Abstract
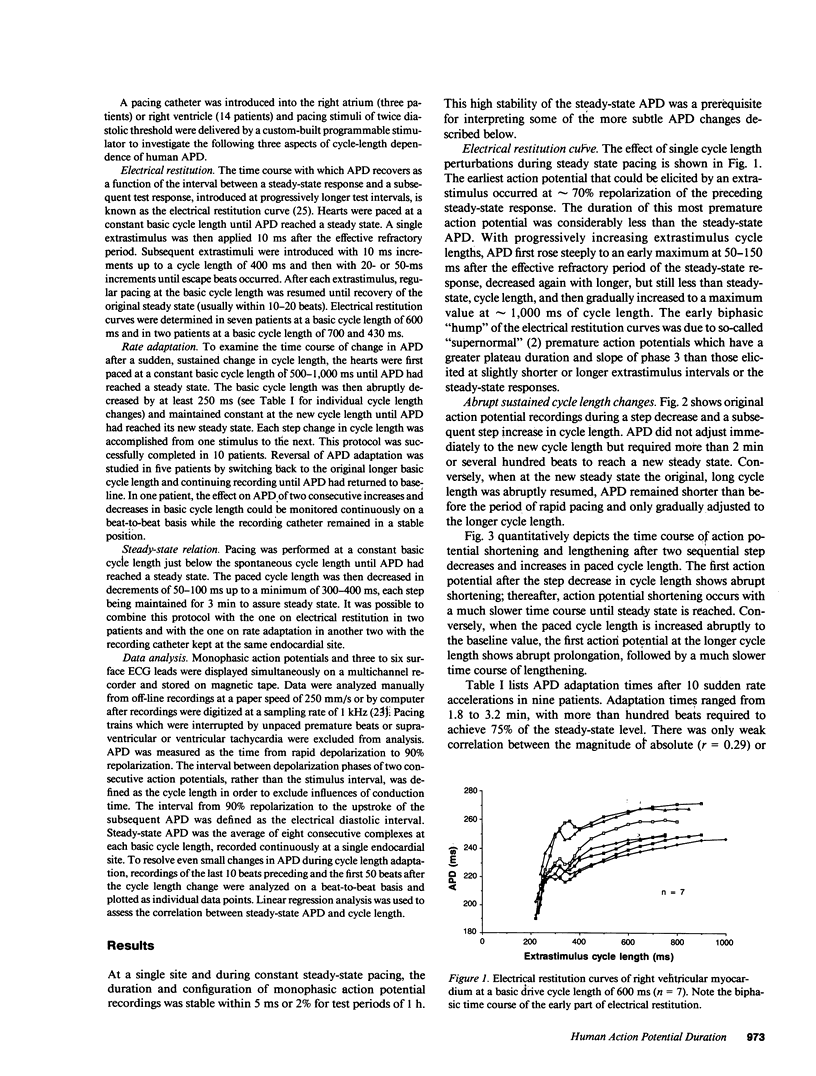
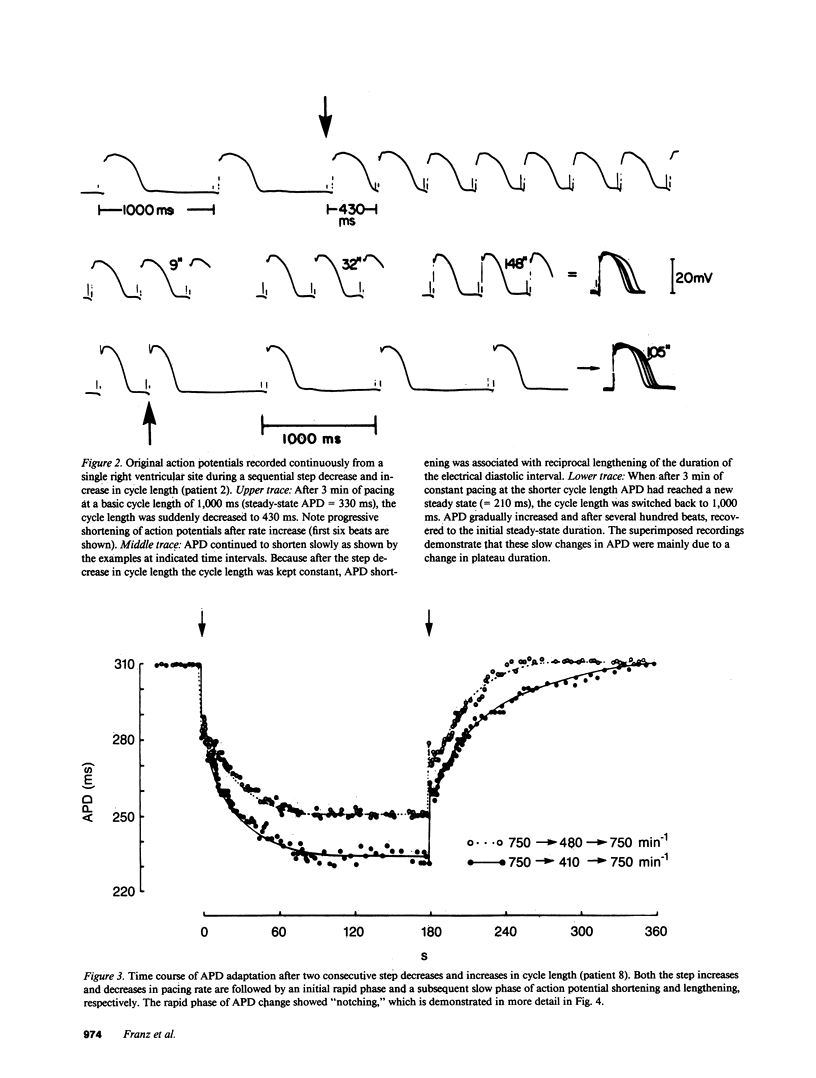
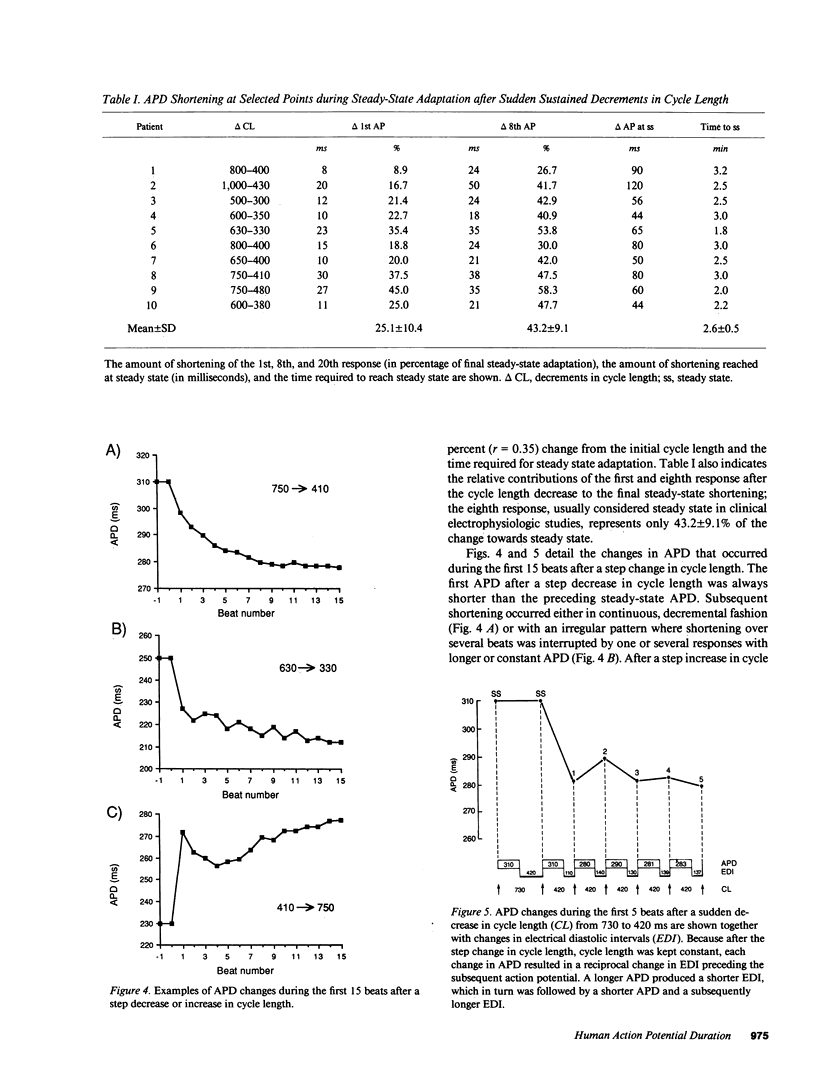
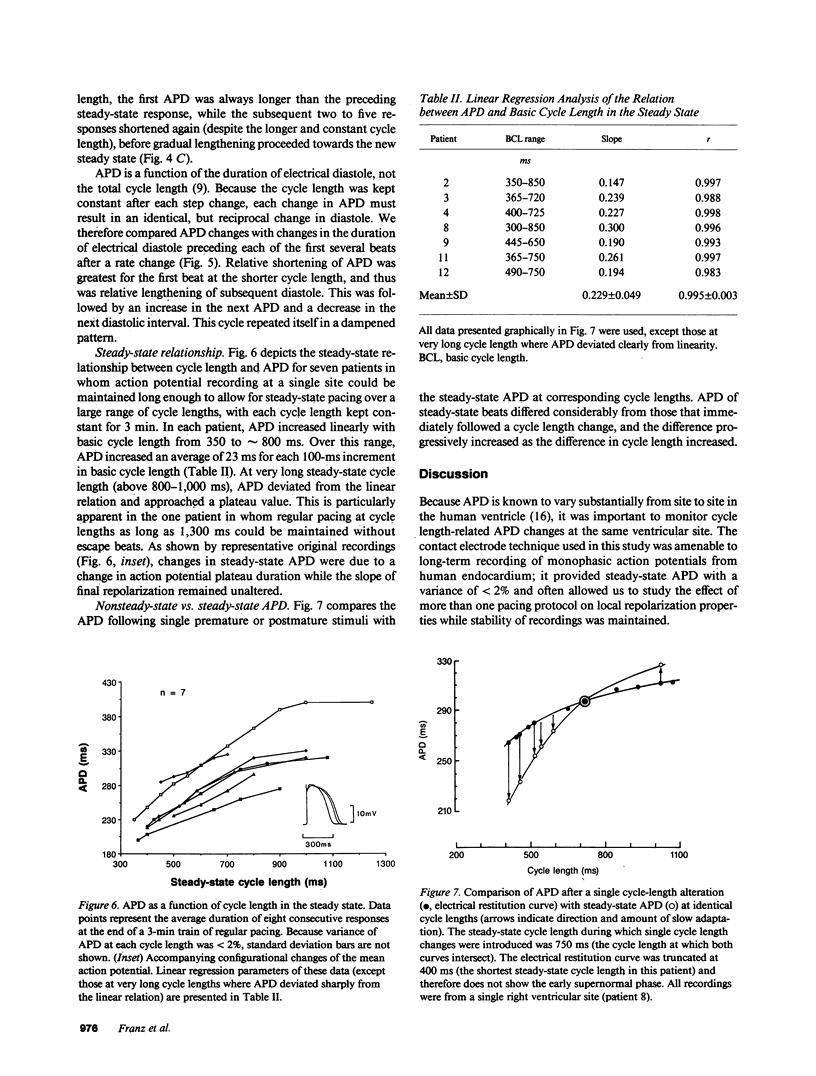
Using a new method for long-term recording of monophasic action potentials from the human heart, we studied in 17 patients the effects on ventricular action potential duration (APD) of three clinically pertinent cycle length perturbations: (1) single extrastimuli, (2) abrupt sustained rate acceleration and deceleration, and (3) different steady-state cycle lengths. Results were: (a) APD after single extrastimuli at progressively longer cycle lengths were related to the extrastimulus cycle length with a biphasic electrical restitution curve which after an initial steep rise and a subsequent transient descent rose again more gradually to a plateau at cycle lengths above 800-1,000 ms. (b) After a sustained step decrease in cycle length, the first APD shortened abruptly while final steady-state adaptation required up to several minutes. The transition between the rapid and slow phase of APD change was characterized by a variable alternans of APD which correlated inversely with the preceding diastolic interval. (c) In the steady state, APD correlated linearly with cycle length, increasing an average of 23 ms per 100 ms cycle length increase (r = 0.995). The divergence between steady-state and non-steady-state APD, and the slowness of steady-state adaptation, are important factors to be considered in clinical electrophysiologic studies and in rate correction algorithms of APD or QT intervals, respectively.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abildskov J. A. The sequence of normal recovery of excitability in the dog heart. Circulation. 1975 Sep;52(3):442–446. doi: 10.1161/01.cir.52.3.442. [DOI] [PubMed] [Google Scholar]
