Abstract
The effectiveness of clinical measures to predict scoliotic progression is unclear. The objective of this study was to identify potential prognostic factors affecting scoliosis progression. Consecutive measurements (181) from 35 non-instrumented adolescent idiopathic scoliosis patients with at least two follow-up assessments were studied. Potential prognostic factors of gender, curve pattern, age, curve magnitude, apex location and lateral deviation and spinal growth were analyzed. Stable and progressed groups were compared (threshold: Cobb angle ≥5° or 10°) with sequential clinical data collected in 6-month intervals. Double curves progressed simultaneously or alternatively on curve regions. Age was not significantly different prior to and at maximal Cobb angle. Maximal Cobb angles were significantly correlated to initial Cobb angles (r = 0.81–0.98). Progressed males had larger initial Cobb angles than progressed females. Apex locations were higher in progressed than stable groups, and at least a half vertebra level higher in females than males. Maximal apex lateral deviations correlated significantly with the initial ones (r = 0.73–0.97) and moderately with maximal Cobb angles (r = 0.33–0.85). In the progressed groups, males had larger apex lateral deviations than females. Spinal growth did not relate to curve progression (r = −0.64 to +0.59) and was not significantly different between groups and genders. Scoliosis may dynamically progress between major and minor curves. Gender, curve magnitude, apex location and lateral deviation have stronger effects on scoliosis progression than age or spinal growth. Females with high apex locations may be expected to progress.
Keywords: Adolescent idiopathic scoliosis, Prognostic factors, Scoliosis progression, Cobb angle, Lateral deviation
Introduction
A major concern of orthopedic surgeons in managing adolescent idiopathic scoliosis (AIS) patients with a minor curvature is identifying which curves will progress to moderate or severe deformities requiring treatment [1, 3, 11, 16, 23]. Previous studies have indicated that various factors have been associated with curve progression, such as gender, age, curve pattern, curve magnitude, apex location and growth velocity [4, 7, 9, 11, 13–15, 18, 20, 21, 25]. However, it is not clear to what extent they can be used in predicting the course of the natural history of the scoliotic curve [3, 16, 19, 24, 26].
When analyzing the progression of spinal deformities, the use of larger or smaller time intervals and various time points for different subjects can skew the progression or growth velocities [9, 13, 26]. It may be more significant to analyze the clinical data prior to the maximum and progressed data in time series data sets than the first clinic visits randomly referred by orthopedic surgeons. Furthermore, the growth velocity of height is different from the spinal growth velocity [13, 25, 26].
Literature also varies in how scoliosis progression is defined, such as incremental Cobb angle (5° and 10°) and the time spans of measurements for identification of progression [9, 10, 13, 15, 23]. This may have resulted in a blinding or canceling of the significances of some prognostic factors.
It is known that the progression of AIS spinal deformity is non-linear with time and difficult to predict [13, 16, 20, 23, 25]. Therefore, the objective of this study was to detect potential prognostic factors affecting scoliosis progression and catch the on-set of progression, using consistent time steps to remove this as a potential confounding variable. Prognostic factors included gender, curve pattern, age, curve magnitude, apex location and lateral deviation, and spinal growth. Data at the onset and prior to the maximum deformity and progression were analyzed for factors of age, curve magnitude, apex lateral deviation and spinal growth. It was hypothesized that spinal deformities of AIS patients smoothly change with time.
Methods
Data acquisition
Data from 35 subjects with AIS [29 females and 6 males, 12.3 years (SD 2.3), range 8.6–17.2 years, Cobb angle 30.2º (SD 12.4°), range 10.1°–69.9° at the first visit] were used for this study. Subjects were selected from the project database (1997–2002) based on the following criteria: (1) a diagnosis of AIS in patients older than 8 years, (2) no instrumentation/surgery during consecutive measurements, and (3) at least two successive follow-ups after the first visit in the 6-month interval. Two standing whole spinal posteroanterior (PA) radiographs at 0° and 20° angled down were obtained for all patients at each visit. The maximum number of available successive measurements for any individual AIS patient was 10, and there were 181 consecutive measurements in total (Table 1). The Conjoint Health Research Ethics Board approved all aspects of the experimental protocol and all subjects gave their informed consent.
