Abstract
A cross-sectional study that targeted a total of 43,630 pupils in Niigata City, Japan was performed. The objective of the study was to evaluate the association between sports activities and low back pain (LBP) in childhood and adolescence in Japan. Regarding risk factors of LBP, a large number of studies have been conducted that have examined gender differences, height and weight, body mass index, sports time, differences in lifestyle, family history, and mental factors; however, no definitive conclusion has yet been made. A questionnaire survey was conducted using 43,630 pupils, including all elementary school pupils from the fourth to sixth grade (21,893 pupils) and all junior high pupils from the first to third year (21,737 pupils) in Niigata City (population of 785,067). 26,766 pupils who were determined to have valid responses (valid response rate 61.3%) were analyzed. Among the 26,766 pupils with valid responses, 2,591 (9.7%) had LBP at the time of the survey, and 8,588 (32.1%) had a history of LBP. The pupils were divided between those who did not participate in sports activities except the physical education in school (No sports group: 5,486, 20.5%) and those who participated in sports activities (Sports group: 21,280, 79.5%), and the difference in lifetime prevalence between No sports group and Sports group was examined. The odds ratio for LBP according to sports activity was calculated by multiple logistic regression analysis adjusted for gender, age, and body mass index. In addition, the severity of LBP was divided into three levels (Level 1: no limitation in any activity, Level 2: necessary to refrain from participating in sports and physical activities, and Level 3: necessary to be absent from school), and Levels 2 and 3 were defined as severe LBP; the severity was compared between No sports group and Sports group and in each sport’s items. Moreover, in Sports group, the amount of time spent participating in sports activities were divided into three groups (Group 1: less than 6 h per week, Group 2: 6–12 h per week, and Group 3: 12.1 h per week or more), and the dose–response between the amount of time spent participating in sports activities and the occurrence of LBP were compared. In No sports group, 21.3% experienced a history of LBP; in Sports group, 34.9% experienced LBP (P < 0.001). In comparison to No sports group, the odds ratio was significantly higher for Sports group (1.57), and also significantly higher for most of the sports items. The severity of LBP was significantly higher in Sports group (20.1 vs. 3.2%, P < 0.001). The amount of time spent participating in sports activities averaged 9.8 h per week, and a history of LBP significantly increased in the group which spent a longer time participating in sports activities (odds ratio 1.43 in Group 3). These findings suggest that sports activity is possible risk factors for the occurrence of LBP, and it might increase the risk for LBP in childhood and adolescence.
Keywords: Low back pain, Childhood, Adolescence, Sports activity, Epidemiology
Introduction
It has recently been recognized that low back pain (LBP) in childhood and adolescence is as common a complaint as that observed in adults [2, 4, 6, 10, 12, 14, 17, 22, 24]. Using a cross-sectional study of 500 pupils between 10 and 16 years of age in Northwest England, Jones et al. [12] determined the average lifetime prevalence of LBP to be 40.2% while the average point prevalence was 15.5%. Leboeuf-Yde et al. [14] performed a cross-sectional study of 29,424 twins ranging from 12 to 41 years of age in Denmark, and reported that the lifetime cumulative incidence surpassed 50% in 18-year-old females and 20-year-old males. In addition, we previously conducted a large-scale cross-sectional study using the questionnaire, and reported that the average point prevalence of LBP was seen in 10.2% of those surveyed, and the average lifetime prevalence of LBP was 28.8%. The prevalence significantly increased as the grade level increased; the lifetime prevalence increased to 42.5% by the third year of junior high school pupils [19].
