Abstract
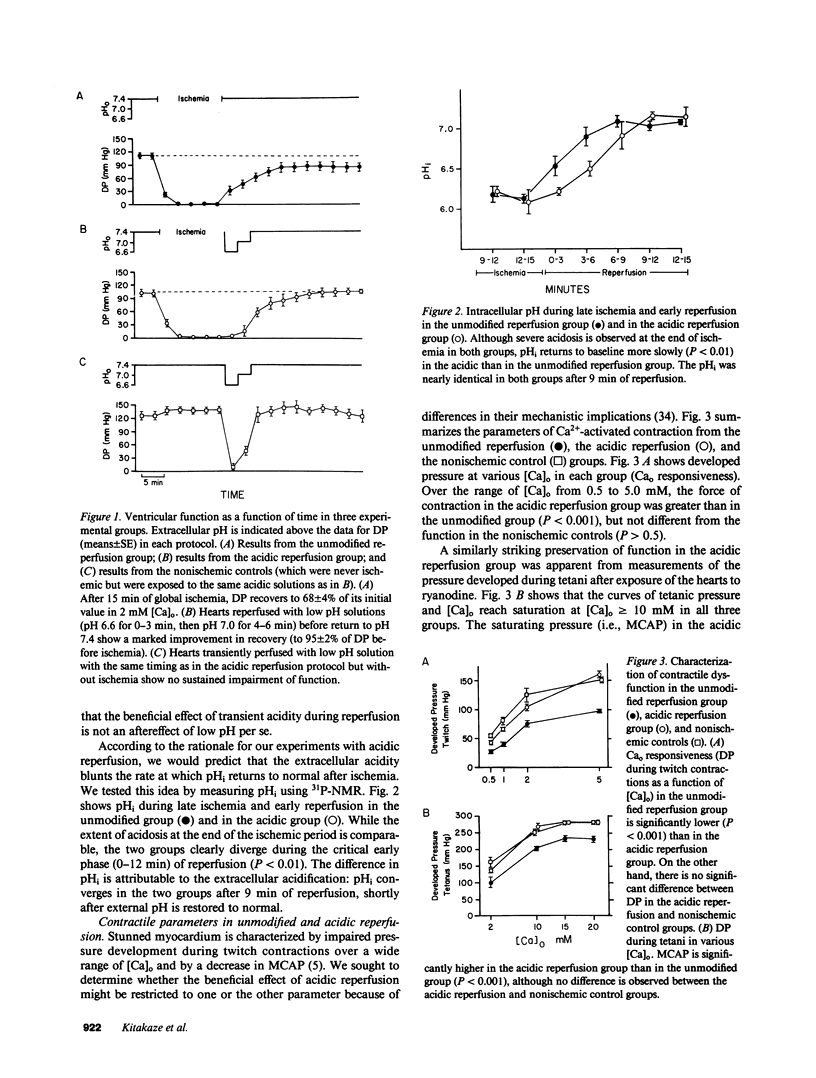
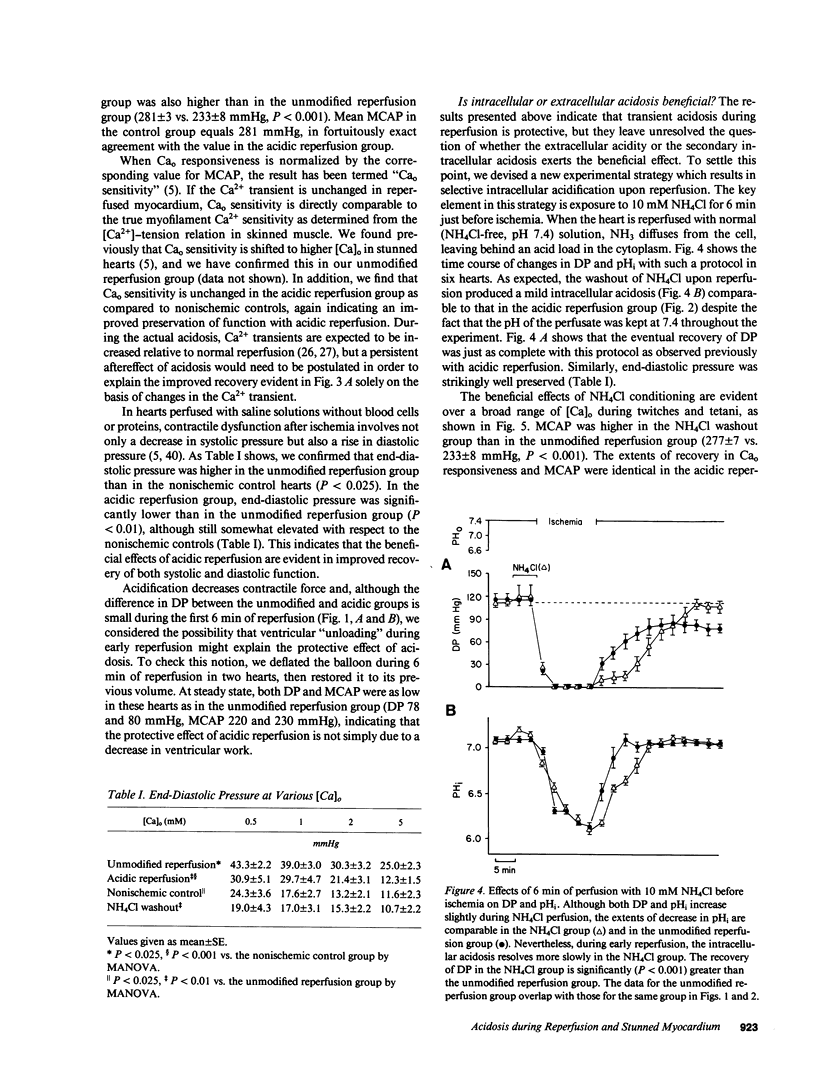
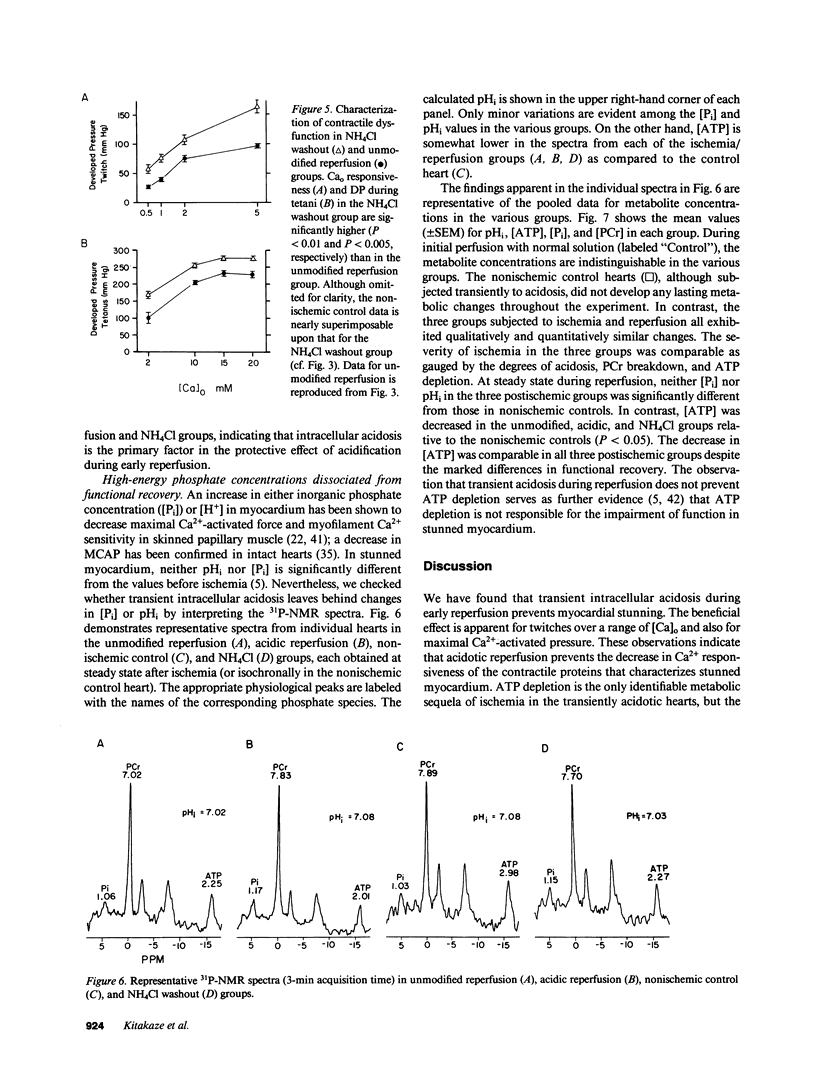
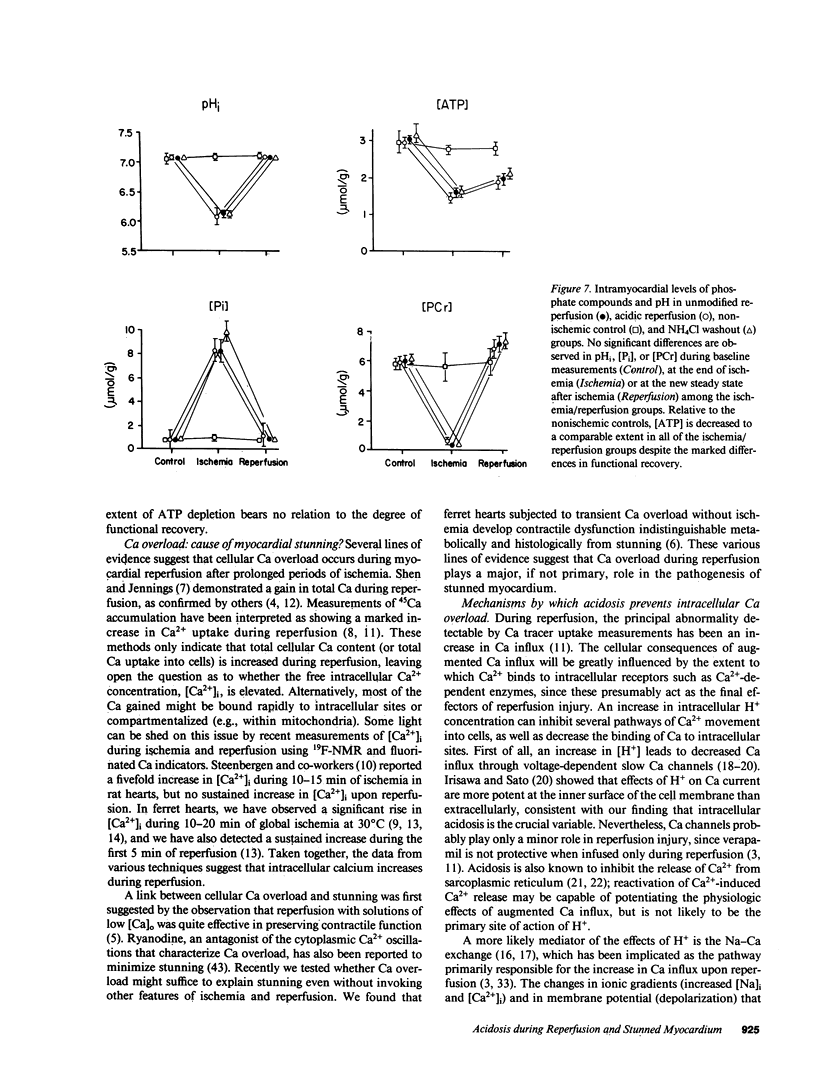
Cellular calcium overload figures prominently in the pathogenesis of the contractile dysfunction observed after brief periods of ischemia (myocardial stunning). Because acidosis is known to antagonize Ca influx and the intracellular binding of Ca, we reasoned that acidosis during reperfusion might prevent Ca overload and ameliorate functional recovery. We measured developed pressure (DP) and 31P-nuclear magnetic resonance spectra in 26 isovolumic Langendorff-perfused ferret hearts. After 15 min of global ischemia, hearts were reperfused either with normal solution (2 mM [Ca]o, Hepes-buffered, pH 7.4 bubbled with 100% O2; n = 6) or with acidic solutions (pH 6.6 during 0-3 min, pH 7.0 during 4-6 min) before returning to the normal perfusate (n = 7). Ventricular function after 30 min of reperfusion was much greater in the acidic group (105 +/- 5 mmHg at 2 mM [Ca]o) than in the unmodified reperfusion group (79 +/- 7 mmHg, P less than 0.001); similar differences in DP were found over a broad range of [Ca]o (0.5-5 mM, P less than 0.001) and during maximal Ca2+ activation (P less than 0.001). Intramyocardial pH (pHi) was lower in the acidic group than in the unmodified group during early reperfusion, but not at steady state. Phosphate compounds were comparable in both groups. To clarify whether the protective effect of acidosis is due to intracellular or extracellular pH, we produced selective intracellular acidosis during early reperfusion by exposure to 10 mM NH4Cl for 6 min just before ischemia (n = 6). For the first 12 min of reperfusion with NH4Cl-free solution (pH = 7.4), pHi was decreased relative to the unmodified group. Recovery of DP was practically complete, and maximal Ca2+-activated pressure was comparable to that in