Table 1.
Distribution of curve patterns and genders of AIS subjects by visits and format of series data
| Curve patterns | Subjects | Female subjects | Visits | Female visits | Format of series dataa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Three data | Four data | Five data | ||||||||
| Female | Male | Female | Male | Female | Male | |||||
| Left lumbar (LL) | 3 | 2 | 16 | 7 | 3 | 7 | 1 | 6 | 0 | 5 |
| Left thoracic (LT) | 2 | 2 | 13 | 13 | 9 | 0 | 7 | 0 | 5 | 0 |
| Left thoracolumbar (LTL) | 1 | 1 | 10 | 10 | 8 | 0 | 7 | 0 | 6 | 0 |
| Right lumbar (RL) | 1 | 1 | 8 | 8 | 6 | 0 | 5 | 0 | 4 | 0 |
| Right thoracic (RT) | 11 | 7 | 28 | 28 | 14 | 13 | 7 | 9 | 3 | 6 |
| Right thoracic and left lumbar (RT–LL) | 17 | 16 | 75 | 75 | 43 | 8 | 27 | 7 | 15 | 6 |
| Total | 35 | 29 | 141 | 141 | – | – | – | – | – | – |
aThree, four and five-series data indicate the number of time series data points
A three-dimensional (3D) geometric model of the whole spine was morphologically represented by linking each vertebra in the thoracolumbar region (T1–L5). A 3D spinal curve was represented by the line passing through the centers of pedicles [2, 12]. The spinal curve was further best fit with a third-order Fourier series with seven coefficients [17].
Prognostic factors
The Cobb angle and apex lateral deviation were computed, similar to the clinical measure (Scoliosis Research Society), as the angle between perpendiculars to the spine curve at inflectional points and the distance of apical point from the vertical line from L5. The apex location was defined as the convex/concave point of a primary curve. Spinal length was the distance from the vertebrae T1 to L5 on the coronal plane or in space. The computed Cobb angle was slightly greater (13%) than the clinical Cobb angle, but was highly correlated with it (r = 0.97) [22]. The inter- and intra-observer variation of computed Cobb angle has been reported at between 0.4° and 1.8° in the frontal plane [6, 12].
Serial data of artificial grouping of the patients
Three sub-databases with three, four or five sequential values were formed by extracting the respective serial data from the foregoing database. All three-, four- and five-serial data sets formed corresponding sub-databases. Each sub-database was further divided into two groups, ‘stable’ and ‘progressed’, by a progression threshold of 5° or 10°. This resulted in three sub-databases, each having one pair of stable and progressed groups. Initial data were defined at the time point prior to the maximal data for the stable group, and at the time point before the progressed data for the progressed group.
Data diagnosis
To study the prognostic factors that affect scoliosis progression, data of prognostic factors at the onset and prior to maximal curvature and progression in serial data sets were analyzed by comparing between two groups (i.e., stable vs. progressed groups) of sequential data sets (i.e., three-, four- and five-serial data). A student’s t test was used to compare between groups, with a statistical significance level of P < 0.05. Relations between factors were determined using Pearson’s correlation coefficient (r).
Results
In total, 111, 76 and 50 serial data sets were formed in the three-, four- and five-serial data sets, respectively (Table 2). Maximal incremental Cobb angles were 3.65° (SD 4.10°), 4.52° (SD 4.04°) and 4.79° (SD 3.91°) in the respective sub-databases. Progressed AIS data sets comprised 35–46% of all data sets at a progression threshold of 5°, while only 12–18% sets were progressed at the 10° threshold.
Table 2.
