C. The increase in absorbance at 420 nm is plotted to follow production of ABTS+·. Data presented is a representative experiment from at least three independent reactions.
Spin Trapping Experiments to Determine the Type of Oxyradicals Produced from Cerium and Hydroperoxides Using Electron Paramagnetic Resonance
All EPR samples were prepared at room temperature and loaded into capillary quartz tubes immediately after mixing of reagents. Each sample was incubated for 45 min at room temperature after the addition of cerium or hydrogen peroxide. DTPA was added (final concentration 50 μM) to confirm that trace metals other than cerium were not contributing to radicals generated. X-band EPR spectra were recorded on a Bruker Elexsys E580 EPR spectrometer. All spectra were collected in perpendicular mode using a Bruker model ER 4122SHQE superhigh Q resonator. Spectral manipulations were performed with the open-source software “xmgrace”. Spectral simulations were performed with the “easyspin” toolbox for MATLAB distributed by Stefan Stoll (26).
Results and Discussion
Cerium Reacts with Hydrogen Peroxide and Induces Damage to DNA
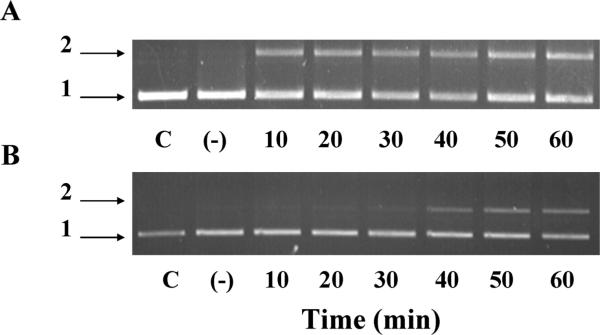
One method commonly used to detect hydroxyl radical formation via Fenton chemistry is through alteration in DNA mobility (strand scission) from a supercoiled form to a nicked form, as used previously in studies of copper-dependent Fenton reactions (27). Fenton chemistry requires the presence of the metal in a reduced state to trigger production of hydroxyl radicals (18). Therefore we tested cerium in the 3+ state to determine whether a Fenton-like reaction would take place with this inner transition metal. In the presence of hydrogen peroxide, addition of cerium chloride (Ce3+ salt, 100 μM) did indeed result in relaxing of supercoiled DNA (Figure 1A). In the absence of added hydrogen peroxide, no DNA damage occurred (data not shown). It is well established that iron can redox cycle in the presence of hydrogen peroxide to produce hydroxyl radical, and this has been extensively used to test the efficacy of radical scavengers (18, 20, 27–30). The DNA damage induced by 100 μM cerium in the presence of peroxide suggests that cerium is producing DNA damage in this experimental system. These results suggest that cerium may be capable of redox cycling in the presence of hydrogen peroxide and exhibit Fenton-like chemistry. Cerium also generated DNA damage when in the presence of tert-butyl hydroperoxide (Figure 1B), indicating that the reactivity with peroxides to generate DNA damaging agents is not specific to hydrogen peroxide. DNA damage was also observed in the presence of DTPA (5 μM), used to effectively chelate low amounts of adventitious metal that could be present in the cerium salt. This would argue against any contaminating metals as being the catalysts for the observed damage.
FIGURE 1.
Plasmid DNA relaxation induced by Fenton-like reaction of cerium with hydrogen peroxide. The migration of supercoiled (form 1) and nicked supercoiled (form 2) plasmid (pBR322) was analyzed upon incubation of cerium with hydrogen peroxide (88 mM) in Tris buffer as described in methods. Aliquots were taken every 10 minutes for 1 h, and the reaction was stopped using 10 mM EDTA. DNA was separated by gel electrophoresis (0.7% agarose gel). C represents control DNA not treated and (–) indicates cerium chloride in the absence of peroxide incubated for 60 min. (A) Cerium chloride (100 μ) or (B) Cerium chloride (20 μM) with tert-butyl hydroperoxide (88 mM). A representative experiment is shown from at least three independent experiments with reproducible results.
