Abstract
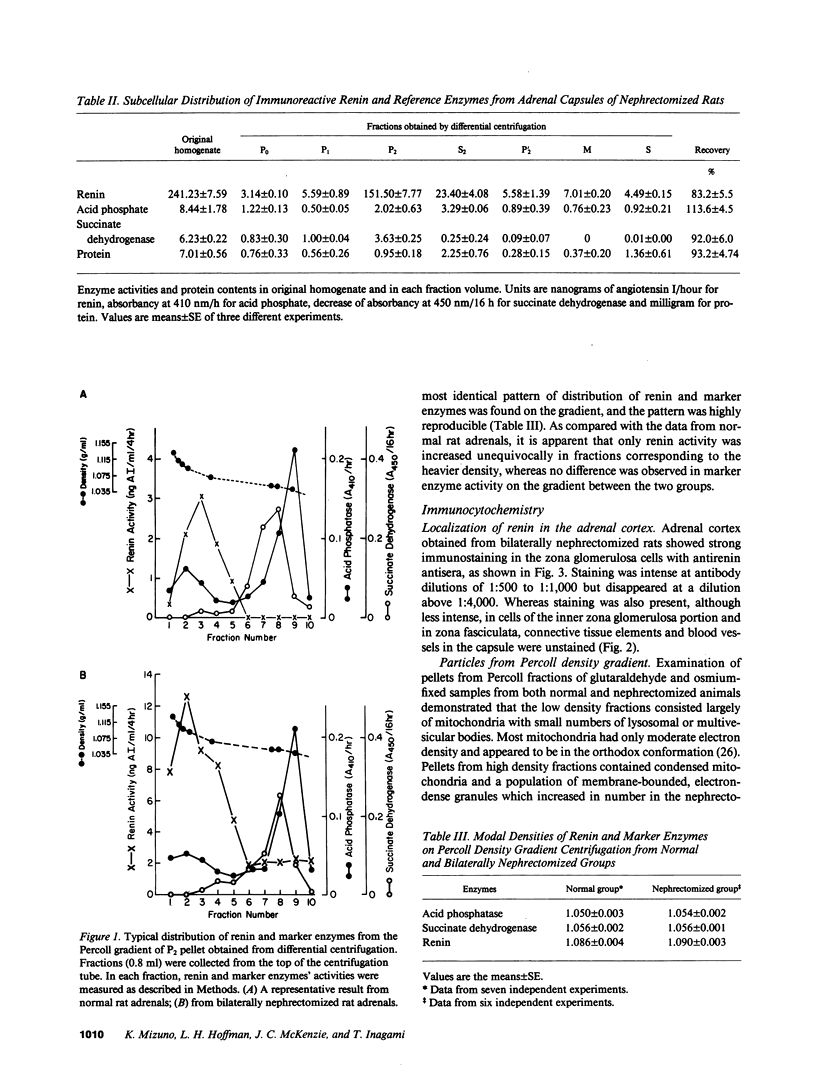
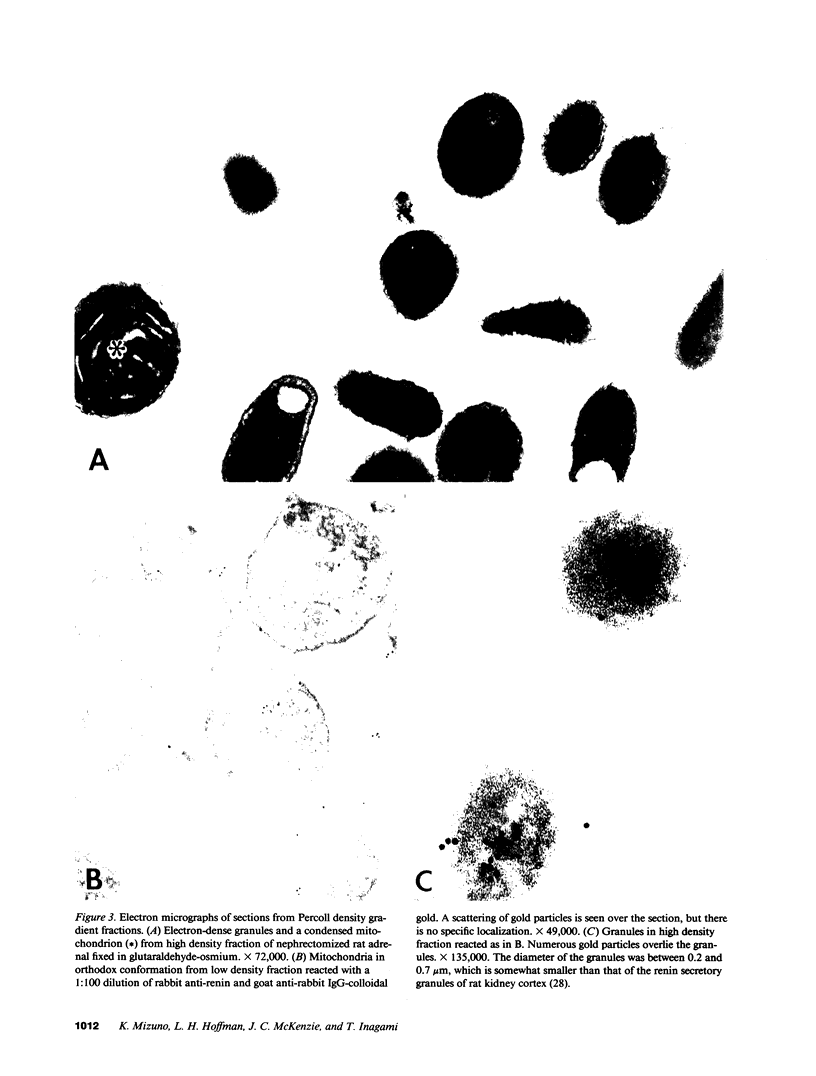
Renin has been identified biochemically and immunohistochemically in the adrenal gland. We examined the subcellular distribution and behavior of adrenal renin. By differential centrifugation of adrenal capsules, we found renin mainly in mitochondrial fractions. By Percoll density gradient centrifugation of this fraction, dense granules were separated from mitochondria and microsomes. The renin activity in the dense granules from the capsules of nephrectomized rats was 15 times greater than that of the intact rat. Immunohistochemical studies revealed that the dense granules increased in number after bilateral nephrectomy. Immunogold staining of these granules showed unequivocally the presence of renin in these granules. Adrenal capsules in organ culture were found to release renin at a steady rate. Renin release from bilaterally nephrectomized rat adrenals was 46 times faster than from the organs of intact animals. The mechanism of the control of renin secretion from the adrenal gland was different from the kidney in that the secretion was stimulated by potassium chloride (10 mM) or angiotensin II (10(-9)-10(-7) M) but not by ACTH (10(-9)-10(-7) M), suggesting stimulation by intracellular calcium. These results provide evidence that the adrenal synthesizes renin, stores it in specific secretory granules and secretes it in a regulated manner.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand-Srivastava M. B. Angiotensin II receptors negatively coupled to adenylate cyclase in rat aorta. Biochem Biophys Res Commun. 1983 Dec 16;117(2):420–428. doi: 10.1016/0006-291x(83)91217-2. [DOI] [PubMed] [Google Scholar]
- Baba K., Doi Y., Franco-Saenz R., Mulrow P. J. Mechanisms by which nephrectomy stimulates adrenal renin. Hypertension. 1986 Nov;8(11):997–1002. doi: 10.1161/01.hyp.8.11.997. [DOI] [PubMed] [Google Scholar]
- Bendayan M., Zollinger M. Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J Histochem Cytochem. 1983 Jan;31(1):101–109. doi: 10.1177/31.1.6187796. [DOI] [PubMed] [Google Scholar]
- Braley L. M., Menachery A. I., Brown E. M., Williams G. H. Comparative effect of angiotensin II, potassium, adrenocorticotropin, and cyclic adenosine 3',5'-monophosphate on cytosolic calcium in rat adrenal cells. Endocrinology. 1986 Sep;119(3):1010–1019. doi: 10.1210/endo-119-3-1010. [DOI] [PubMed] [Google Scholar]