- Arnold L., Page J., Attwell D., Cannell M., Eisner D. A. The dependence on heart rate of the human ventricular action potential duration. Cardiovasc Res. 1982 Oct;16(10):547–551. doi: 10.1093/cvr/16.10.547. [DOI] [PubMed] [Google Scholar]
- Attwell D., Cohen I., Eisner D. A. The effects of heart rate on the action potential of guinea-pig and human ventricular muscle. J Physiol. 1981;313:439–461. doi: 10.1113/jphysiol.1981.sp013675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass B. G. Restitution of the action potential in cat papillary muscle. Am J Physiol. 1975 Jun;228(6):1717–1724. doi: 10.1152/ajplegacy.1975.228.6.1717. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Jewell B. R. A study of the factors responsible for rate-dependent shortening of the action potential in mammalian ventricular muscle. J Physiol. 1978 Dec;285:359–380. doi: 10.1113/jphysiol.1978.sp012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Jewell B. R. Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Prog Biophys Mol Biol. 1980;36(1):1–52. doi: 10.1016/0079-6107(81)90003-1. [DOI] [PubMed] [Google Scholar]
- CARMELIET E. Modification de la durée du potentiel d'action cardiaque sous l'influence des excitants. J Physiol (Paris) 1958 Mar;50(2):204–207. [PubMed] [Google Scholar]
- Carmeliet E. Repolarisation and frequency in cardiac cells. J Physiol (Paris) 1977;73(7):903–923. [PubMed] [Google Scholar]
- Colatsky T. J., Hogan P. M. Effects of external calcium, calcium channel-blocking agents, and stimulation frequency on cycle length-dependent changes in canine cardiac action potential duration. Circ Res. 1980 Apr;46(4):543–552. doi: 10.1161/01.res.46.4.543. [DOI] [PubMed] [Google Scholar]