Radicals Produced by Cerium and Peroxide Followed by ABTS Radical Cation Formation
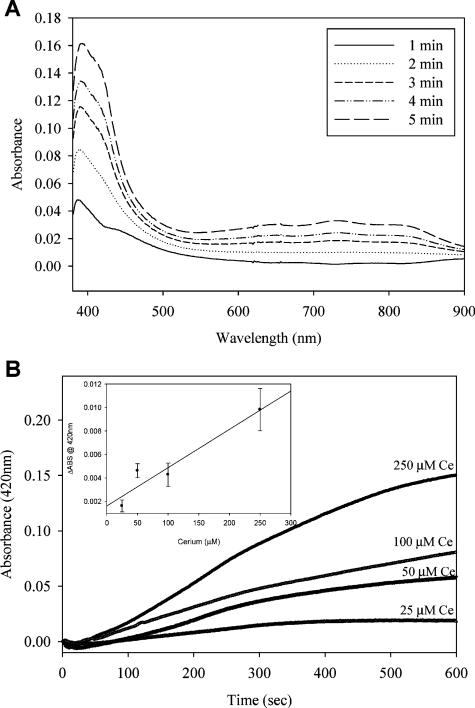
To further probe the production of radicals, presumably hydroxyl radical, by cerium in the presence of hydrogen peroxide we used the chromagen ABTS. This compound can react with short-lived radicals such as hydroxyl radical and the resulting cation radical that is formed (ABTS+·) is far more stable and has a characteristic absorption spectrum with a peak near 400 nm (Figure 2A). In the presence of cerium chloride, ABTS+· production accelerated in a time dependent manner over a 10 minute incubation at 25 °C (Figure 2A and B). Addition of increasing amounts of cerium chloride resulted in a near linear increase in the rate of ABTS radical formation (Figure 2B). These results suggest this method provides a useful alternative to follow the kinetics of radical formation that occurred in plasmid relaxation experiments (Figure 1). This also independently supports the hypothesis that cerium exhibits Fenton-like chemistry in the presence of peroxides.
FIGURE 2.
Radical production followed by reaction with ABTS to form cation radical. Increases in absorbance of ABTS–· as assessed by UV–visible spectroscopy. (A) The characteristic spectrum of the ABTS radical cation as previously described (23) increases upon incubation with cerium chloride and hydrogen peroxide. Spectra from one minute intervals of a reaction containing 100 μM cerium chloride and 88 mM hydrogen peroxide are shown. (B) Increases in ABTS radical formation dependent on increasing cerium concentrations. ABTS–· formation was followed spectrophotometrically at 420 nm for 10 minutes under the same experimental conditions described above. Cerium chloride concentrations are indicated in the plot. Inset shows rate of ABTS absorbance per μM Cerium concentration.
Oxygen radical formation, similar to that catalyzed by free iron, could pose a serious threat to biological macromolecules, such as proteins and nucleic acids, in vivo. Unlike iron and copper, there are no known roles for cerium or any rare earth metal in mammalian physiology, and thus the cell's capability to withstand exposure to such agents is suspect. To further probe the nature of the radicals produced by this Fenton-like reaction, we tested several radical scavengers to determine whether the reactivity of these compounds could give insight into the species of radicals produced by cerium and peroxide.
Spin Trap DEPMPO Prevents Formation of ABTS Radical from Cerium and Peroxide
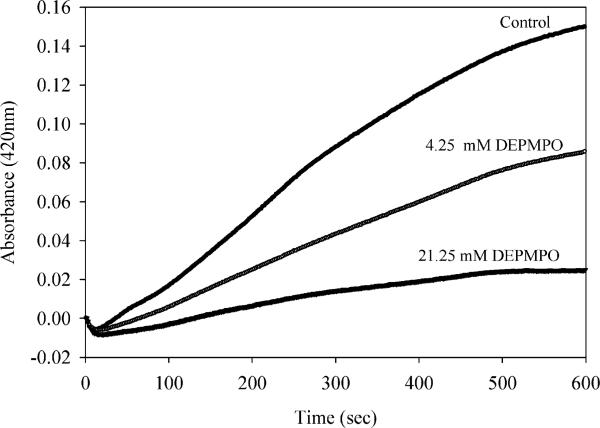
Spin traps are commonly used to detect the presence of radicals in redox-dependent processes (31–33). We followed the production of ABTS+· in the presence of DEPMPO to determine if competition by a spin trap would suppress production of the chromagenic radical cation. Indeed addition of DEPMPO did reduce production of ABTS+· in a concentration dependent manner (Figure 3). These data strongly suggests that a radical species is produced by reaction of cerium with hydrogen peroxide, and this radical is being trapped by DEPMPO. However the nature of the radical produced can not be directly assessed by reaction with DEPMPO using this indirect assay, since this spin trap is well-known to react both with superoxide and hydroxyl radicals (34).