a nonischemic control group (n = 5). These results indicate that transient intracellular acidosis can prevent myocardial stunning, presumably owing to a reduction of Ca influx into cells and/or competition of H+ for intracellular Ca2+ binding sites during early reperfusion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barcenas-Ruiz L., Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in heart: membrane currents and changes in [Ca2+]i. Science. 1987 Dec 18;238(4834):1720–1722. doi: 10.1126/science.3686010. [DOI] [PubMed] [Google Scholar]
- Bing O. H., Brooks W. W., Messer J. V. Heart muscle viability following hypoxia: protective effect of acidosis. Science. 1973 Jun 22;180(4092):1297–1298. doi: 10.1126/science.180.4092.1297. [DOI] [PubMed] [Google Scholar]
- Bourdillon P. D., Poole-Wilson P. A. Effects of ischaemia and reperfusion on calcium exchange and mechanical function in isolated rabbit myocardium. Cardiovasc Res. 1981 Mar;15(3):121–130. doi: 10.1093/cvr/15.3.121. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985 Nov;76(5):1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982 Dec;66(6):1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- Chesnais J. M., Coraboeuf E., Sauviat M. P., Vassas J. M. Sensitivity to H, Li and Mg ions of the slow inward sodium current in frog atrial fibres. J Mol Cell Cardiol. 1975 Sep;7(9):627–642. doi: 10.1016/0022-2828(75)90140-6. [DOI] [PubMed] [Google Scholar]
- Donaldson S. K., Hermansen L., Bolles L. Differential, direct effects of H+ on Ca2+ -activated force of skinned fibers from the soleus, cardiac and adductor magnus muscles of rabbits. Pflugers Arch. 1978 Aug 25;376(1):55–65. doi: 10.1007/BF00585248. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty J. T., Weisfeldt M. L., Bulkley B. H., Gardner T. J., Gott V. L., Jacobus W. E. Mechanisms of ischemic myocardial cell damage assessed by phosphorus-31 nuclear magnetic resonance. Circulation. 1982 Mar;65(3):561–570. doi: 10.1161/01.cir.65.3.561. [DOI] [PubMed] [Google Scholar]