- Denker S., Lehmann M., Mahmud R., Gilbert C., Akhtar M. Facilitation of ventricular tachycardia induction with abrupt changes in ventricular cycle length. Am J Cardiol. 1984 Feb 1;53(4):508–515. doi: 10.1016/0002-9149(84)90022-5. [DOI] [PubMed] [Google Scholar]
- Denker S., Shenasa M., Gilbert C. J., Akhtar M. Effects of abrupt changes in cycle length on refractoriness of the His-Purkinje system in man. Circulation. 1983 Jan;67(1):60–68. doi: 10.1161/01.cir.67.1.60. [DOI] [PubMed] [Google Scholar]
- Edmands R. E., Greenspan K., Fisch C. Effect of cycle-length alteration upon the configuration of the canine ventricular action potential. Circ Res. 1966 Sep;19(3):602–610. doi: 10.1161/01.res.19.3.602. [DOI] [PubMed] [Google Scholar]
- Elharrar V., Atarashi H., Surawicz B. Cycle length-dependent action potential duration in canine cardiac Purkinje fibers. Am J Physiol. 1984 Dec;247(6 Pt 2):H936–H945. doi: 10.1152/ajpheart.1984.247.6.H936. [DOI] [PubMed] [Google Scholar]
- Franz M. R., Bargheer K., Rafflenbeul W., Haverich A., Lichtlen P. R. Monophasic action potential mapping in human subjects with normal electrocardiograms: direct evidence for the genesis of the T wave. Circulation. 1987 Feb;75(2):379–386. doi: 10.1161/01.cir.75.2.379. [DOI] [PubMed] [Google Scholar]
- Franz M. R., Burkhoff D., Spurgeon H., Weisfeldt M. L., Lakatta E. G. In vitro validation of a new cardiac catheter technique for recording monophasic action potentials. Eur Heart J. 1986 Jan;7(1):34–41. doi: 10.1093/oxfordjournals.eurheartj.a061954. [DOI] [PubMed] [Google Scholar]
- Franz M. R. Long-term recording of monophasic action potentials from human endocardium. Am J Cardiol. 1983 Jun;51(10):1629–1634. doi: 10.1016/0002-9149(83)90199-6. [DOI] [PubMed] [Google Scholar]
- GIBBS C. L., JOHNSON E. A. Effect of changes in frequency of stimulation upon rabbit ventricular action potential. Circ Res. 1961 Jan;9:165–170. doi: 10.1161/01.res.9.1.165. [DOI] [PubMed] [Google Scholar]