FIGURE 3.
Hydroxyl radical scavenger DEPMPO inhibits ABTS radical formation. ABTS–· formation was followed as in Figure 2, with the addition of DEPMPO at the concentrations indicated just prior to addition of cerium chloride (100 μM).
Trolox, a Vitamin E Analog, Reacts with Radicals Produced by Cerium and Hydrogen Peroxide
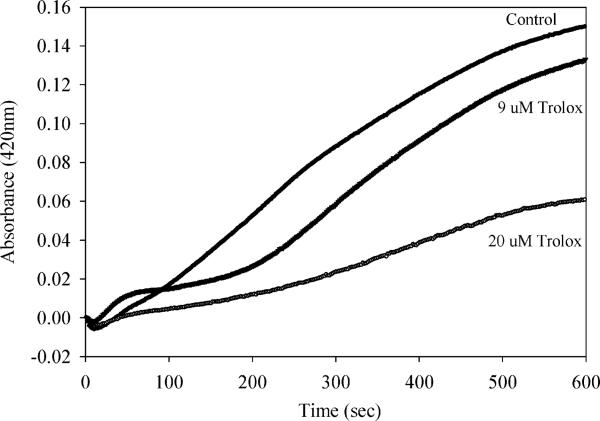
Vitamin E (alpha-tocopherol) is a key antioxidant that serves to prevent free radical mediated oxidative damage to lipids. Trolox, a vitamin E analog that is water soluble, has been characterized as a free radical scavenger that is capable of reacting with several free radical species including hydroxyl radical as well as thiyl radicals (35). However, Trolox was found not to affect the normal rate of decay of superoxide formed from pulse radiolysis (35). Addition of Trolox at low micromolar concentrations significantly inhibited the production of ABTS radical in the presence of cerium and hydrogen peroxide (Figure 4), and in a concentration dependent manner. This efficient scavenging of the radical species produced by cerium and hydrogen peroxide suggests the radical produced is indeed hydroxyl radical (OH · ). This would be the most likely radical species formed if cerium is behaving similar to iron in a Fenton-like reaction.
FIGURE 4.
Scavenging of radicals produced from cerium and peroxide by Trolox. ABTS–· production was followed as in Figure 2, with the addition of Trolox at the concentrations indicated just prior to addition of cerium chloride (100 μM).
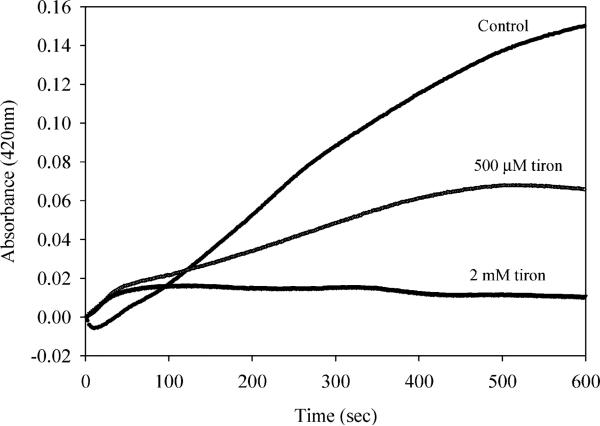
Tiron, an SOD-Mimetic, Also Reduces ABTS Cation Radical Formation in the Presence of Cerium and Hydrogen Peroxide
Tiron has been characterized as a spin trap and also characterized by its SOD mimetic activity (36). To determine whether superoxide could be produced as a result of the reaction of cerium with hydrogen peroxide, we followed the level of ABTS radical cation formation in the presence of Tiron, cerium and hydrogen peroxide. High micromolar levels (500 μM) of Tiron did significantly reduce the level of ABTS cation radical formed (Figure 5). Higher concentrations of Tiron (2 mM) essentially abolished radical formation using this assay. These results are somewhat contradictory, if one makes the assumption that Tiron is specific for scavenging superoxide. It should be noted that a recent report on redox cycling of chromium actually confirmed production of both hydroxyl and superoxide radicals, as assessed by electron spin resonance spectroscopy (34). Clearly these findings support the hypothesis that radicals are produced from reactions with cerium and hydrogen peroxide. To further probe the nature of radicals produced we used electron spin resonance techniques (34).
