Abstract
 The management of spinal tuberculosis, especially in children, is controversial. In children, vertebral destruction is more severe than adults because of the cartilaginous nature of their bone. Modern chemotherapy has significantly decreased mortality in spinal tuberculosis, but morbidity remains high. Without early surgery, patients can develop severe kyphosis leading to respiratory insufficiency, painful costopelvic impingement and paraplegia. Lumbar kyphosis results in early degenerative lumbar canal stenosis and is cosmetically unacceptable. We report a paediatric case of atypical spinal tuberculosis demonstrating the need for early surgical intervention to prevent significant spinal instability and neurologic deficit. A 12-year-old girl presented with increasing ambulatory difficulty and double incontinence 4 months after initiating treatment for pulmonary tuberculosis. There was no history of traumatic injury. Examination revealed severe lower limb neurologic deficit, with hypotonia, areflexia, marked sensory loss, and grade 0/5 power in both lower limbs. Plain radiographs and magnetic resonance imaging (MRI) demonstrated grade IV posterior listhesis of the L2 vertebral body over L3, cauda equina compression and bilateral psoas abscesses. Erosion of both the body and pedicle of L2 was observed. Both serology and pus drained from the psoas abscesses were negative for microorganisms. The patient underwent an L2 vertebrectomy via a left retroperitoneal approach. A titanium cage packed with autologous bone graft was inserted, and the spine was stabilized by fixation with screw and rods. Histopathology confirmed a diagnosis of tuberculosis. Eighteen months following the procedure, the patient has regained some power in her right leg and has completed her course of anti-tuberculous chemotherapy, but remains wheelchair-bound. To our knowledge, this is the first reported case of posterior listhesis secondary to spinal tuberculosis. Here, we discuss the possible management options in such a case, and the indications for surgery. As the global HIV/AIDS epidemic causes a resurgence in tuberculosis, increased awareness among the medical community regarding the atypical presentations of spinal tuberculosis is necessitated; both in the developing world where advanced clinical presentations are common, and in the developed world where spinal tuberculosis is an often-neglected diagnosis.
The management of spinal tuberculosis, especially in children, is controversial. In children, vertebral destruction is more severe than adults because of the cartilaginous nature of their bone. Modern chemotherapy has significantly decreased mortality in spinal tuberculosis, but morbidity remains high. Without early surgery, patients can develop severe kyphosis leading to respiratory insufficiency, painful costopelvic impingement and paraplegia. Lumbar kyphosis results in early degenerative lumbar canal stenosis and is cosmetically unacceptable. We report a paediatric case of atypical spinal tuberculosis demonstrating the need for early surgical intervention to prevent significant spinal instability and neurologic deficit. A 12-year-old girl presented with increasing ambulatory difficulty and double incontinence 4 months after initiating treatment for pulmonary tuberculosis. There was no history of traumatic injury. Examination revealed severe lower limb neurologic deficit, with hypotonia, areflexia, marked sensory loss, and grade 0/5 power in both lower limbs. Plain radiographs and magnetic resonance imaging (MRI) demonstrated grade IV posterior listhesis of the L2 vertebral body over L3, cauda equina compression and bilateral psoas abscesses. Erosion of both the body and pedicle of L2 was observed. Both serology and pus drained from the psoas abscesses were negative for microorganisms. The patient underwent an L2 vertebrectomy via a left retroperitoneal approach. A titanium cage packed with autologous bone graft was inserted, and the spine was stabilized by fixation with screw and rods. Histopathology confirmed a diagnosis of tuberculosis. Eighteen months following the procedure, the patient has regained some power in her right leg and has completed her course of anti-tuberculous chemotherapy, but remains wheelchair-bound. To our knowledge, this is the first reported case of posterior listhesis secondary to spinal tuberculosis. Here, we discuss the possible management options in such a case, and the indications for surgery. As the global HIV/AIDS epidemic causes a resurgence in tuberculosis, increased awareness among the medical community regarding the atypical presentations of spinal tuberculosis is necessitated; both in the developing world where advanced clinical presentations are common, and in the developed world where spinal tuberculosis is an often-neglected diagnosis.