As for the risk factors of LBP, a large number of studies have been conducted that have examined the gender differences, height and weight, body mass index (BMI), physical factors such as the mobility of the spinal column and muscle strength, sports activities, differences in lifestyles such as the amount of activity, time spent sitting in chairs, time spent watching TV or playing video games, mechanical load factors such as the weight of school bags, image findings such as X-rays and MRI scans, family histories, and mental factors. However, no definitive conclusion has yet been reached [1, 2, 4–6, 8, 9, 11, 13, 15–18, 21–23, 25, 27]. Regarding sports activities, the risk factors for LBP in childhood and adolescence also remain controversial. Cardon and Balagué reported that competitive sports activities and a high level of physical activity were associated with an increased risk of LBP [7]. Using a questionnaire study given to 1,775 children 8–16 years of age, Balagué et al. [3] reported that competitive sports activity significantly increased the risk for LBP among children. On the other hand, using a cross-sectional survey of 481 children aged 8–10 years and 325 adolescents aged 14–16 years, Wedderkopp et al. reported no association between the objectively measured level of physical activity and back pain [26]. In addition, most previous reports have been conducted in Europe, whereas few such reports have been published in Japan. The purpose of this study was to evaluate the association between sports activities and LBP in childhood and adolescence in Japan using a large-scale cross-sectional epidemiological study based on a questionnaire administered to all children living in a defined area, Niigata City.
Materials and methods
The present study includes all schoolchildren consisting of a total of 43,630 pupils (22,356 males and 21,274 females), including all elementary school pupils from the fourth to sixth grade (110 schools, 21,893 pupils) and all junior high pupils from the first to third year (57 schools, 21,737 pupils) in Niigata City (at longitude 139° east and latitude 37° north, located on the west coast of Japan with an area of 649 km2 and a population of 785,067 as on September 30, 2005). In the Japanese school system, elementary school consists of a 6-year program including the first to sixth grades, while junior high school is a 3-year program comprising the first year, the second year, and the third year levels. The fourth grade elementary school pupils thus correspond to children ranging from 9 to 10 years of age (E4); the fifth grade children ranging from 10 to 11 years of age (E5), and the sixth grade children ranging from 11 to 12 years of age (E6). The first-year junior high school pupils correspond to children ranging from 12 to 13 years of age (J1); the second-year pupils comprise children ranging from 13 to 14 years of age (J2), and the third-year pupils comprise children ranging from 14 to 15 years of age (J3).
An anonymous questionnaire, which carefully protected any personal information, was made and distributed to each school. The questionnaire was taken home by all elementary school children to fill out together with their parents or guardians, and thereafter, they were collected at the school. For junior high school students, it was filled out by each themselves and collected at the school. The data were collected from October 17, 2005 to November 11, 2005.
At first, basic information, such as the name of their school, their grade, gender, height, and weight were filled in on the answer form. Next, any experience of LBP was described by means of multiple choice answers in Question 1 of the questionnaire (Fig. 1), with the options divided between the present and the past, and the details of LBP were continuously surveyed using the selection answer form for students who had experienced LBP. In addition, regardless of the presence of LBP, the details of sports activities other than school physical education classes, musical activities, and social activities were reported in the questionnaire (Fig. 2). The amount of time spent participating in sports activities (hours per week) was also filled in on the answer form.
Fig. 1.
Details of Question 1 in the questionnaire
Fig. 2.
Details of question for sports activities
Regarding the definition of LBP, there was no information for LBP in the questionnaire, and it depended only on their judgment as LBP. A valid response was the ones that had also appropriately answered their grade, gender, height, weight, and Question 1. Responses to the questionnaire were received from 34,830 of 43,630 pupils (response rate 79.8%), and 26,766 pupils who were determined to have valid responses (valid response rate 61.3%) were thus analyzed. Among the 26,766 pupils with valid responses, 2,591 (9.7%; 57% males and 43% females) had LBP at the time of the survey, and 8,588 (32.1%; 54% males and 46% females) had a history of LBP.
The pupils were divided between those who did not participate in sports activities except the physical education in school (No sports group) and those who participated in sports activities (Sports group) (Table 1). The lifetime prevalence of LBP was compared between the two groups, and the odds ratio for LBP according to sports activity was calculated between No sports group and Sports group and in each sports items. The severity of LBP was divided into three levels (Level 1: no limitation in any activity, Level 2: necessary to refrain from participating in sports and physical activities, and Level 3: necessary to be absent from school), and LBP that is necessary to refrain from participating in sports and physical activities and necessary to be absent from school was defined as severe LBP (Levels 2 and 3); the severe LBP was compared between No sports group and Sports group and in each sport’s items. Regarding the dose–response between the amount of time spent participating in sports activities and the occurrence of LBP, the amount of time spent participating in sports activities were divided into three groups (Group 1: less than 6 h per week, Group 2: 6–12 h per week, and Group 3: 12.1 h per week or more), and then they were compared.