- Gettes L. S., Morehouse N., Surawicz B. Effect of premature depolarization on the duration of action potentials in Purkinje and ventricular fibers of the moderator band of the pig heart. Role of proximity and the duration of the preceding action potential. Circ Res. 1972 Jan;30(1):55–66. doi: 10.1161/01.res.30.1.55. [DOI] [PubMed] [Google Scholar]
- Gettes L. S., Reuter H. Slow recovery from inactivation of inward currents in mammalian myocardial fibres. J Physiol. 1974 Aug;240(3):703–724. doi: 10.1113/jphysiol.1974.sp010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan K., Edmands R. E., Fisch C. Effects of cycle-length alteration on canine cardiac action potentials. Am J Physiol. 1967 Jun;212(6):1416–1420. doi: 10.1152/ajplegacy.1967.212.6.1416. [DOI] [PubMed] [Google Scholar]
- Greenspan K., Edmonds R. E., Fisch C. The relation of contractile enhancement to action potential change in canine myocardium. Circ Res. 1967 Mar;20(3):311–320. doi: 10.1161/01.res.20.3.311. [DOI] [PubMed] [Google Scholar]
- HOFFMAN B. F., SUCKLING E. E. Effect of heart rate on cardiac membrane potentials and the unipolar electrogram. Am J Physiol. 1954 Oct;179(1):123–130. doi: 10.1152/ajplegacy.1954.179.1.123. [DOI] [PubMed] [Google Scholar]
- Hausworth O., Noble D., Tsien R. W. The dependence of plateau currents in cardiac Purkinje fibres on the interval between action potentials. J Physiol. 1972 Apr;222(1):27–49. doi: 10.1113/jphysiol.1972.sp009786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y., Kodama I., Iwamura N., Shimizu T., Toyama J., Yamada K. Effects of verapamil on canine Purkinje fibres and ventricular muscle fibres with particular reference to the alternation of action potential duration after a sudden increase in driving rate. Cardiovasc Res. 1979 Jan;13(1):1–8. doi: 10.1093/cvr/13.1.1. [DOI] [PubMed] [Google Scholar]