Keywords: Posterior listhesis, Spinal tuberculosis, Lumbar spine, Vertebrae, Pott’s disease
Case presentation
A 12-year-old Bangladeshi girl with a recent history of pulmonary tuberculosis presented with a 5-day history of immobility, loss of sensation below the mid-thigh bilaterally and bowel and bladder incontinence. She was on anti-tuberculous therapy at admission—rifampicin and isoniazid—for pulmonary tuberculosis diagnosed 4 months previously. Severe lower back and anterior thigh pain had developed 1 month before admission, diagnosed at another centre as tuberculosis of the L2 vertebra; bed rest and continuation of anti-tuberculous therapy was advised at that time. There was no history of traumatic injury. Past medical history revealed a Dandy-Walker cyst, untreated due to lack of parental consent. There was no history of abnormal mental state, but performance in school was subnormal. Examination revealed macrocephaly in keeping with hydrocephalus, but normal higher functions and cranial nerves. Bilateral upper-extremity muscle tone, power, and reflexes were normal. Both lower limbs were wasted without fasciculation but demonstrated hypotonia, grade 0/5 muscle power and areflexia. Abdominal reflexes were present and plantars were down-going bilaterally, but anal reflex was absent. Sensation below the level of L3 was absent on the right and vastly reduced on the left. At the level of L2, gibbus was present with severe tenderness, as well as scoliosis with convexity to the right. Owing to the patient’s ambulatory status, gait was unable to be assessed. Routine hematological and biochemical investigations, including erythrocyte sedimentation rate (ESR), were normal. Plain radiographs of the dorso-lumbar spine (anteroposterior and lateral) revealed Meyerding’s Grade IV posterior listhesis (total intervertebral foramina occlusion) of L2 over L3, with evidence of erosion of the L2 body and pedicle (Fig. 1). MRI of the same region demonstrated cauda equina compression, hypointensity of the L2 vertebra, minor hypointense signal changes of the L1 and L3 vertebrae (Fig. 2) and bilateral psoas abscesses (Fig. 3). Cerebral MRI revealed the Dandy–Walker malformation and hydrocephalus, without peri-ventricular leukomalacia. Both serology and pus drained from the psoas abscesses failed to grow microorganisms, and no acid-fast bacilli were demonstrated on Ziehl–Neelsen staining.
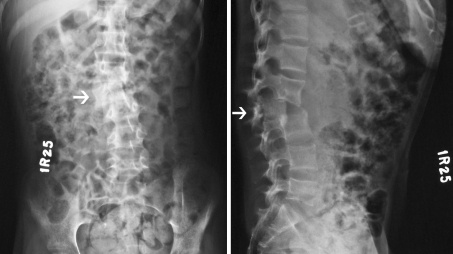
Fig. 1.
Admission anteroposterior (left) and lateral (right) plain radiographs of the spine demonstrating Grade IV posterior listhesis of the L2 vertebra over L3 (arrows) and scoliosis
Fig. 2.