- Fry C. H., Poole-Wilson P. A. Effects of acid-base changes on excitation--contraction coupling in guinea-pig and rabbit cardiac ventricular muscle. J Physiol. 1981;313:141–160. doi: 10.1113/jphysiol.1981.sp013655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene H. L., Weisfeldt M. L. Determinants of hypoxic and posthypoxic myocardial contracture. Am J Physiol. 1977 May;232(5):H526–H533. doi: 10.1152/ajpheart.1977.232.5.H526. [DOI] [PubMed] [Google Scholar]
- Grinwald P. M. Calcium uptake during post-ischemic reperfusion in the isolated rat heart: influence of extracellular sodium. J Mol Cell Cardiol. 1982 Jun;14(6):359–365. doi: 10.1016/0022-2828(82)90251-6. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Sato R. Intra- and extracellular actions of proton on the calcium current of isolated guinea pig ventricular cells. Circ Res. 1986 Sep;59(3):348–355. doi: 10.1161/01.res.59.3.348. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Hecht H. H. Editorial: the early "pump" failure of the ischemic heart. Am J Med. 1969 Oct;47(4):497–502. doi: 10.1016/0002-9343(69)90180-6. [DOI] [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Cragoe E. J., Jr, Smith T. W. Relations among sodium pump inhibition, Na-Ca and Na-H exchange activities, and Ca-H interaction in cultured chick heart cells. Circ Res. 1987 Feb;60(2):185–193. doi: 10.1161/01.res.60.2.185. [DOI] [PubMed] [Google Scholar]
- Kim D., Smith T. W. Altered Ca fluxes and contractile state during pH changes in cultured heart cells. Am J Physiol. 1987 Jul;253(1 Pt 1):C137–C146. doi: 10.1152/ajpcell.1987.253.1.C137. [DOI] [PubMed] [Google Scholar]
- Kitakaze M., Weisman H. F., Marban E. Contractile dysfunction and ATP depletion after transient calcium overload in perfused ferret hearts. Circulation. 1988 Mar;77(3):685–695. doi: 10.1161/01.cir.77.3.685. [DOI] [PubMed] [Google Scholar]