- Iinuma H., Kato K. Mechanism of augmented premature responses in canine ventricular muscle. Circ Res. 1979 May;44(5):624–629. doi: 10.1161/01.res.44.5.624. [DOI] [PubMed] [Google Scholar]
- Janse M. J., van der Steen A. B., van Dam R. T. Refractory period of the dog's ventricular myocardium following sudden changes in frequency. Circ Res. 1969 Feb;24(2):251–262. doi: 10.1161/01.res.24.2.251. [DOI] [PubMed] [Google Scholar]
- Kuo C. S., Munakata K., Reddy C. P., Surawicz B. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983 Jun;67(6):1356–1367. doi: 10.1161/01.cir.67.6.1356. [DOI] [PubMed] [Google Scholar]
- MOORE E. N., PRESTON J. B., MOE G. K. DURATIONS OF TRANSMEMBRANE ACTION POTENTIALS AND FUNCTIONAL REFRACTORY PERIODS OF CANINE FALSE TENDON AND VENTRICULAR MYOCARDIUM: COMPARISONS IN SINGLE FIBERS. Circ Res. 1965 Sep;17:259–273. doi: 10.1161/01.res.17.3.259. [DOI] [PubMed] [Google Scholar]
- Marchlinski F. E. Characterization of oscillations in ventricular refractoriness in man after an abrupt increment in heart rate. Circulation. 1987 Mar;75(3):550–556. doi: 10.1161/01.cir.75.3.550. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Wallace A. G., Feezor M. D. A quantitative comparison of the relation between the shape of the action potential and the pattern of stimulation in canine ventricular muscle and Purkinje fibers. J Mol Cell Cardiol. 1971 Mar;2(1):3–19. doi: 10.1016/0022-2828(71)90074-5. [DOI] [PubMed] [Google Scholar]
- Noble D., Cohen I. The interpretation of the T wave of the electrocardiogram. Cardiovasc Res. 1978 Jan;12(1):13–27. doi: 10.1093/cvr/12.1.13. [DOI] [PubMed] [Google Scholar]