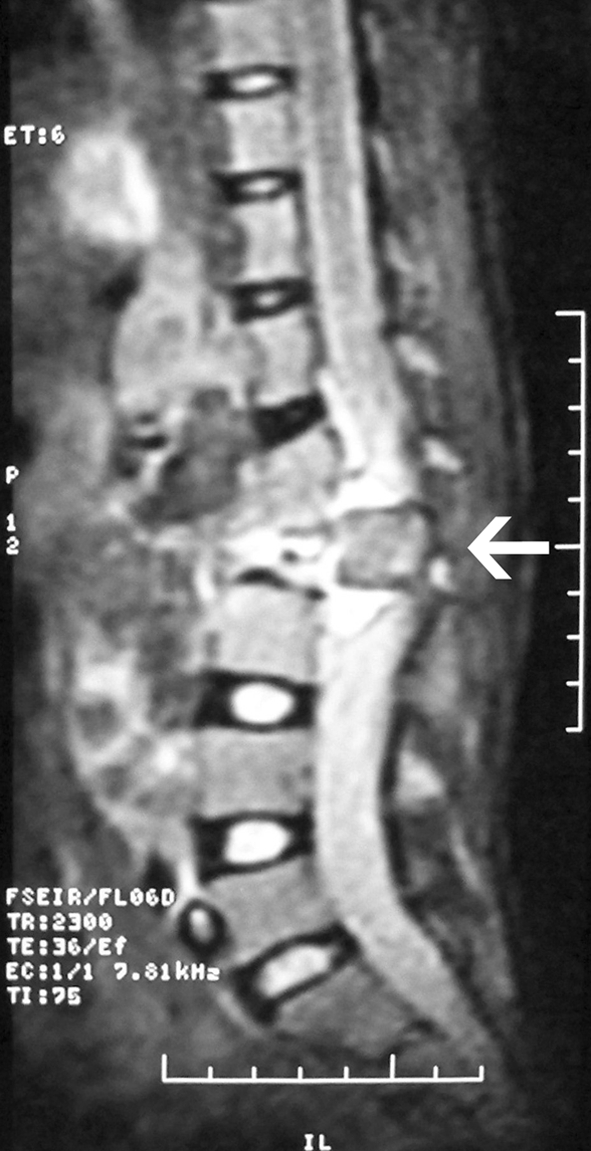
Sagittal T2-weighted MRI demonstrating posterior listhesis of the hypointense L2 vertebra over L3, and cauda equina compression
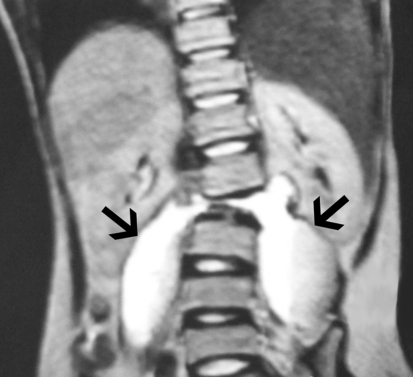
Fig. 3.
Coronal T2-weighted MRI depicting bilateral psoas abscesses (arrows) and scoliosis
Diagnostic imaging (Figs. 1, 2, 3)
Historical review of the condition, epidemiology, diagnosis, pathology, and differential diagnosis
Tuberculosis, an ancient scourge of humanity, symptomatically affects 30 million people worldwide and kills three million annually [1]. Skeletal involvement is present in 1–3% of affected individuals and, of these, half have spinal involvement [2, 3]. Spinal tuberculosis was described in 1779 by Percival Pott as “…that kind of palsy of lower limbs which is frequently found to accompany a curvature of the spine” [4]; nowadays, it is considered a disease of children in developing nations and elderly in the industrialized world. Tuberculosis has a predilection for the thoracic vertebrae, with only 3–5% of spinal infections located in the cervical or lumbar regions [2].