- Campbell W. B., Graham R. M., Jackson E. K. Role of renal prostaglandins in sympathetically mediated renin relase in the rat. J Clin Invest. 1979 Aug;64(2):448–456. doi: 10.1172/JCI109482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catt K. J., Cain M. C. Measurement of angiotensin II in blood. Lancet. 1967 Nov 11;2(7524):1005–1007. doi: 10.1016/s0140-6736(67)90285-1. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Cornwall M. C., Williams G. H. Spatially resolved cytosolic calcium response to angiotensin II and potassium in rat glomerulosa cells measured by digital imaging techniques. J Biol Chem. 1987 Feb 25;262(6):2919–2927. [PubMed] [Google Scholar]
- Doi Y., Atarashi K., Franco-Saenz R., Mulrow P. Adrenal renin: a possible regulator of aldosterone production. Clin Exp Hypertens A. 1983;5(7-8):1119–1126. doi: 10.3109/10641968309048845. [DOI] [PubMed] [Google Scholar]
- Elliott M. E., Goodfriend T. L. Angiotensin alters 45Ca2+ fluxes in bovine adrenal glomerulosa cells. Proc Natl Acad Sci U S A. 1981 May;78(5):3044–3048. doi: 10.1073/pnas.78.5.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakunding J. L., Catt K. J. Dependence of aldosterone stimulation in adrenal glomerulosa cells on calcium uptake: effects of lanthanum nd verapamil. Endocrinology. 1980 Nov;107(5):1345–1353. doi: 10.1210/endo-107-5-1345. [DOI] [PubMed] [Google Scholar]
- Fisher E. R. Lysosomal nature of juxtaglomerular granules. Science. 1966 Jun 24;152(3730):1752–1753. doi: 10.1126/science.152.3730.1752. [DOI] [PubMed] [Google Scholar]
- Ganten D., Ganten U., Kubo S., Granger P., Nowaczynski W., Boucher R., Genest J. Influence of sodium, potassium, and pituitary hormones on iso-renin in rat adrenal glands. Am J Physiol. 1974 Jul;227(1):224–229. doi: 10.1152/ajplegacy.1974.227.1.224. [DOI] [PubMed] [Google Scholar]
- Gomba S., Soltész B. M. Histochemistry of lysosomal enzymes in juxtaglomerular cells. Experientia. 1969 May 15;25(5):513–513. doi: 10.1007/BF01900791. [DOI] [PubMed] [Google Scholar]
- Grammer R. T., Naruse M., Inagami T. The subcellular distribution of renin in hog anterior pituitary. Endocrinology. 1983 Jul;113(1):344–347. doi: 10.1210/endo-113-1-344. [DOI] [PubMed] [Google Scholar]