Distribution of AIS subjects in sub-databases in database and progressed groups by gender, and for simultaneously and alternatively progressed double curves (RT-LL)
| Progression threshold | Format | Database | Progressed group | Progressed group/database | Progressed double curve (RT–LL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female (F) | Male (M) | F/(F + M) | Female (F) | Male (M) | F/(F + M) | F/F | M/M | (F + M)/(F + M) | RT + LL | RT | LL | Total | ||
| Cobb angle 5º | 3-data | 83 | 28 | 0.75 | 25 | 14 | 0.64 | 0.30 | 0.50 | 0.35 | 11 | 5 | 7 | 34 |
| 4-data | 54 | 22 | 0.71 | 17 | 14 | 0.55 | 0.31 | 0.64 | 0.41 | 10 | 2 | 5 | 27 | |
| 5-data | 33 | 17 | 0.66 | 10 | 13 | 0.43 | 0.30 | 0.76 | 0.46 | 7 | 1 | 4 | 19 | |
| Cobb angle 10º | 3-data | 83 | 28 | 0.75 | 7 | 6 | 0.54 | 0.08 | 0.21 | 0.12 | 1 | 3 | 2 | 7 |
| 4-data | 54 | 22 | 0.71 | 6 | 6 | 0.50 | 0.11 | 0.27 | 0.16 | 1 | 3 | 2 | 7 | |
| 5-data | 33 | 17 | 0.66 | 4 | 5 | 0.44 | 0.12 | 0.29 | 0.18 | 1 | 2 | 2 | 6 | |
RT right thoracic, LL left lumbar
Gender and curve pattern
As demonstrated in Table 2, AIS patients were predominantly female (66–75% in three sub-databases). Approximately, 30% of females showed a progression at the 5° threshold compared to an average of 60% for males. Approximately, 10% of females made a progression greater than or equal to 10° compared to an average of 26% for males. In females with a double curve, 42–47% progressed at the 5° threshold, and 5–7% progressed at the 10° threshold. All double curves had a major RT curve. Double curves (RT-LL) did not consistently progress in the same curve region (primary or minor) (Table 2).
Age
There were no significant differences for initial ages and ages with maximal Cobb angle between stable and respective progressed groups in three sub-databases for both progression thresholds. Subject ages with maximal Cobb angle in the progressed groups had a tendency to be approximately 6 months older than the ones in the stable groups, but this was not significant. In progressed groups, male subjects were generally 6 months younger than females, and in the case of the five-serial data sets at a progression threshold of 5°, the finding was significant.
Curve magnitude
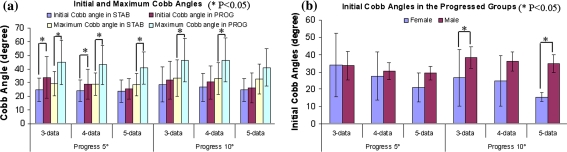
Initial Cobb angles in the stable groups in sub-databases of three- and four-serial data sets were significantly different from those in the corresponding progressed groups (Fig. 1a). Maximal Cobb angles were significantly different between all stable and progressed groups except in the sub-database of five-serial data sets. Maximal Cobb angles were significantly correlated to initial Cobb angles in both stable and progressed groups (r = 0.81–0.98), moderately correlated to age with initial and maximal Cobb angles in the stable groups (r = 0.31–0.56 and 0.21–0.56, respectively) and poorly in the progressed groups (r = −0.31 to +0.36 and −0.27 to +0.36, respectively). Initial Cobb angles of females and males were significantly different in the two progressed groups of three- and five-serial data sets for the progression threshold of 10° (Fig. 1b), and the initial Cobb angle in male subjects had a tendency to be greater than in females (9°). The incidences of spontaneous improvement were 18, 7 and 4% in the three sub-databases, respectively. The most improved curves were RT–LL, followed by RT, left thoracic (LT), LL and left thoracolumbar (LTL) by 1.7°–5.4° between consecutive measurements.
Fig. 1.
a Average initial and maximum Cobb angles in the stable (‘STAB’) and progressed (‘PROG’) groups. b Average initial Cobb angles by gender in the progressed groups
Apex location and lateral deviation
In 264 consecutive curves, curve regions were consistent with time. Apex locations in the progressed group were significantly higher (~1.2 vertebra levels) than those in the stable group for the progression threshold of 5°. There was a tendency for females to have higher apex locations (at least half vertebra level) than males in the progressed groups.