Table 1.
Number of participants by grade and gender differences in participation in sports activities between Sports group and No sports group
| Total | E4 | E5 | E6 | J1 | J2 | J3 | |
|---|---|---|---|---|---|---|---|
| No. | 26,766 | 4,006 | 4,118 | 4,045 | 5,127 | 4,766 | 4,704 |
| No sports group (%) | 5,486 (20.5) | 1,249 (31) | 1,157 (28) | 1,112 (28) | 480 (9) | 572 (12) | 916 (20) |
| Male (%) | 2,122 (39) | 399 (32) | 377 (33) | 359 (32) | 207 (43) | 279 (49) | 501 (55) |
| Female (%) | 3,364 (61) | 850 (68) | 780 (67) | 753 (68) | 273 (57) | 293 (51) | 415 (45) |
| Sports group (%) | 21,280 (79.5) | 2,757 (69) | 2,961 (72) | 2,933 (72) | 4,647 (91) | 4,194 (88) | 3,788 (80) |
| Male (%) | 12,214 (57) | 1,522 (55) | 1,620 (55) | 1,612 (55) | 2,606 (56) | 2,535 (60) | 2,319 (61) |
| Female (%) | 9,066 (43) | 1,235 (45) | 1,341 (45) | 1,321 (45) | 2,041 (44) | 1,659 (40) | 1,469 (39) |
The SPSS software program (Version 14) for Windows was used to perform a statistical analysis; the χ2 test was used for comparison of the lifetime prevalence of LBP and the severe LBP between No sports group and Sports group, and a multiple logistic regression was used to calculate the association between sports activities and experiencing of LBP and comparisons between among three groups regarding the dose–response between the amount of time spent participating in sports activities and the occurrence of LBP. In general, there are grade differences, gender differences, and build differences between each sports item; therefore, all analysis was corrected for grade, gender, and BMI. In all the cases, significance was set at P < 0.05.
Results
No sports group versus Sports group
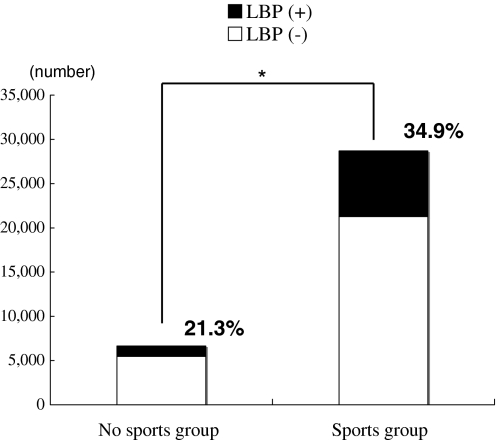
In No sports group, 21.3% of participants experienced a history of LBP; in Sports group, 34.9% experienced LBP, thus indicating it to be significantly higher in Sports group (P < 0.001) (Fig. 3). In addition, compared to No sports group, the odds ratio which shows the association between sports activities and experiencing LBP was significantly higher for Sports group (1.57) and also significantly higher for most sports items, especially in volleyball, athletics, judo, gymnastics, golf, and rugby, where the odds ratio exceeded 2 (Table 2).
Fig. 3.
The lifetime prevalence of LBP between No sports group and Sports group. The lifetime prevalence of LBP was significantly higher in Sports group (*P < 0.001: χ2 analysis; grade, gender, and BMI corrected)
Table 2.