- Haber E., Koerner T., Page L. B., Kliman B., Purnode A. Application of a radioimmunoassay for angiotensin I to the physiologic measurements of plasma renin activity in normal human subjects. J Clin Endocrinol Metab. 1969 Oct;29(10):1349–1355. doi: 10.1210/jcem-29-10-1349. [DOI] [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. II. Electron transport-linked ultrastructural transformations in mitochondria. J Cell Biol. 1968 May;37(2):345–369. doi: 10.1083/jcb.37.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayduk K., Boucher R., Genest J. Renin activity content in various tissues od dogs under different physiopathological states. Proc Soc Exp Biol Med. 1970 May;134(1):252–255. doi: 10.3181/00379727-134-34770. [DOI] [PubMed] [Google Scholar]
- Kawamura M., McKenzie J. C., Hoffman L. H., Tanaka I., Parmentier M., Inagami T. The storage form of renin in renin granules from rat kidney cortex. Hypertension. 1986 Aug;8(8):706–711. doi: 10.1161/01.hyp.8.8.706. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Man in 't Veld A. J., Wenting G. J., Schalekamp M. A. Does captopril lower blood pressure in anephric patients? Br Med J. 1979 Nov 3;2(6198):1110–1110. doi: 10.1136/bmj.2.6198.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie J., Gaillard R. C., Schoenenberg P., Jard S., Bockaert J. Pharmacological characterization of the angiotensin receptor negatively coupled with adenylate cyclase in rat anterior pituitary gland. Endocrinology. 1985 Mar;116(3):1044–1050. doi: 10.1210/endo-116-3-1044. [DOI] [PubMed] [Google Scholar]
- Marie J., Jard S. Angiotensin II inhibits adenylate cyclase from adrenal cortex glomerulosa zone. FEBS Lett. 1983 Aug 8;159(1-2):97–101. doi: 10.1016/0014-5793(83)80424-4. [DOI] [PubMed] [Google Scholar]
- McKenzie J. C., Naruse K., Inagami T. The renin-angiotensin system in the rat anterior pituitary: colocalization of renin and angiotensin II in gonadotrophs. Anat Rec. 1985 Jun;212(2):158–166. doi: 10.1002/ar.1092120209. [DOI] [PubMed] [Google Scholar]
- Meyer D. I., Burger M. M. Isolation of a protein from the plasma membrane of adrenal medulla which binds to secretory vesicles. J Biol Chem. 1979 Oct 10;254(19):9854–9859. [PubMed] [Google Scholar]
- Michelakis A. M., Caudle J., Liddle G. W. In vitro stimulation of renin production by epinephrine, norepinephrine, and cyclic AMP. Proc Soc Exp Biol Med. 1969 Mar;130(3):748–753. doi: 10.3181/00379727-130-33647. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Ojima M., Gotoh M., Hashimoto S., Fukuchi S. True renin in human pituitary tissue. J Neurochem. 1985 Feb;44(2):633–636. doi: 10.1111/j.1471-4159.1985.tb05459.x. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Ojima M., Hashimoto S., Fukuchi S. Renin and angiotensin-converting enzyme in human neuroblastoma tissue. J Neurochem. 