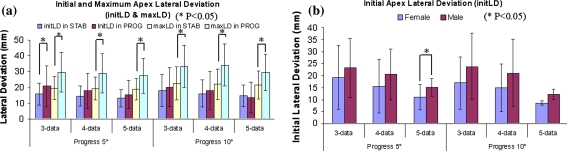
Maximal apex lateral deviations were significantly different between the stable and respective progressed groups, but not for initial apex lateral deviations (Fig. 2a). Maximal apex lateral deviations were strongly correlated with initial lateral deviations (r = 0.73–0.97) in all sub-databases, moderately correlated with maximal Cobb angles (r = 0.33–0.78) in the stable groups and strongly correlated (r = 0.77–0.85) in the progressed groups. Maximal Cobb angles had moderate correlations with initial apex lateral deviations in the stable groups (r = 0.36–0.79) and in the progressed groups (r = 0.58–0.83).
Fig. 2.
a Average initial and maximum apex lateral deviations in the stable (‘STAB’) and progressed (‘PROG’) groups. b Average initial apex lateral deviations by gender in the progressed groups
In progressed groups, male AIS patients had larger initial and maximal apex lateral deviations and apex lateral deviations at the initial and maximal Cobb angles when compared to females (Fig. 2b), varying by 13 mm within a 95% confidence interval.
Spinal growth
Coincidental times with maximal spinal length in the coronal plane were similar to those in space but not the same as those with maximal Cobb angles (a variation of 9 months within a 95% confidence interval) and with maximal apex lateral deviations (a variation of 7 months within a 95% confidence interval). Spinal growth in the coronal plane and in 3D had poor or fair correlations with the curve progression in the stable groups (r = 0.0–0.38 and 0.09–0.43, respectively) and in the progressed groups (r = −0.64 to 0.59 and −0.52 to 0.01, respectively). Also, maximal spinal lengths on the coronal plane and in 3D poorly correlated with maximal apex lateral deviations in the stable groups (r = 0.08–0.43 and 0.03–0.32, respectively) and in the progressed groups (r = 0.0–0.29 and −0.07 to 0.2, respectively). There was no evidence for significant differences in spinal growths in the coronal plane and in 3D between genders in progressed groups.
Discussion
The present study presented a retrospective review of AIS patients from time series measurements at equal time intervals (6 months). The AIS progression was defined as an increase of at least 5° or 10° of Cobb angle in the 6-month interval within consecutive measurements. The incidence of progression of serial data sets were 35, 41 and 46% in three sub-databases with a progression threshold of 5°, respectively, which were comparable to previous reports of 68, 60 and 56% [4, 15, 24]. With a progression threshold of 10°, the incidence of progression was 12, 16 and 18%, respectively, which were similar to other studies (e.g., 5.0, 6.8 and 14.7%) [3, 18, 20]. The incidence of spontaneous improvement of 18% in the sub-data of three-serial data sets was similar to 22.0 and 27.4% reported in literature [3, 20]. There were only 7 and 4% of improvement incidence for four- and five-serial data sets in the current study, respectively, which may be caused by the long time interval (2 and 2.5 years) of consecutive measurements when assessing the improvement in this study. The resolved curves were primarily double curves, followed by right thoracic curves.
There were no significant differences for initial ages and ages with maximal curve magnitudes between stable and respective progressed groups, suggesting that there was not a direct linear relation between age and the incidence of progression, similar to previous reports [10, 11, 16, 20]. It was shown that AIS patients had greater curve magnitudes and greater chance of progression with adolescent growth. The present study also found that AIS males may progress at similar or even younger ages than females, which had not been noted in previous reports.
Scoliotic progression directly correlated to the magnitudes of initial scoliotic curve, the initial and maximal Cobb angles were significantly different between the stable and progressed groups, and the maximal Cobb angles excellently correlated with the initial Cobb angles, similar to previous reports [8, 14, 20, 21].
As reported previously [26], the current study found that the major and minor curves of double curve RT-LL made spinal deformity progressions simultaneously and alternatively. In addition, curve regions were consistent with time, varying within a quarter of one vertebral offset within a 95% confidence interval. These results may be individually explained by the mechanical bulking theory [5]. AIS patients with a higher apex location had a greater incidence of progression, which was in agreement with previous reports [1, 16, 24]. The finding that progressed females had higher apex locations in the progressed groups may be a factor for the high incidence of AIS among females.