FIGURE 5.
Tiron, a vitamin E analog, also scavenges radicals produced by cerium in the presence of hydrogen peroxide. ABTS–· formation was followed as in Figure 2, with the addition of Tiron at the concentrations indicated with Tiron added just prior to addition of cerium chloride (100 μM).
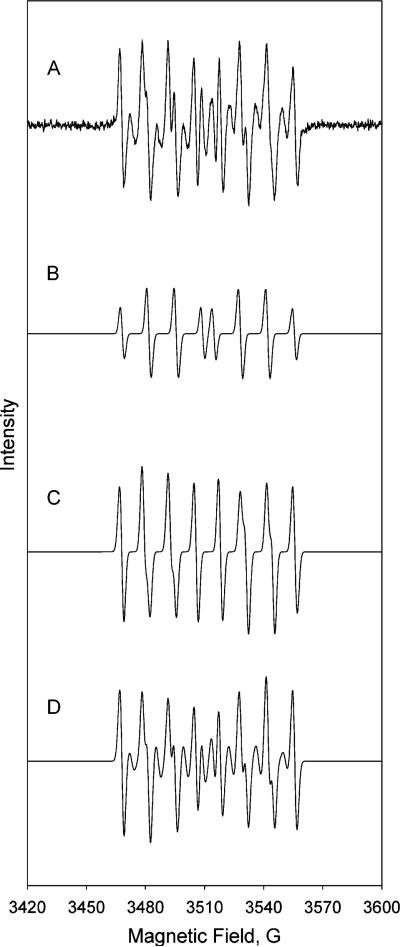
EPR Analysis Suggests Both Superoxide and Hydroxyl Radical Are Produced in the Presence of Cerium and Peroxide
To identify the radical products of the Fenton-like reaction occurring with cerium and hydrogen peroxide, we used a spin trap, DEPMPO and EPR. A chelating agent, DTPA, was also included at a concentration that should remove efficiently any potential adventitious contaminating metals. In absence of either cerium or hydrogen peroxide alone no detectable signal was observed (data not shown). In the presence of cerium chloride and hydrogen peroxide we observed a significant level of DEPMPO radical adducts (Figure 6). Using published values for both the hydroxyl and superoxide anion radical adducts (37) simulations were carried out to determine the relative contribution of each of these species in the experimental sample. These simulations (Figure 6) indicate that three different species are present; an OH adduct representing 32% of the species, an OOH adduct contributing 48% and an unknown adduct (needed to complete the simulation of the experimental spectrum) giving 20%. Although the nature of the unknown adduct has not been identified, the presence of both hydroxyl and superoxide anion radicals is clear from this analysis. This data confirms the characteristics seen when either Tiron or Trolox were used as scavengers. These results are similar to that recently observed in studies of radicals produced during redox cycling of chromium (34). We can predict, based on the results obtained in this study, that hydroxyl radical and superoxide anion could be produced from a series of reactions analogous to Fenton/Haber Weiss reactions:
FIGURE 6.
Evidence that superoxide and hydroxyl radicals are produced in the presence of cerium and hydrogen peroxide. Reaction contained 10 mM H2O2, 30 mM DEPMPO, 50 μM DTPA and 100 μM Tris buffer pH 7.0. (A) One mM cerium chloride with hydrogen peroxide (baseline corrected signal); (B) simulated hydroxyl radical adduct; (C) simulated superoxide radical adduct; (D) combined hydroxyl and superoxide radical adduct. EPR parameters were as follows: 20 mW microwave power, 20.48 ms time constant, 81.92 conversion time, 0.5 Gauss modulation amplitude, field set at 3514 with a sweep width of 200 Gauss.