Mycobacterium tuberculosis usually enters the body via the respiratory tract, before undergoing hematogenous dissemination. Vertebral involvement often results from dissemination of tuberculous bacilli via the intercostal lumbar arteries and, to a lesser extent, the vertebral venous plexus (Batson’s plexus) or proximal affected para-aortic lymph nodes; the result is destruction of first cancellous and then cortical bone, followed by gradual spread to adjacent vertebrae via the intervertebral disc space. In advanced stages of disease as in our case, progressive vertebral collapse leads to spinal instability with kyphosis, gibbus formation, and neurologic sequelae. Single vertebral involvement in tuberculosis is extremely rare [5], and posterior listhesis of a vertebra has never been associated with tuberculosis in the current literature. Further, of the thirteen reported cases of coexisting spinal tuberculosis and anterolisthesis [6–9], in only three instances did tuberculosis definitely predate the development of anterolisthesis [7, 9]. The case reported by Nissen-Lie (quoted by Ratliff [7]) had no evidence of a defect in the pars interarticularis before tuberculosis, believing that the tuberculosis led to extensive vertebral destruction and a ‘stress lesion’ of the neural arch, precipitating anterolisthesis. In two of the three cases of coexisting anterolisthesis and tuberculosis reported by Chadha et al. [9], the authors reported that tuberculosis of the posterior elements probably led to secondary anterolisthesis; in the other case they reported, the tubercular process was confined to the anterior (paradiscal) site without involving the posterior elements, and hence appears to be unrelated to the anterolisthesis observed. Similarly, anterolisthesis was definitely present before the tubercular process commenced in the case reported by Ratliff [7], and the details of two cases described by Newman [6] and six by Tuli [8] are obscure. A pre-existing vertebral defect was unlikely in our case given the child’s age, radiological picture, relatively sudden onset and lack of a history of trauma. Further, there is no evidence in the literature that an infection predisposes to anterior or posterior listhesis of a vertebra. Instead, tuberculous invasion and erosion of both the body and pedicle of the L2 vertebra probably led to posterior listhesis of L2 over L3, completely obliterating the intervertebral foramina and causing cauda equina compression.
Clinically, spinal tuberculosis usually takes an indolent course, presenting with back pain, low grade fever, chills, weight loss, and non-specific constitutional symptoms. Only occasionally is paraplegia the first sign, and usually this is a result of tuberculous granulation tissue, pus, extruded sequestrae, intervertebral disc fragments and vertebral collapse. The mainstay of diagnosis rests on high clinical suspicion. Documented history of tuberculosis with involvement of posterior vertebral elements and presence of psoas abscesses is almost pathognomonic of spinal tuberculosis even when lacking positive cultures [10]. Purified protein derivative (PPD) testing should be performed in all suspected patients, but a positive result does not confirm the diagnosis, and false-negative results in extrapulmonary disease may approach 30% [2]. Polymerase chain reaction (PCR) is valuable where available, but sensitivity for non-respiratory specimens varies. Antitubercular antibodies by enzyme-linked immunosorbent assay (ELISA) may be sought if molecular techniques are unavailable [2]. MRI is the most useful diagnostic tool for typical and atypical spinal tuberculosis; it affords detection of the disease several months earlier than conventional methods, provides greater detail of soft tissue and disc, and allows better visualization of posterior vertebral elements [1]. However, a confident diagnosis of spinal tuberculosis from radiological findings is made in less than half of cases and, since three-quarters of cases demonstrate paravertebral soft tissue mass and spinal abscesses [2], both bacteriologic and histocytologic evidence must be collected by image-guided fine needle aspiration (FNA) for diagnostic confirmation before initiating treatment, especially for atypical presentations [5]. Acid-fast bacilli staining or culture is only positive in around 30% of cases [9]. Further, the sad irony is that access to these investigations in resource-limited settings—where spinal tuberculosis is at its most prevalent—can be meagre. It is for this reason that patients such as the one described here present with such advanced pathology.
The main differential diagnoses of spinal tuberculosis include pyogenic and fungal osteomyelitis, trauma, multiple myeloma, lymphoma, eosinophilic granuloma, and metastatic deposits; the latter, although not an uncommon cause of acute paraplegia, is unlikely in our patient of only 12 years old.
Rationale for treatment and evidenced-based literature
Treatment of spinal tuberculosis is guided by the severity and extent of disease. Eradication of infection should be the primary aim, and if present, the correction or prevention of an increase in angular deformity and the recovery of any neurological deficits. Those with early clinical disease lacking neurological signs can potentially recover fully through bed rest and at least 18 months chemotherapy incorporating isoniazid (so-called conservative management) [1]. Patient compliance with chemotherapy is vital for success, hence directly observed therapy (DOT) is recommended.