- Kléber A. G. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res. 1983 Apr;52(4):442–450. doi: 10.1161/01.res.52.4.442. [DOI] [PubMed] [Google Scholar]
- Kusuoka H., Porterfield J. K., Weisman H. F., Weisfeldt M. L., Marban E. Pathophysiology and pathogenesis of stunned myocardium. Depressed Ca2+ activation of contraction as a consequence of reperfusion-induced cellular calcium overload in ferret hearts. J Clin Invest. 1987 Mar;79(3):950–961. doi: 10.1172/JCI112906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuoka H., Weisfeldt M. L., Zweier J. L., Jacobus W. E., Marban E. Mechanism of early contractile failure during hypoxia in intact ferret heart: evidence for modulation of maximal Ca2+-activated force by inorganic phosphate. Circ Res. 1986 Sep;59(3):270–282. doi: 10.1161/01.res.59.3.270. [DOI] [PubMed] [Google Scholar]
- Lakatta E. G., Nayler W. G., Poole-Wilson P. A. Calcium overload and mechanical function in posthypoxic myocardium: biphasic effect of pH during hypoxia. Eur J Cardiol. 1979 Jul;10(1):77–87. [PubMed] [Google Scholar]
- Langer G. A. The effect of pH on cellular and membrane calcium binding and contraction of myocardium. A possible role for sarcolemmal phospholipid in EC coupling. Circ Res. 1985 Sep;57(3):374–382. doi: 10.1161/01.res.57.3.374. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Smith N., Mohabir R., Clusin W. T. Cytosolic calcium transients from the beating mammalian heart. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7793–7797. doi: 10.1073/pnas.84.21.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Kusuoka H., Porterfield J. K., Yue D. T., Chacko V. P. Intracellular free calcium concentration measured with 19F NMR spectroscopy in intact ferret hearts. Proc Natl Acad Sci U S A. 1987 Aug;84(16):6005–6009. doi: 10.1073/pnas.84.16.