The association between sports activities and LBP
| Type of sports | No. (%) | LBP (%) | Odds ratio | 95% CI | P value | Severe LBP (%) |
|---|---|---|---|---|---|---|
| Swimming | 5,662 (19) | 27.5 | 1.41 | 1.27−1.55 | <0.001 | 17.5 |
| Basketball | 3,726 (12) | 37.9 | 1.79 | 1.61−1.99 | <0.001 | 22.9 |
| Soccer | 3,534 (12) | 34.9 | 1.77 | 1.56−2.02 | <0.001 | 22.8 |
| Baseball | 3,525 (12) | 37.5 | 1.82 | 1.60−2.06 | <0.001 | 21.9 |
| Tennis | 2,097 (7) | 34.3 | 1.24 | 1.09−1.42 | <0.01 | 15.3 |
| Wind-instrument music | 1,872 (6) | 37.3 | 1.32 | 1.15−1.51 | <0.001 | 12.8 |
| Table tennis | 1,486 (5) | 34.7 | 1.05 | 0.89−1.21 | 0.63 | 18.1 |
| Volleyball | 1,445 (5) | 46.6 | 2.14 | 1.86−2.46 | <0.001 | 21.6 |
| Athletics | 1,324 (4) | 48.6 | 2.18 | 1.89−2.52 | <0.001 | 27.4 |
| Kendo | 993 (3) | 35.5 | 1.39 | 1.18−1.65 | <0.001 | 24.7 |
| Karate | 897 (3) | 31.9 | 1.57 | 1.31−1.87 | <0.001 | 22 |
| Badminton | 771 (3) | 39.8 | 1.51 | 1.26−1.80 | <0.001 | 25.6 |
| Ballet | 669 (2) | 30.3 | 1.63 | 1.33−1.99 | <0.001 | 17.5 |
| Dance | 582 (2) | 34.7 | 1.75 | 1.42−2.15 | <0.001 | 20 |
| Judo | 569 (2) | 51.1 | 2.12 | 1.73−2.52 | <0.001 | 31.1 |
| Gymnastics | 560 (2) | 36.3 | 2.05 | 1.67−2.51 | <0.001 | 29.4 |
| Golf | 102 (<1) | 51 | 2.2 | 1.45−3.35 | <0.001 | 27.2 |
| Dodgeball | 95 (<1) | 32.6 | 1.59 | 0.95−2.55 | 0.08 | 20.8 |
| Rugby | 70 (<1) | 51.4 | 2.58 | 1.56−4.27 | <0.001 | 29 |
| Sumo wrestling, wrestling | 48 (<1) | 35.4 | 0.85 | 0.42−1.71 | 0.65 | 25 |
| Archery | 23 (<1) | 39.1 | 1.1 | 0.42−2.88 | 0.84 | 16.7 |
| Total | 30,050* | |||||
| Sports group | 21,280/26,766 | 34.9 | 1.57 | 1.45−1.70 | <0.001 | 20.1 |
| No sports group | 5,486/26,766 | 21.3 | 1 | 3.2 |
The odds ratio and the severity of LBP between No Sports group and Sports group and in each sports item were calculated by multiple logistic regression analysis and χ2 analysis (grade, gender, and BMI corrected)
* Repeated answers in sports items were included
Severe LBP (%) the ratio of severe LBP among subjects with a history of LBP, CI confidence interval
Severity of LBP
When comparing the two groups, in Sports group, 20.1% of subjects with a history of LBP had had severe LBP, whereas only 3.2% in No sports group had had severe LBP. The severity was significantly higher in Sports group (P < 0.001) (Table 2). Comparing sports items, athletics, badminton, judo, gymnastics, golf, rugby, and sumo wrestling, wrestling accounted for 25% or more cases of severe LBP (Table 2).
Dose–response between the amount of time spent participating in sports activities and the occurrence of LBP
The amount of time spent participating in sports activities averaged 9.8 (±6.6) h per week in Sports group. When comparing the findings among the three groups, a history of LBP was seen in 20.7% in Group 1, in 30.4% in Group 2, and in 40.5% in Group 3, and it significantly increased in the group which spent a longer time participating in sports activities (Table 3).
Table 3.