Metal catalyzed oxidation of proteins, lipid and DNA represent a major source of oxidative stress in cells (18, 20, 21, 29, 38). The biologically relevant metals such as iron, copper, and nickel have been well studied with respect to trafficking in cells and mammals, and yet any “free” metal is still considered a potential source of oxidative stress via Fenton reactions. Cerium is a rare earth inner transition metal that is being used in its oxide form to create novel nanomaterials (CeO2–x) that also contain reduced cerium sites at the surface. Though most of the cerium within these structures is present as Ce4+, the reactive sites are presumed to be redox active and present in the Ce3+ state in the absence of oxidants. This rare earth metal has been studied extensively in various engineering applications, but studies on its interaction with biological cells are limited. Recent reports indicate that at high concentrations ceria nanoparticles can cause oxidative stress in cell culture models (15, 17), as well as a bacterial model (39). It should be noted that the concentrations of nanoparticles in these studies was quite high (micromolar or higher levels) as compared to studies that have shown a benefit from nanoceria (9, 10, 13, 14, 40).
The reactivity of the surface associated Ce3+ sites is the most likely source of reactive oxygen species in these studies, yet that has yet to be shown conclusively. In this study we used a simpler model (Ce3+ salt) to ask the fundamental question regarding the production of radicals in the presence of peroxides. Further work is clearly needed to determine whether radicals can be generated from oxygen vacancies at the surface of cerium oxide materials. It seems that cerium oxide based materials have clearly shown protection against oxidative stress (10, 11, 40), and also exhibited toxicity to mammalian cells (15, 17). These seemingly contradictory results are likely due to differences in particle uptake and metabolism, an area that is in need of further investigation in both culture and animal models. Any redox active compound that can act to scavenge radicals when present at low concentrations could conceivably also produce radicals when present at high concentrations. Indeed this is one of the key reasons that detection of superoxide radicals in cells and tissues is difficult (41, 42).
The results described in this report give credence to the necessity to design nanostructures (predominantly cerium oxide) of ceria that are resistant to degradation in vivo. We have presented substantial evidence, including EPR spin-trapping analysis, that oxygen-derived radicals are produced in the presence of cerium metal and hydrogen peroxide. The scavenging of these radicals by both Trolox and Tiron further confirm that both superoxide and hydroxyl radicals are being produced.
The cells and/or tissues that will be exposed to cerium would likely be directly from environmental exposure, e.g. skin, lungs, gastrointestinal systems. To test the safety and efficacy of cerium oxide based materials, future research should focus on the impact of these materials on cell culture models as well as animal models to rigorously assess safety.
Acknowledgments
We thank Witcha Imaram and Dr. Alex Angerhofer (Department of Chemistry, University of Florida, Gainesville, Florida) for their assistance in the EPR experiments and analysis. This work was funded in part by two grants from the National Science Foundation (CBET-0711239 and CBET 0541516) to S.S. and W.S.
Literature Cited
- 1.Esch F, Fabris S, Zhou L, Montini T, Africh C, Fornasiero P, Comelli G, Rosei R. Electron localization determines defect formation on ceria substrates. Science. 2005;309(5735):752–5. doi: 10.1126/science.1111568. [DOI] [PubMed] [Google Scholar]
- 2.Sayle TX, Parker SC, Sayle DC. Oxidising CO to CO2 using ceria nanoparticles. Phys. Chem. Chem. Phys. 2005;7(15):2936–41. doi: 10.1039/b506359k. [DOI] [PubMed] [Google Scholar]
- 3.Campbell CT, Peden CH. Chemistry. Oxygen vacancies and catalysis on ceria surfaces. Science. 2005;309(5735):713–4. doi: 10.1126/science.1113955. [DOI] [PubMed] [Google Scholar]