- Olsson S. B. Right ventricular monophasic action potentials during regular rhythm. A heart catheterization study in man. Acta Med Scand. 1972 Mar;191(3):145–157. [PubMed] [Google Scholar]
- Seed W. A., Noble M. I., Oldershaw P., Wanless R. B., Drake-Holland A. J., Redwood D., Pugh S., Mills C. Relation of human cardiac action potential duration to the interval between beats: implications for the validity of rate corrected QT interval (QTc). Br Heart J. 1987 Jan;57(1):32–37. doi: 10.1136/hrt.57.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchou P. J., Lehmann M. H., Dongas J., Mahmud R., Denker S. T., Akhtar M. Effect of sudden rate acceleration on the human His-Purkinje system: adaptation of refractoriness in a dampened oscillatory pattern. Circulation. 1986 May;73(5):920–929. doi: 10.1161/01.cir.73.5.920. [DOI] [PubMed] [Google Scholar]
- Vick R. L. Action potential duration in canine Purkinje tissue: effects of preceding excitation. J Electrocardiol. 1971;4(2):105–115. doi: 10.1016/s0022-0736(71)80003-1. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Rautaharju P. M., McDonald T. F. Ventricular action potentials, ventricular extracellular potentials, and the ECG of guinea pig. Circ Res. 1985 Sep;57(3):362–373. doi: 10.1161/01.res.57.3.362. [DOI] [PubMed] [Google Scholar]
- Wiener I., Kunkes S., Rubin D., Kupersmith J., Packer M., Pitchon R., Schweitzer P. Effects of sudden change in cycle length on human atrial, atrioventricular nodal and ventricular refractory periods. Circulation. 1981 Aug;64(2):245–248. doi: 10.1161/01.cir.64.2.245. [DOI] [PubMed] [Google Scholar]
- von Savigny L., Hohnloser S., Antoni H. Effects of changes in frequency on guinea pig ventricular action potential duration and on QT interval under different experimental conditions. Basic Res Cardiol. 1981 May-Jun;76(3):276–288. doi: 10.1007/BF01907772. [DOI] [PubMed] [Google Scholar]