The dose–response between the amount of time spent participating in sports activities and the occurrence of LBP
| Group 1 (n = 7,095) | Group 2 (n = 6,720) | Group 3 (n = 5,526) | |
|---|---|---|---|
| Time spent participating in sports activities (h/week) | −6.0 | 6.1–12.0 | 12.3+ |
| Experience of a history of LBP | 1,470 (20.7) | 2,046 (30.4) | 2,239 (40.5) |
| Odds ratio (95% CI) | 1.00 | 1.13 (0.99–1.28)* | 1.43 (1.26–1.64)† |
The comparison between the amount of time spent participating in sports activities and the occurrence of LBP in three groups were calculated by multiple logistic regression analysis (grade, gender, and BMI corrected)
* P = 0.072, † P < 0.001
Discussion
In this study, we focused on sports activities as a risk factor for LBP in childhood and adolescence. Regarding physical activity, Cardon and Balagué [7] reported that competitive sports activities and a high level of physical activity were associated with an increased risk of LBP. Using descriptive statistics and logistic regression in a self-administered questionnaire given to 1,775 children 8–16 years of age, Balagué et al. [3] reported that competitive sports activity significantly increased the risk for LBP among children, controlling for the child’s age and gender (adjusted odds ratio 1.73; P = 0.003). On the other hand, using a cross-sectional survey of 481 children aged 8–10 years and 325 adolescents aged 14–16 years, Wedderkopp et al. [26] reported no association between the objectively measured level of physical activity and back pain. Previously, we reported and concluded that the age of first onset of LBP in third year junior high school students increased rapidly from the first year of junior high school onward, and 90.2% of them experienced LBP during the first to third year of junior high school. The time spent participating in athletic activities increased approximately twofold, from an average 6.6 h/week for the sixth grade of elementary school pupils to 12.2 h/week for the first year of junior high school students. In Japan, many children join club activities when they enter junior high school, and this result means that the opportunities to participate in sports activities rapidly increased during this period. These findings suggested that the amount of time spent participating in athletic activity might be one of the risk factors for the occurrence of LBP in childhood and adolescence [19]. In this study, comparing the amount of time spent participating in sports activities in Sports group, a history of LBP was significantly increased in the group which spent a longer time participating in sports activities, and the odds ratio was 1.43 in Group 3 (12.1 h per week or more) in comparison to Group 1 (less than 6 h per week). There was a dose–response between the amount of time spent participating in sports activities and the occurrence of LBP. Moreover, from the results of this study, the lifetime prevalence of LBP was significantly higher in Sports group, and the odds ratio for the risk of experiencing of LBP was 1.57 compared to No sports group. Additionally, as for the severity of LBP, LBP that is necessary to refrain from participating in sports and physical activities and necessary to be absent from school was defined as severe LBP; 20.1% had severe LBP in Sports group and 3.2% in No sports group. The severe LBP was significantly higher in Sports group compared to No sports group. These findings also suggest that sports activity increased the risk for LBP in childhood and adolescence.
Regarding the sports items, Balagué et al. [2, 3] reported that volleyball, body-building, aerobics, tennis, and cycling were associated with an increased prevalence of LBP. Using multiple logistic regressions in a survey of 546 schoolchildren aged 15–16 years, Skoffer et al. [20] reported that among sports activities, only swimming was associated with a decreased LBP prevalence. In this study, the odds ratio for the risk of experiencing LBP was significantly higher in most sports items compared with No sports group, especially in volleyball, athletics, judo, gymnastics, golf, and rugby, where the odds ratio exceeded 2. As reported in the previous study, the prevalence of LBP was increased in sports items that would repeatedly put a load on the low back. Moreover, it was thought that direct injury to the low back was a cause of LBP in competitive sports such as judo and rugby. As for the severity of LBP, comparing the sports items, athletics, badminton, judo, gymnastics, golf, rugby, and sumo wrestling, wrestling accounted for 25% or more of the cases of severe LBP. Playing a wind instrument accounted for 12.8% of the cases of severe LBP: archery 16.7% and swimming and ballet 17.5%. The lower incidence of severe LBP in swimmers supports Skoffer’s [20] findings.