1985 Aug;45(2):626–629. doi: 10.1111/j.1471-4159.1985.tb04032.x. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Ojima M., Hashimoto S., Tani M., Niimura S., Kunii N., Yabe R., Watari H., Inagami T., Fukuchi S. Multiple forms of immunoreactive renin in human adrenocortical tumour tissue from patients with primary aldosteronism. Clin Sci (Lond) 1987 Jun;72(6):699–704. doi: 10.1042/cs0720699. [DOI] [PubMed] [Google Scholar]
- Nakamaru M., Misono K. S., Naruse M., Workman R. J., Inagami T. A role for the adrenal renin-angiotensin system in the regulation of potassium-stimulated aldosterone production. Endocrinology. 1985 Nov;117(5):1772–1778. doi: 10.1210/endo-117-5-1772. [DOI] [PubMed] [Google Scholar]
- Naruse K., Inagami T., Celio M. R., Workman R. J., Takii Y. Immunohistochemical evidence that angiotensins I and II are formed by intracellular mechanism in juxtaglomerular cells. Hypertension. 1982 May-Jun;4(3 Pt 2):70–74. [PubMed] [Google Scholar]
- Naruse M., Naruse K., Inagaki T., Inagami T. Immunoreactive renin in mouse adrenal gland. Localization in the inner cortical region. Hypertension. 1984 Mar-Apr;6(2 Pt 1):275–280. [PubMed] [Google Scholar]
- Naruse M., Sussman C. R., Naruse K., Jackson R. V., Inagami T. Renin exists in human adrenal tissue. J Clin Endocrinol Metab. 1983 Sep;57(3):482–487. doi: 10.1210/jcem-57-3-482. [DOI] [PubMed] [Google Scholar]
- OSTROWSKI W., TSUGITA A. Purification of acid phosphomonoesterase from the human prostate gland. Arch Biochem Biophys. 1961 Jul;94:68–78. doi: 10.1016/0003-9861(61)90012-1. [DOI] [PubMed] [Google Scholar]
- Ohkubo H., Nakayama K., Tanaka T., Nakanishi S. Tissue distribution of rat angiotensinogen mRNA and structural analysis of its heterogeneity. J Biol Chem. 1986 Jan 5;261(1):319–323. [PubMed] [Google Scholar]
- Okamura T., Clemens D. L., Inagami T. Generation of angiotensins in cultured pheochromocytoma cells. Neurosci Lett. 1984 May 4;46(2):151–156. doi: 10.1016/0304-3940(84)90433-6. [DOI] [PubMed] [Google Scholar]
- Okamura T., Clemens D. L., Inagami T. Renin, angiotensins, and angiotensin-converting enzyme in neuroblastoma cells: evidence for intracellular formation of angiotensins. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6940–6943. doi: 10.1073/pnas.78.11.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey K. N., Inagami T. Regulation of renin angiotensins by gonadotropic hormones in cultured murine Leydig tumor cells. Release of angiotensin but not renin. J Biol Chem. 1986 Mar 25;261(9):3934–3938. [PubMed] [Google Scholar]