- 4.Fertizz RM, Gorte RJ, Vohs JM. Determining the Ce2O2SCeOx phase boundary for conditions relevant to adsorption and catalysis. Appl. Catal. B. 2003;43(3):273–280. [Google Scholar]
- 5.Trovarelli A. Catalysis by Ceria and Related Materials. Imperial College Press; London: 2002. p. 508. [Google Scholar]
- 6.Czerwinski F, Szpunar JA. The nanocrystalline ceria sol-gel coatings for high temperature applications. J. Sol-Gel Sci. Technol. 1997;9(1):103–114. [Google Scholar]
- 7.Mamontov E, Egami T, Brezny R, Koranne M, Tyagi S. Lattice defects and oxygen storage capacity of nanocrystalline ceria and ceria-zirconia. J. Phys. Chem. B. 2000;104(47):11110–11116. [Google Scholar]
- 8.Skorodumova NV, Simak SI, Lundqvist BI, Abrikosov IA, Johansson B. Quantum origin of the oxygen storage capability of ceria. Phys. Rev. Lett. 2002;89(16):166601. doi: 10.1103/PhysRevLett.89.166601. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Patil S, Seal S, McGinnis JF. Rare Earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nature Nanotechnol. 2006;1(2):142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 10.Korsvik C, Patil S, Seal S, Self WT. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007;10:1056–1058. doi: 10.1039/b615134e. [DOI] [PubMed] [Google Scholar]
- 11.Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc. Res. 2007;73(3):549–59. doi: 10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rzigalinski BA. Nanoparticles and cell longevity. Technol Cancer Res Treat. 2005;4(6):651–60. doi: 10.1177/153303460500400609. [DOI] [PubMed] [Google Scholar]
- 13.Rzigalinski BA, Bailey D, Chow L, Kuiry SC, Patil S, Merchant S, Seal S. Cerium oxide nanoparticles increase the lifespan of cultured brain cells and protect against free radical and mechanical trauma. FASEB J. 2003;17(4):A606–A606. [Google Scholar]
- 14.Tarnuzzer RW, Colon J, Patil S, Seal S. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett. 2005;5(12):2573–7. doi: 10.1021/nl052024f. [DOI] [PubMed] [Google Scholar]
- 15.Lin WS, Huang YW, Zhou XD, Ma YF. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int. J. Toxicol. 2006;25(6):451–457. doi: 10.1080/10915810600959543. [DOI] [PubMed] [Google Scholar]
- 16.Limbach LK, Li YC, Grass RN, Brunner TJ, Hintermann MA, Muller M, Gunther D, Stark WJ. Oxide nanoparticle uptake in human lung fibroblasts: Effects of particle size, agglomeration, and diffusion at low concentrations. Environ. Sci. Technol. 2005;39(23):9370–9376. doi: 10.1021/es051043o. [DOI] [PubMed] [Google Scholar]
- 17.Park EJ, Choi J, Park YK, Park K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology. 2008;245(1-2):90–100. doi: 10.1016/j.tox.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240(4852):640–2. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 19.Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005;12(10):1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 20.Sugden KD, Geer RD, Rogers SJ. Oxygen radical-mediated DNA damage by redox-active Cr(III) complexes. Biochemistry. 1992;31(46):11626–31. doi: 10.1021/bi00161a049. [DOI] [PubMed] [Google Scholar]
- 21.Shi X, Dalal NS. Vanadate-mediated hydroxyl radical generation from superoxide radical in the presence of NADH: Haber-Weiss vs Fenton mechanism. Arch. Biochem. Biophys. 1993;307(2):336–41. doi: 10.1006/abbi.1993.1597. [DOI] [PubMed] [Google Scholar]
- 22.Requena JR, Levine RL, Stadtman ER. Recent advances in the analysis of oxidized proteins. Amino Acids. 2003;25(3-4):221–6. doi: 10.1007/s00726-003-0012-1. [DOI] [PubMed] [Google Scholar]
- 23.Yim MB, Chock PB, Stadtman ER. Enzyme function of copper, zinc superoxide dismutase as a free radical generator. J. Biol. Chem. 1993;268(6):4099–105. [PubMed] [Google Scholar]
- 24.Wolfenden BS, Willson RL. Radical-cations as reference chromogens in kinetic-studies of one-electron transfer-reactions—Pulse-radiolysis studies of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfphonate). J. Chem. Soc., Perkin Trans. 2. 1982;(7):805–812. [Google Scholar]