However, this study was a questionnaire survey and therefore had some limitations. To closely evaluate the relationship between LBP and sports activity, more information regarding the pupils was needed, including, basic information such as their school name, grade, gender, height and weight, and answer in Question 1. Therefore, the pupils who completely filled out of them were limited, and number of valid responses was only 61.3%. In this study, various evaluations were performed only for the pupils who provided valid responses, and therefore, the details regarding the pupils who gave invalid responses remained unclear. A comparison should therefore have been performed between the two groups. In addition, the definition of LBP used in this study might also be insufficient. The definition of LBP used in this study seemed to depend on only their judgment, which could thus lead to some bias. As a result, more information regarding how LBP was determined, and a clear definition of the criteria was needed. Moreover, regarding the risk factors of LBP in childhood and adolescence, this study examined physical and sports activities only. As shown in previous reports, the risk factors of LBP cannot be explained by a single cause. In future studies, it will be necessary to evaluate other risk factors [5, 7, 11].
Conclusion
We conducted a large-scale cross-sectional study using a questionnaire to study the actual conditions of LBP among childhood and adolescence in Japan. The lifetime prevalence of LBP was significantly higher in Sports group, and the odds ratio for the risk of experiencing LBP was 1.57 compared to No sports group. Moreover, Sports group had had more severe LBP compare to No sports group (20.1 vs. 3.2%, P < 0.001). The amount of time spent participating in sports activities averaged 9.8 h per week, and a history of LBP significantly increased in the group which spent a longer time participating in sports activities (odds ratio 1.43 in Group 3). These findings suggest that sports activity is possible risk factors for the occurrence of LBP, and it might increase the risk for LBP in childhood and adolescence.
Acknowledgments
We would like to sincerely thank the members of the Niigata City board of education and others associated with the elementary schools and junior high schools in Niigata City for their valuable cooperation in making this study.
References
- 1.Anderson LB, Wedderkopp N, Leboeuf-Yde C. Association between back pain and physical fitness in adolescents. Spine. 2006;31:1740–1744. doi: 10.1097/01.brs.0000224186.68017.e0. [DOI] [PubMed] [Google Scholar]
- 2.Balagué F, Dutoit G, Waldburger M. Low back pain in schoolchildren. An epidemiological study. Scand J Rehabil Med. 1988;20:175–179. [PubMed] [Google Scholar]
- 3.Balagué F, Nordin M, Skovron ML, Dutoit G, Yee A, Waldburger M. Non-specific low-back pain among schoolchildren: a field survey with analysis of some associated factors. J Spinal Disord. 1994;7:374–379. [PubMed] [Google Scholar]
- 4.Balagué F, Skovron ML, Nordin M, Dutoti G, Pol LR, Waldburger M. Low back pain in schoolchildren—a study of familial and psychological factors. Spine. 1995;20:1265–1270. doi: 10.1097/00007632-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Balagué F, Troussier B, Salminen JJ. Non-specific low back pain in children and adolescents: risk factors. Eur Spine J. 1999;8:429–438. doi: 10.1007/s005860050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton AK, Clarke RD, McClune TD, Tillotson KM. The natural history of low back pain in adolescents. Spine. 1996;21:2323–2328. doi: 10.1097/00007632-199610150-00004. [DOI] [PubMed] [Google Scholar]
- 7.Cardon G, Balagué F. Low back pain prevention’s effects in schoolchildren. What is the evidence? Eur Spine J. 2004;13:663–679. doi: 10.1007/s00586-004-0749-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harreby M, Neergaard K, Hesselsøe G, Kjer J. Are radiologic changes in the thoracic and lumbar spine of adolescents risk factors for low back pain in adults? Spine. 1995;20:2298–2302. doi: 10.1097/00007632-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Harreby M, Nygaard B, Jessen T, Larsen E, Storr-Paulsen A, Lindahl A, Fisker I, Laegaard E. Risk factors for low back pain in a cohort of 1389 Danish school children: an epidemiologic study. Eur Spine J. 1999;8:444–450. doi: 10.1007/s005860050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hestbaek L, Leboeuf-Yde C, Kyvik KO, Manniche CM. The Course of low back pain from adolescence to adulthood. Eight-year follow-up of 9600 twins. Spine. 2006;31:468–472. doi: 10.1097/01.brs.0000199958.04073.d9. [DOI] [PubMed] [Google Scholar]