Later curve progression had a strong correlation with initial apex lateral deviation. However, initial and maximal apex lateral deviations were not coincident with initial and maximal Cobb angles. Thus, the scoliosis spinal deformity may not be a pure mechanical bulking problem, and the vertebral rotation with time should be considered. Both Cobb angle and apex lateral deviation may be independent factors for prediction of scoliotic progression.
Substantial height growth velocity may be caused mainly by the rapid growth of lower limbs and partially by spinal growth. The present study analyzed the spinal growth, instead of using the height growth velocity as in previous studies [13, 25, 26]. There were no linear relations between maximal spinal length and maximal spinal curvature (i.e., Cobb angle and apex lateral deviation). One reason may have been that the vertebral rotation affected the spinal deformities, which was reflected in the spinal curvatures. High spinal growth velocity was not a clear cause for high incidence of progression.
Conclusion
Scoliosis has a stable curve region, and progression may dynamically occur between major and minor curve regions. Gender, curve magnitude, apex location and lateral deviation have stronger effects on scoliosis progression than age and spinal growth. Substantial progression can be expected from curves with high apex locations. Females with high apex locations can be expected to progress even with small initial curvatures (Cobb angles and lateral deviations). Cobb angle and apex lateral deviation should be simultaneously evaluated for scoliotic progression. Torso twist and vertebral rotation may be prognostic factors for progression. Apex lateral deviations changed with curve magnitudes and spinal growth, but curve magnitudes did not always increase with spinal growth.
Acknowledgments
The authors would like to recognize the contributions of the Canadian Institutes of Health Research (CIHR), the Alberta Children’s Hospital Foundation, the Fraternal Order of Eagles (Alberta & Saskatchewan), and the Natural Sciences and Engineering Research Council of Canada (NSERC).
References
- 1.Ascani E, Bartolozzi P, Logroscino CA, Marchetti PG, Ponte A, Savini R, Travaglini F, Binazzi R, Di Silvestre M. Natural history of untreated idiopathic scoliosis after skeletal maturity. Spine. 1986;11:784–789. doi: 10.1097/00007632-198610000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Aubin CE, Dansereau J, Parent F, Labelle H, Guise JA. Morphometric evaluations of personalised 3D reconstructions and geometric models of the human spine. Med Biol Eng Comput. 1997;35:611–618. doi: 10.1007/BF02510968. [DOI] [PubMed] [Google Scholar]
- 3.Brooks HL, Azen SP, Gerberg E, Brooks R, Chan L. Scoliosis: a prospective epidemiological study. J Bone Joint Surg Am. 1975;57:968–972. [PubMed] [Google Scholar]
- 4.Bunnell WP. The natural history of idiopathic scoliosis before skeletal maturity. Spine. 1986;11:773–776. doi: 10.1097/00007632-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Chajes A. Principles of structural stability theory. Englewood Cliffs NJ: Prentice-Hall; 1974. [Google Scholar]
- 6.Delorme S, Petit Y, Guise JA, Labelle H, Aubin CE, Dansereau J. Assessment of the 3-D reconstruction and high-resolution geometrical modeling of the human skeletal trunk from 2-D radiographic images. IEEE Trans Biomed Eng. 2003;50:989–998. doi: 10.1109/TBME.2003.814525. [DOI] [PubMed] [Google Scholar]
- 7.Duval-Beaupere G. Rib hump and supine angle as prognostic factors for mild scoliosis. Spine. 1992;17:103–107. doi: 10.1097/00007632-199201000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Fustier T (1980) Evolution radiologique spontanee des scolioses idiopathiques de moins de 45 degrees en periode de croissance. Etude graphique retrospective de cente dossiers du centre de readaptation fonctionnelle des massues. These, University Claude, Bernard, Lyon