- Reid I. A., Stockigt J. R., Goldfien A., Ganong W. F. Stimulation of renin secretion in dogs by theophylline. Eur J Pharmacol. 1972 Mar;17(3):325–332. doi: 10.1016/0014-2999(72)90112-4. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- SLATER E. C., BORNER W. D., Jr The effect of fluoride on the succinic oxidase system. Biochem J. 1952 Oct;52(2):185–196. doi: 10.1042/bj0520185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple P. F., Boyd A. S., Dawes P. M., Morton J. J. Angiotensin II and its heptapeptide (2-8), hexapeptide (3-8), and pentapeptide (4-8) metabolites in arterial and venous blood of man. Circ Res. 1976 Nov;39(5):671–678. doi: 10.1161/01.res.39.5.671. [DOI] [PubMed] [Google Scholar]
- Takii Y., Figueiredo A. F., Inagami T. Application of immunochemical methods to the identification and characterization of rat kidney inactive renin. Hypertension. 1985 Mar-Apr;7(2):236–243. doi: 10.1161/01.hyp.7.2.236. [DOI] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P., Détraz M. Comparative studies of intracellular transport of secretory proteins. J Cell Biol. 1978 Dec;79(3):694–707. doi: 10.1083/jcb.79.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault G., Garcia R., Gutkowska J., Bilodeau J., Lazure C., Seidah N. G., Chrétien M., Genest J., Cantin M. The propeptide Asn1-Tyr126 is the storage form of rat atrial natriuretic factor. Biochem J. 1987 Jan 1;241(1):265–272. doi: 10.1042/bj2410265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A. Y., Anderson J. K., Siminoski K., Mole J. E., Murphy R. A. Cellular and subcellular colocalization of nerve growth factor and epidermal growth factor in mouse submandibular glands. Anat Rec. 1985 Nov;213(3):365–376. doi: 10.1002/ar.1092130302. [DOI] [PubMed] [Google Scholar]
- Weinberger M. H., Wade M. B., Aoi W., Usa T., Dentino M., Luft F., Grim C. E. An extrarenal source of "renin-like" activity in anephric man. Circ Res. 1977 May;40(5 Suppl 1):I1–I4. [PubMed] [Google Scholar]
- Woodcock E. A., Johnston C. I. Inhibition of adenylate cyclase by angiotensin II in rat renal cortex. Endocrinology. 1982 Nov;111(5):1687–1691. doi: 10.1210/endo-111-5-1687. [DOI] [PubMed] [Google Scholar]
- Woodcock E. A., Johnston C. I. Inhibition of adenylate cyclase in rat adrenal glomerulosa cells by angiotensin II. Endocrinology. 1984 Jul;115(1):337–341. doi: 10.1210/endo-115-1-337. [DOI] [PubMed] [Google Scholar]