Our case demonstrates the significant spinal instability and neurologic deficit that can result from spinal tuberculosis. If surgery is planned, it is vital to assess the extent of destruction as well as the possibility of instability and neurologic involvement [5]. Individuals developing new tubercular lesions or those without adequate clinicoradiologic healing response may require surgery; difficulty arises in deciding how long to wait before making this decision [11]. Cases lacking classic clinicoradiologic findings require surgical decompression and tissue biopsy for histopathological examination to confirm the diagnosis. Early surgical decompression and stabilization should be performed in all cases where a neurologic deficit is developing, remaining stationary or worsening with chemotherapy and bed rest alone [11]. Acute-onset paraplegia and neural arch involvement require early surgical decompression [11]. In our case of acute-onset paraplegia with posterior lumbar listhesis, surgery was clearly indicated; although extremely rare, patients with simultaneous involvement of the anterior and posterior elements of a single vertebra have been reported as being optimally managed by posterior decompression and stabilization [5]. The best outcomes from stabilization in spinal tuberculosis have resulted from using a titanium cage packed with autologous bone graft and anterior screw and rod fixation [12, 13]. Iliac crest bone graft offers good conductivity and osteoinduction, and is the graft material of choice at all levels of the spine despite harvesting area morbidity [11]. It is for these reasons that in our patient a titanium cage packed with autologous bone from the ipsilateral iliac crest was utilized.
Adjuvant chemotherapy is essential in improving outcomes following surgery, and for this reason our patient continued with her prior rifampicin and isoniazid therapy, as well as commencing ethambutol and pyrazinamide on admission. Modern chemotherapy has decreased mortality of skeletal tuberculosis by 75% [14], but morbidity remains high; 20% of survivors develop paraplegia along with residual spinal deformity [15]. In the long-term, severe kyphosis results in respiratory insufficiency, painful costopelvic impingement and paraplegia [11, 16]. In children, vertebral destruction is more severe than adults because most bone is cartilaginous, and growth adds to the angulation due to growth retardation of the anterior column and unrestricted growth of the posterior column [17]. Lumbar kyphosis results in early degenerative lumbar canal stenosis and is cosmetically unacceptable. This highlights the need for a proactive approach to management, not least in the paediatric age group.
Procedure
Two days after admission, the patient underwent an L2 vertebrectomy via a left retroperitoneal approach under general anaesthetic. On direct visualization it was clear that the left pedicle of the L2 vertebra was partially destroyed, and the L2 vertebra was displaced postero-laterally to the right, stretching the thecal sac. The thecal sac and roots were decompressed, and a titanium cage (GESCO, Chennai, India) packed with autologous bone graft taken from the ipsilateral iliac crest was inserted for stabilization, along with rod-and-screw fixation (Fig. 4). Both psoas muscles were found to be significantly swollen due to cold abscess and granulation tissue, and underwent debridement.
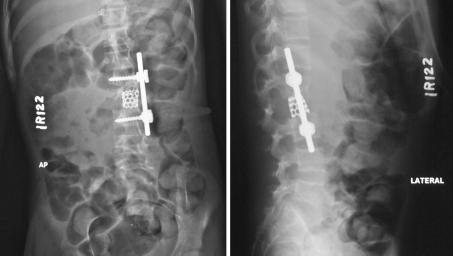
Fig. 4.
Postoperative anteroposterior (left) and lateral (right) plain radiographs of the spine showing satisfactory fixation with rod and screws and stabilization with titanium cage packed with autologous bone graft
Procedure imaging (Fig. 4)
Outcome and follow-up
Histopathology of L2 vertebral tissue and paraspinal granulation tissue revealed a histiocytic granuloma consistent with tuberculosis. The post-operative period was uneventful and the patient was discharged 1 month after admission, to complete a 2-month course of rifampicin, isoniazid, pyrazinamide and ethambutol, and a further 16 months of rifampicin and isoniazid alone. Two months following the surgery, power improved to grade 1/5 in the right lower limb only. Otherwise, there has been no further improvement in her neurological symptoms since her initial presentation. She remains wheelchair-bound 18 months post-operatively, having completed her course of medication, and is followed up in clinic regularly.
