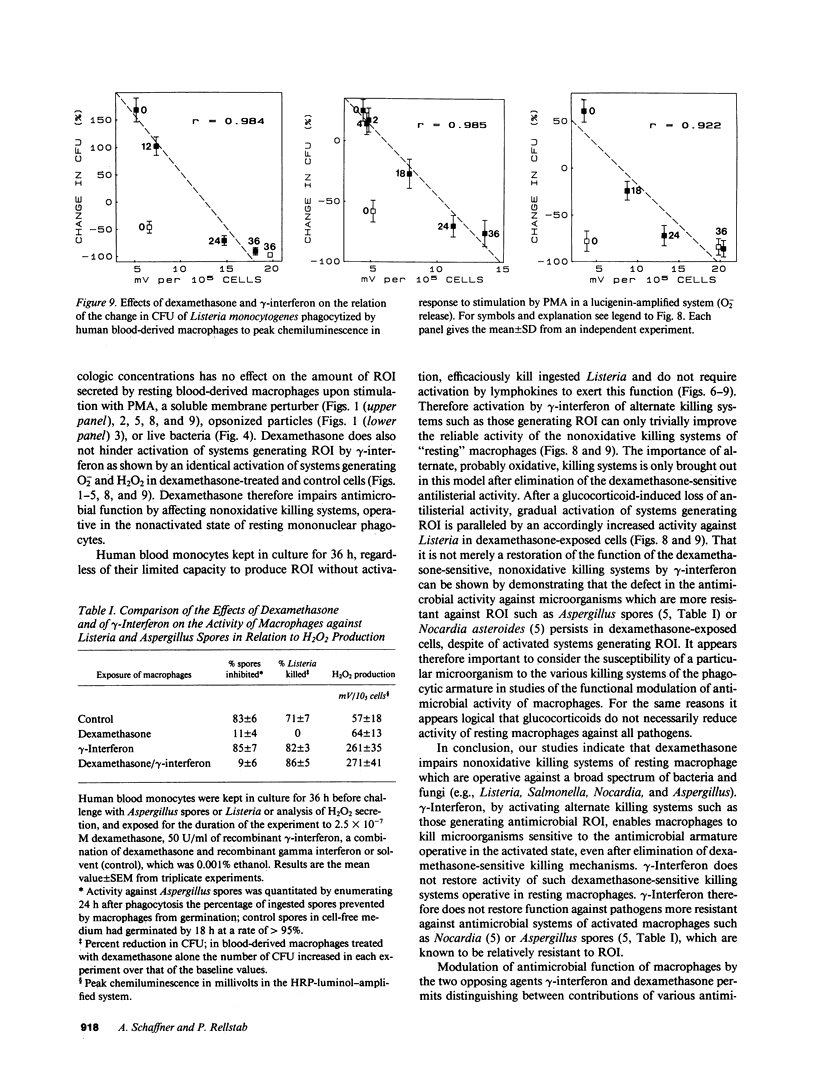
Abstract
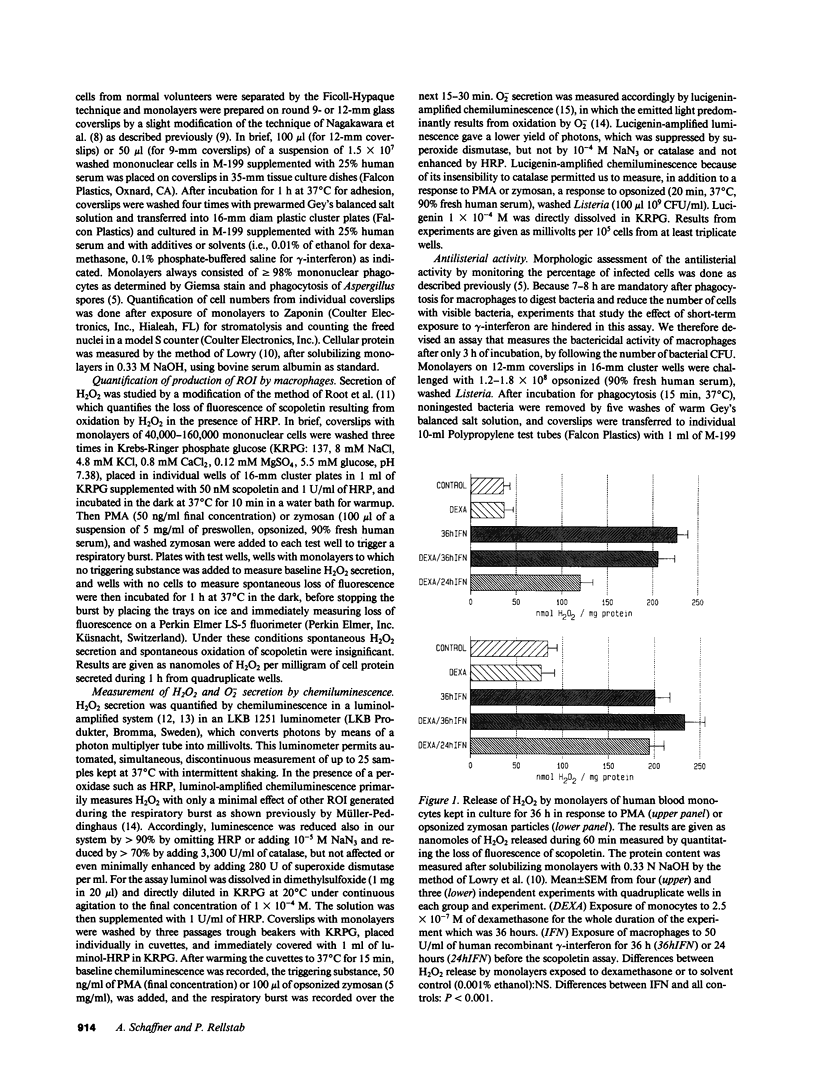
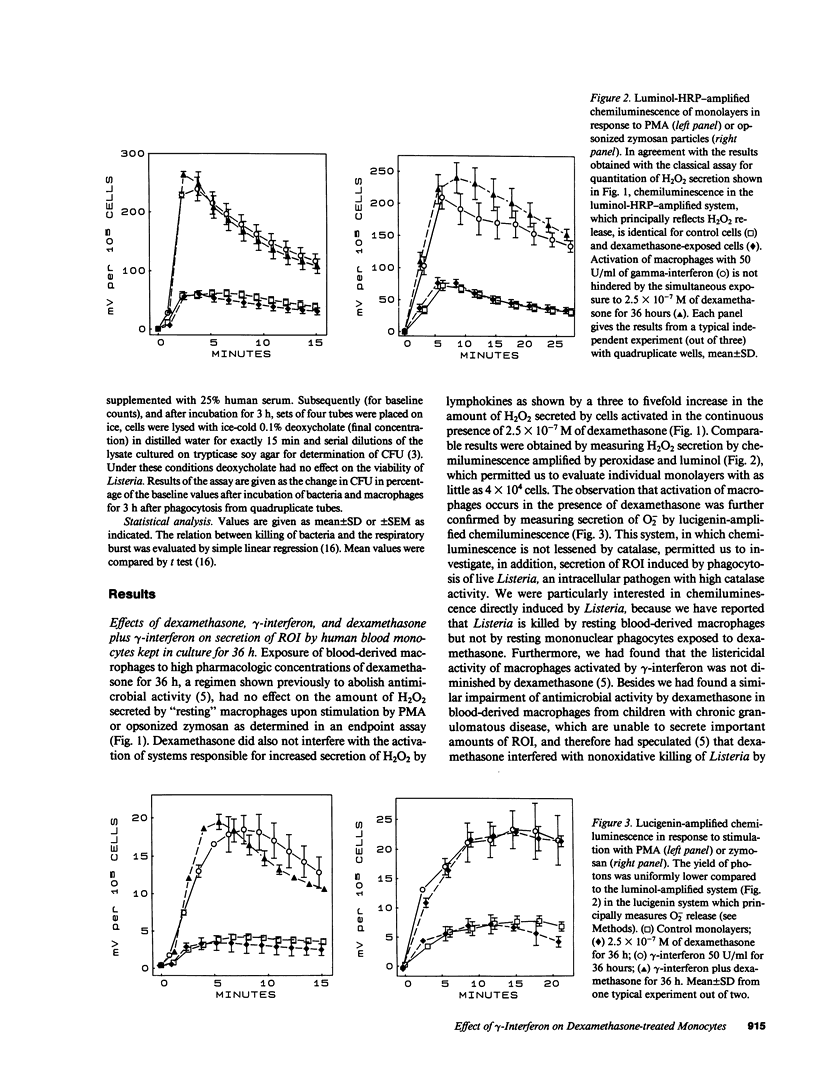
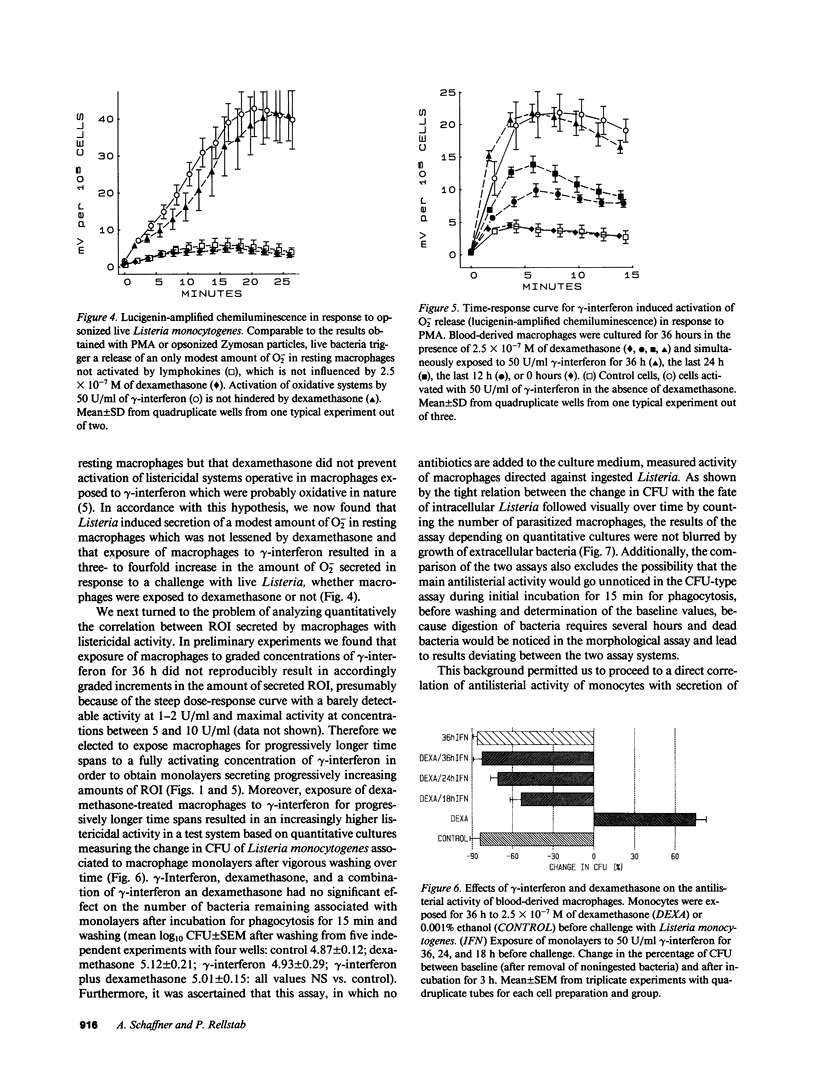
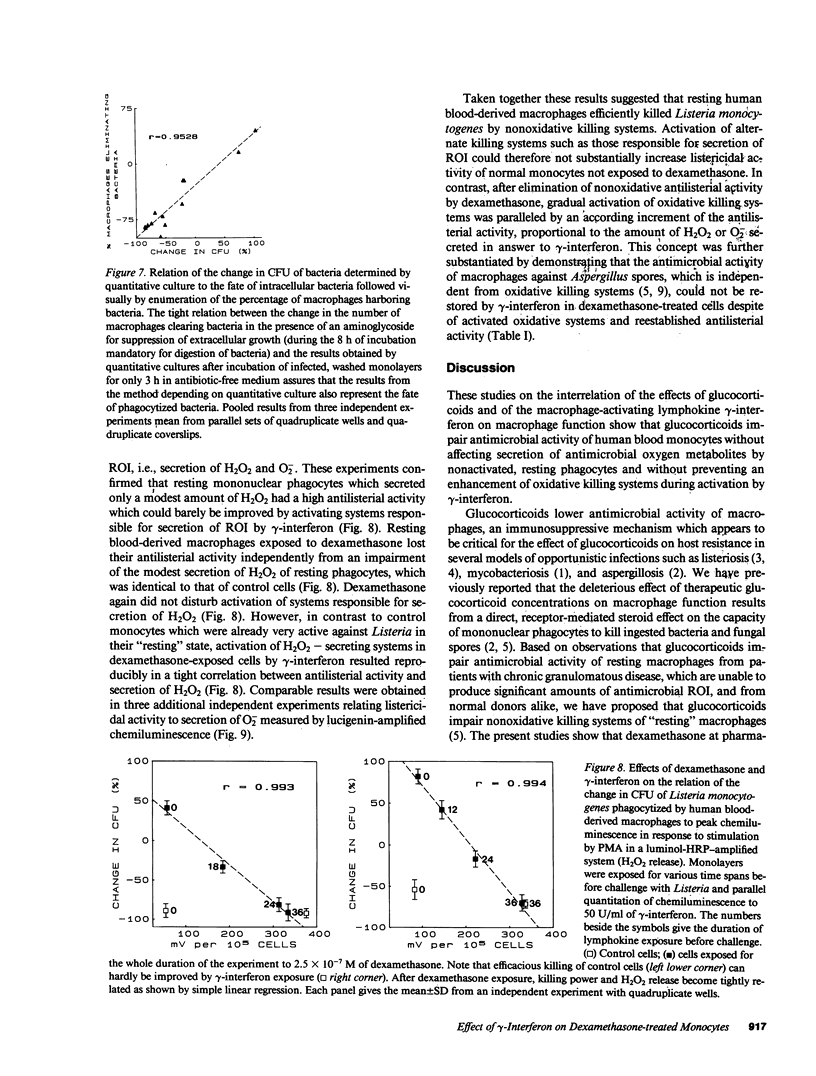
By preventing macrophages from destroying phagocytized microorganisms, glucocorticoids lower natural resistance of the host against many pathogens. gamma-Interferon has an opposite effect and restores activity of dexamethasone-treated mononuclear phagocytes against some but not all microorganisms. In the present studies we show that dexamethasone impairs activity of human blood monocytes kept in culture for 36 h against Listeria monocytogenes, without impairing H2O2 or O2- secretion. Likewise dexamethasone does not interfere with activation of systems generating oxygen metabolites by lymphokines. Thus gamma-interferon increases three- to fivefold the capacity of dexamethasone-treated monocytes to secrete