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban E., Kusuoka H., Yue D. T., Weisfeldt M. L., Wier W. G. Maximal Ca2+-activated force elicited by tetanization of ferret papillary muscle and whole heart: mechanism and characteristics of steady contractile activation in intact myocardium. Circ Res. 1986 Sep;59(3):262–269. doi: 10.1161/01.res.59.3.262. [DOI] [PubMed] [Google Scholar]
- Nakamaru Y., Schwartz A. The influence of hydrogen ion concentration on calcium binding and release by skeletal muscle sarcoplasmic reticulum. J Gen Physiol. 1972 Jan;59(1):22–32. doi: 10.1085/jgp.59.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G. Calcium and cell death. Eur Heart J. 1983 May;4 (Suppl 100):33–41. doi: 10.1093/eurheartj/4.suppl_c.33. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Ferrari R., Poole-Wilson P. A., Yepez C. E. A protective effect of a mild acidosis on hypoxic heart muscle. J Mol Cell Cardiol. 1979 Oct;11(10):1053–1071. doi: 10.1016/0022-2828(79)90394-8. [DOI] [PubMed] [Google Scholar]
- Orchard C. H. The role of the sarcoplasmic reticulum in the response of ferret and rat heart muscle to acidosis. J Physiol. 1987 Mar;384:431–449. doi: 10.1113/jphysiol.1987.sp016462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson K. D., Bersohn M. M., Nishimoto A. Y. Effects of pH on Na+-Ca2+ exchange in canine cardiac sarcolemmal vesicles. Circ Res. 1982 Feb;50(2):287–293. doi: 10.1161/01.res.50.2.287. [DOI] [PubMed] [Google Scholar]
- Regan T. J., Broisman L., Haider B., Eaddy C., Oldewurtel H. A. Dissociation of myocardial sodium and potassium alterations in mild versus severe ischemia. Am J Physiol. 1980 Apr;238(4):H575–H580. doi: 10.1152/ajpheart.1980.238.4.H575. [DOI] [PubMed] [Google Scholar]
- Renlund D. G., Gerstenblith G., Lakatta E. G., Jacobus W. E., Kallman C. H., Weisfeldt M. L. Perfusate sodium during ischemia modifies post-ischemic functional and metabolic recovery in the rabbit heart. J Mol Cell Cardiol. 1984 Sep;16(9):795–801. doi: 10.1016/s0022-2828(84)80003-6. [DOI] [PubMed] [Google Scholar]