- 25.Poeggeler B, Thuermann S, Dose A, Schoenke M, Burkhardt S, Hardeland R. Melatonin's unique radical scavenging properties - roles of its functional substituents as revealed by a comparison with its structural analogs. J Pineal Res. 2002;33(1):20–30. doi: 10.1034/j.1600-079x.2002.01873.x. [DOI] [PubMed] [Google Scholar]
- 26.Stoll S, Schweiger A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006;178(1):42–55. doi: 10.1016/j.jmr.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Humphreys KJ, Johnson AE, Karlin KD, Rokita SE. Oxidative strand scission of nucleic acids by a multinuclear copper(II) complex. J Biol Inorg Chem. 2002;7(7-8):835–42. doi: 10.1007/s00775-002-0369-8. [DOI] [PubMed] [Google Scholar]
- 28.Kawanishi S, Inoue S, Sano S. Mechanism of DNA cleavage induced by sodium chromate(VI) in the presence of hydrogen peroxide. J. Biol. Chem. 1986;261(13):5952–8. [PubMed] [Google Scholar]
- 29.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biol. Med. 1995;18(2):321–36. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Park JW. A manganese porphyrin complex is a novel radiation protector. Free Radical Biol. Med. 2004;37(2):272–83. doi: 10.1016/j.freeradbiomed.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 31.Roubaud V, Sankarapandi S, Kuppusamy P, Tordo P, Zweier JL. Quantitative measurement of superoxide generation and oxygen consumption from leukocytes using electron paramagnetic resonance spectroscopy. Anal. Biochem. 1998;257(2):210–7. doi: 10.1006/abio.1997.2542. [DOI] [PubMed] [Google Scholar]
- 32.Roubaud V, Sankarapandi S, Kuppusamy P, Tordo P, Zweier JL. Quantitative measurement of superoxide generation using the spin trap 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide. Anal. Biochem. 1997;247(2):404–11. doi: 10.1006/abio.1997.2067. [DOI] [PubMed] [Google Scholar]
- 33.Dambrova M, Baumane L, Kalvinsh I, Wikberg JE. Improved method for EPR detection of DEPMPO-superoxide radicals by liquid nitrogen freezing. Biochem. Biophys. Res. Commun. 2000;275(3):895–8. doi: 10.1006/bbrc.2000.3387. [DOI] [PubMed] [Google Scholar]
- 34.Borthiry GR, Antholine WE, Kalyanaraman B, Myers JM, Myers CR. Reduction of hexavalent chromium by human cytochrome b(5): Generation of hydroxyl radical and superoxide. Free Radical Biol. Med. 2007;42(6):738–55. doi: 10.1016/j.freeradbiomed.2006.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies MJ, Forni LG, Willson RL. Vitamin E analogue Trolox C. E.s.r. and pulse-radiolysis studies of free-radical reactions. Biochem. J. 1988;255(2):513–22. [PMC free article] [PubMed] [Google Scholar]
- 36.Ledenev AN, Konstantinov AA, Popova E, Ruuge EK. A simple assay of the superoxide generation rate with Tiron as an EPR-visible radical scavenger. Biochem. Int. 1986;13(2):391–6. [PubMed] [Google Scholar]
- 37.Frejaville C, Karoui H, Tuccio B, Le Moigne F, Culcasi M, Pietri S, Lauricella R, Tordo P. 5-(Diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide: a new efficient phosphorylated nitrone for the in vitro and in vivo spin trapping of oxygen-centered radicals. J. Med. Chem. 1995;38(2):258–65. doi: 10.1021/jm00002a007. [DOI] [PubMed] [Google Scholar]
- 38.van der Loo B, Fenton MJ, Erusalimsky JD. Cytochemical detection of a senescence-associated beta-galactosidase in endothelial and smooth muscle cells from human and rabbit blood vessels. Exp. Cell Res. 1998;241(2):309–15. doi: 10.1006/excr.1998.4035. [DOI] [PubMed] [Google Scholar]
- 39.Thill A, Zeyons O, Spalla O, Chauvat F, Rose J, Auffan M, Flank AM. Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ. Sci. Technol. 2006;40(19):6151–6. doi: 10.1021/es060999b. [DOI] [PubMed] [Google Scholar]
- 40.Das M, Patil S, Bhargava N, Kang JF, Riedel LM, Seal S, Hickman JJ. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28(10):1918–25. doi: 10.1016/j.biomaterials.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fridovich I. Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J. Biol. Chem. 1997;272(30):18515–7. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 42.Faulkner K, Fridovich I. Luminol and lucigenin as detectors for O2. Free Radical Biol. Med. 1993;15(4):447–51. doi: 10.1016/0891-5849(93)90044-u. [DOI] [PubMed] [Google Scholar]