Conflict of interest
None.
References
- 1.Tuli SM. Tuberculosis of the spine: a historical review. Clin Orthop Relat Res. 2007;460:29–38. doi: 10.1097/BLO.0b013e318065b75e. [DOI] [PubMed] [Google Scholar]
- 2.Kourbeti IS, Tsiodras S, Boumpas DT. Spinal infections: evolving concepts. Curr Opin Rheumatol. 2008;20(4):471–479. doi: 10.1097/BOR.0b013e3282ff5e66. [DOI] [PubMed] [Google Scholar]
- 3.Tuli SM, Srivastava TP, Varma BP, Sinha GP. Tuberculosis of spine. Acta Orthop Scand. 1967;38(4):445–458. doi: 10.3109/17453676708989653. [DOI] [PubMed] [Google Scholar]
- 4.Bick KM. Classics of orthopaedics. Philadelphia: JB Lippincott Co.; 1976. [Google Scholar]
- 5.Pande KC, Babhulkar SS. Atypical spinal tuberculosis. Clin Orthop Relat Res. 2002;398:67–74. doi: 10.1097/00003086-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Newman PH. The etiology of spondylolisthesis. J Bone Joint Surg (Br) 1963;45:39–59. [Google Scholar]
- 7.Ratliff AHC. Tuberculosis at the site of spondylolisthesis. Br J Surg. 1956;43:502–504. doi: 10.1002/bjs.18004318109. [DOI] [PubMed] [Google Scholar]
- 8.Tuli SM. Tuberculosis of the skeletal system. New Delhi: Jaypee Brothers; 1997. [Google Scholar]
- 9.Chadha M, Agarwal A, Kumar S. Spinal tuberculosis with concomitant spondylolisthesis: coexisting entities or ‘cause and effect’? Spinal Cord. 2006;44(6):399–404. doi: 10.1038/sj.sc.3101852. [DOI] [PubMed] [Google Scholar]
- 10.Turunc T, Demiroglu YZ, Uncu H, et al. A comparative analysis of tuberculous, brucellar and pyogenic spontaneous spondylodiscitis patients. J Infect. 2007;55:158–163. doi: 10.1016/j.jinf.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Jain AK, Dhammi IK. Tuberculosis of the spine: a review. Clin Orthop Relat Res. 2007;460:39–49. doi: 10.1097/BLO.0b013e318073bd29. [DOI] [PubMed] [Google Scholar]
- 12.Christodoulou AG, Givissis P, Karataglis D, Symeonidis PD, Pournaras J. Treatment of tuberculous spondylitis with anterior stabilization and titanium cage. Clin Orthop Relat Res. 2006;444:60–65. doi: 10.1097/01.blo.0000201175.87635.28. [DOI] [PubMed] [Google Scholar]
- 13.Dai LY, Jiang LS, Wang W, Cui YM. Single-stage anterior autogenous bone grafting and instrumentation in the surgical management of spinal tuberculosis. Spine. 2005;30:2342–2349. doi: 10.1097/01.brs.0000182109.36973.93. [DOI] [PubMed] [Google Scholar]
- 14.Konstam PG. Spinal tuberculosis in Nigeria. Ann R Coll Surg Engl. 1963;32:99–114. [PMC free article] [PubMed] [Google Scholar]
- 15.Moon MS, Woo YK, Lee KS, Ha KY, Kim SS, Sun DH. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine. 1995;20(17):1910–1916. doi: 10.1097/00007632-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Bilsel N, Aydingoz O, Hanci M, Erdogan F. Late onset Pott’s paraplegia. Spinal Cord. 2000;38:669–674. doi: 10.1038/sj.sc.3101082. [DOI] [PubMed] [Google Scholar]
- 17.Mushkin AY, Kovalenko KN. Neurological complications of spinal tuberculosis in children. Int Orthop. 1999;23:210–212. doi: 10.1007/s002640050352. [DOI] [PMC free article] [PubMed] [Google Scholar]