- Sharma A. D., Saffitz J. E., Lee B. I., Sobel B. E., Corr P. B. Alpha adrenergic-mediated accumulation of calcium in reperfused myocardium. J Clin Invest. 1983 Sep;72(3):802–818. doi: 10.1172/JCI111051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Kinetics of calcium accumulation in acute myocardial ischemic injury. Am J Pathol. 1972 Jun;67(3):441–452. [PMC free article] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Myocardial calcium and magnesium in acute ischemic injury. Am J Pathol. 1972 Jun;67(3):417–440. [PMC free article] [PubMed] [Google Scholar]
- Steenbergen C., Hill M. L., Jennings R. B. Cytoskeletal damage during myocardial ischemia: changes in vinculin immunofluorescence staining during total in vitro ischemia in canine heart. Circ Res. 1987 Apr;60(4):478–486. doi: 10.1161/01.res.60.4.478. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Murphy E., Levy L., London R. E. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ Res. 1987 May;60(5):700–707. doi: 10.1161/01.res.60.5.700. [DOI] [PubMed] [Google Scholar]
- Weisfeldt M. L., Bishop R. L., Greene H. L. Effects of pH and pCO2 on performance of ischemic myocardium. Recent Adv Stud Cardiac Struct Metab. 1975;10:355–364. [PubMed] [Google Scholar]
- Wexler L. F., Weinberg E. O., Ingwall J. S., Apstein C. S. Acute alterations in diastolic left ventricular chamber distensibility: mechanistic differences between hypoxemia and ischemia in isolated perfused rabbit and rat hearts. Circ Res. 1986 Nov;59(5):515–528. doi: 10.1161/01.res.59.5.515. [DOI] [PubMed] [Google Scholar]
- Wilde A. A., Kléber A. G. The combined effects of hypoxia, high K+, and acidosis on the intracellular sodium activity and resting potential in guinea pig papillary muscle. Circ Res. 1986 Feb;58(2):249–256. doi: 10.1161/01.res.58.2.249. [DOI] [PubMed] [Google Scholar]