- 9.Goldberg CJ, Dowling FE, Hall JE, Emans JB. A statistical comparison between natural history of idiopathic scoliosis and brace treatment in skeletally immature adolescent girls. Spine. 1993;18:902–908. doi: 10.1097/00007632-199306000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg MS, Mayo NE, Poitras B, Scott S, Hanley J. The Ste-Justine Adolescent Idiopathic Scoliosis Cohort Study. Part ii: perception of health, self and body image, and participation in physical activities. Spine. 1994;19:1562–1572. doi: 10.1097/00007632-199407001-00004. [DOI] [PubMed] [Google Scholar]
- 11.Karol LA, Johnston CE, 2nd, Browne RH, Madison M. Progression of the curve in boys who have idiopathic scoliosis. J Bone Joint Surg Am. 1993;75:1804–1810. doi: 10.2106/00004623-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Labelle H, Dansereau J, Bellefleur C, Jequier JC. Variability of geometric measurements from three-dimensional reconstructions of scoliotic spines and rib cages. Eur Spine J. 1995;4:88–94. doi: 10.1007/BF00278918. [DOI] [PubMed] [Google Scholar]
- 13.Little DG, Song KM, Katz D, Herring JA. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis in girls. J Bone Joint Surg Am. 2000;82:685–693. doi: 10.2106/00004623-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Lonstein JE. Natural history and school screening for scoliosis. Orthop Clin North Am. 1988;19:227–237. [PubMed] [Google Scholar]
- 15.Nachemson AL, Lonstein JE, Weinstein S. Report of the prevalence and natural history committee of the Scoliosis Research Society. Denver, Colorado: Annual Meeting of the Scoliosis Research Society; 1982. [Google Scholar]
- 16.Peterson LE, Nachemson AL. Prediction of progression of the curve in girls who have adolescent idiopathic scoliosis of moderate severity. Logistic regression analysis based on data from the brace study of the Scoliosis Research Society. J Bone Joint Surg Am. 1995;77:823–827. doi: 10.2106/00004623-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Poncet P, Dansereau J, Labelle H. Geometric torsion in idiopathic scoliosis: three-dimensional analysis and proposal for a new classification. Spine. 2001;26:2235–2243. doi: 10.1097/00007632-200110150-00015. [DOI] [PubMed] [Google Scholar]
- 18.Rogala EJ, Drummond DS, Gurr J. Scoliosis: incidence and natural history. A prospective epidemiological study. J Bone Joint Surg Am. 1978;60:173–176. [PubMed] [Google Scholar]
- 19.Siu King Cheung C, Tak Keung Lee W, Kit Tse Y, Ping Tang S, Man Lee K, Guo X, Qin L, Chun Yiu Cheng J. Abnormal peri-pubertal anthropometric measurements and growth pattern in adolescent idiopathic scoliosis: a study of 598 patients. Spine. 2003;28:2152–2157. doi: 10.1097/01.BRS.0000084265.15201.D5. [DOI] [PubMed] [Google Scholar]
- 20.Soucacos PN, Zacharis K, Gelalis J, Soultanis K, Kalos N, Beris A, Xenakis T, Johnson EO. Assessment of curve progression in idiopathic scoliosis. Eur Spine J. 1998;7:270–277. doi: 10.1007/s005860050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stirling AJ, Howel D, Millner PA, Sadiq S, Sharples D, Dickson RA. Late-onset idiopathic scoliosis in children six to fourteen years old. A cross-sectional prevalence study. J Bone Joint Surg Am. 1996;78:1330–1336. doi: 10.2106/00004623-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Stokes IA, Shuma-Hartswick D, Moreland MS. Spine and back-shape changes in scoliosis. Acta Orthop Scand. 1988;59:128–133. [PubMed] [Google Scholar]
- 23.Theologis TN, Fairbank JC, Turner-Smith AR, Pantazopoulos T. Early detection of progression in adolescent idiopathic scoliosis by measurement of changes in back shape with the integrated shape imaging system scanner. Spine. 1997;22:1223–1227. doi: 10.1097/00007632-199706010-00010. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein SL, Ponseti IV. Curve progression in idiopathic scoliosis. J Bone Joint Surg Am. 1983;65:447–455. [PubMed] [Google Scholar]
- 25.Ylikoski M. Height of girls with adolescent idiopathic scoliosis. Eur Spine J. 2003;12:288–291. doi: 10.1007/s00586-003-0527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ylikoski M. Growth and progression of adolescent idiopathic scoliosis in girls. J Pediatr Orthop B. 2005;14:320–324. doi: 10.1097/01202412-200509000-00002. [DOI] [PubMed] [Google Scholar]