O2- or H2O2 upon stimulation by opsonized zymosan, live bacteria, or phorbol myristate acetate. Concurrently gamma-interferon restores listericidal activity of dexamethasone-treated monocytes. After gradual activation by exposure to gamma-interferon for progressive time periods, listericidal activity becomes tightly correlated (r = 0.922-0.994) with the amount of H2O2 or O2- secreted by dexamethasone-treated monocytes. Activation of oxidative systems by the lymphokine is, however, not correlated with the restoration of activity against Aspergillus spores, lost during dexamethasone treatment, which does not depend on antimicrobial oxygen metabolites. Taken together, these observations lend to the hypothesis that glucocorticoids impair nonoxidative defense mechanisms of "resting" macrophages, while gamma-interferon restores macrophage function impaired by glucocorticoids by activating alternate killing systems, which are at least partly of oxidative nature.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Blackwood L. L., Pennington J. E. Dose-dependent effect of glucocorticosteroids on pulmonary defenses in a steroid-resistant host. Am Rev Respir Dis. 1982 Dec;126(6):1045–1049. doi: 10.1164/arrd.1982.126.6.1045. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Müller-Peddinghaus R. In vitro determination of phagocyte activity by luminol- and lucigenin-amplified chemiluminescence. Int J Immunopharmacol. 1984;6(5):455–466. doi: 10.1016/0192-0561(84)90084-5. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Davis C. E., Schaffner T., Markert M., Douglas H., Braude A. I. In vitro susceptibility of fungi to killing by neutrophil granulocytes discriminates between primary pathogenicity and opportunism. J Clin Invest. 1986 Aug;78(2):511–524. doi: 10.1172/JCI112603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. I., Davis C. E. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect Immun. 1983 Dec;42(3):1109–1115. doi: 10.1128/iai.42.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982 Mar;69(3):617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Davis C. E. Models of T cell deficiency in listeriosis: the effects of cortisone and cyclosporin A on normal and nude BALB/c mice. J Immunol. 1983 Jul;131(1):450–453. [PubMed] [Google Scholar]
- Schaffner A. Therapeutic concentrations of glucocorticoids suppress the antimicrobial activity of human macrophages without impairing their responsiveness to gamma interferon. J Clin Invest. 1985 Nov;76(5):1755–1764. doi: 10.1172/JCI112166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Kleinschmidt W. J. Functional identity between murine gamma interferon and macrophage activating factor. Nature. 1983 Sep 15;305(5931):239–240. doi: 10.1038/305239a0. [DOI] [PubMed] [Google Scholar]