- 11.Jones GT, Macfarlane GJ. Epidemiology of low back pain in children and adolescents. Arch Dis Child. 2005;90:312–316. doi: 10.1136/adc.2004.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones MA, Stratton G, Reilly T, Unnithan VB. A school-based survey of recurrent non-specific low-back pain prevalence and consequences in children. Health Educ Res. 2004;19:284–289. doi: 10.1093/her/cyg025. [DOI] [PubMed] [Google Scholar]
- 13.Kelsey JK, White AA. Epidemiology and impact of low back pain. Spine. 1985;5:133–142. doi: 10.1097/00007632-198003000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Leboeuf-Yde C, Kyvik KO. At what age dose low back pain become a common problem? A study of 29,424 individuals aged 12–41 years. Spine. 1998;23:228–234. doi: 10.1097/00007632-199801150-00015. [DOI] [PubMed] [Google Scholar]
- 15.Limon S, Valinsky LJ, Ben-Shalom Y. Children at risk. Risk factors for low back pain in the elementary school environment. Spine. 2004;29:697–702. doi: 10.1097/01.BRS.0000116695.09697.22. [DOI] [PubMed] [Google Scholar]
- 16.Poussa MS, Heliövaara MM, Seitsamo JT, Könönen MH, Huemerinta KA, Nissinen MJ. Anthropometric measurements and growth as predictors of low-back pain: a cohort study of children followed up from the age of 11 to 22 years. Eur Spine J. 2005;14:595–598. doi: 10.1007/s00586-004-0872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salminen JJ, Pentti J, Terho P. Low back pain and disability in 14-year-old schoolchildren. Acta Paediatr. 1992;81:1035–1039. doi: 10.1111/j.1651-2227.1992.tb12170.x. [DOI] [PubMed] [Google Scholar]
- 18.Salminen JJ, Erkintalo M, Laine M, Pentti J. Low back pain in the young. A prospective three-year follow-up study of subjects with and without low back pain. Spine. 1995;20:2101–2108. doi: 10.1097/00007632-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Sato T, Ito T, Hirano T, Morita O, Kikuchi R, Endo N, Tanabe N. Low back pain in childhood and adolescence: a cross-sectional study in Niigata City. Eur Spine J. 2008;17:1441–1447. doi: 10.1007/s00586-008-0788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skoffer B, Foldspang A. Physical activity and low-back pain in schoolchildren. Eur Spine J. 2008;17:373–379. doi: 10.1007/s00586-007-0583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szpalski M, Gunzburg R, Balagué F, Nordin M, Mélot C. A 2-year prospective longitudinal study on low back pain in primary school children. Eur Spine J. 2002;11:459–464. doi: 10.1007/s00586-002-0385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taimela S, Kujala UM, Salminen JJ, Viljanen T. The prevalence of low back pain among children and adolescents. A nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132–1136. doi: 10.1097/00007632-199705150-00013. [DOI] [PubMed] [Google Scholar]
- 23.Tertti MO, Salminen JJ, Paajanen HE, Terho PH, Kormano MJ. Low-back pain and disk degeneration in children: a case-control MR imaging study. Radiology. 1991;180:503–507. doi: 10.1148/radiology.180.2.1829844. [DOI] [PubMed] [Google Scholar]
- 24.Watson KD, Papageorgiou AC, Jones GT, Taylor S, Symmons DPM, Silman AJ, Macfarlane GJ. Low back pain in schoolchildren: occurrence and characteristics. Pain. 2002;97:87–92. doi: 10.1016/S0304-3959(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 25.Watson KD, Papageorgiou AC, Jones GT, Taylor S, Symmons DPM, Silman AJ, Macfarlane GJ. Low back pain in schoolchildren: the role of mechanical and psychosocial factors. Arch Dis Child. 2003;88:12–17. doi: 10.1136/adc.88.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wedderkopp N, Leboeuf-Yde C, Andersen LB, Karsten F. Back pain in children: no association with objectively measured level of physical activity. Spine. 2003;28:2019–2024. doi: 10.1097/01.BRS.0000083238.78155.31. [DOI] [PubMed] [Google Scholar]
- 27.Widhe T. Spine: posture, mobility and pain. A longitudinal study from childhood to adolescence. Eur Spine J. 2001;10:118–123. doi: 10.1007/s005860000230. [DOI] [PMC free article] [PubMed] [Google Scholar]