Abstract
The zebrafish pharyngeal cartilage is derived from the pharyngeal apparatus, a vertebrate-specific structure derived from all three germ layers. Developmental aberrations of the pharyngeal apparatus lead to birth defects such as Treacher Collins and DiGeorge syndromes. While interactions between endoderm and neural crest (NC) are known to be important for cartilage formation, the full complement of molecular players involved and their roles remain to be elucidated. Activated leukocyte cell adhesion molecule a (alcama), a member of the immunoglobulin (Ig) superfamily, is among the prominent markers of pharyngeal pouch endoderm, but to date no role has been assigned to this adhesion molecule in the development of the pharyngeal apparatus. Here we show that alcama plays a crucial, non-autonomous role in pharyngeal endoderm during zebrafish cartilage morphogenesis. alcama knockdown leads to defects in NC differentiation, without affecting NC specification or migration. These defects are reminiscent of the phenotypes observed when Endothelin1 (Edn1) signaling, a key regulator of cartilage development is disrupted. Using gene expression analysis and rescue experiments we show that Alcama functions downstream of Edn1 signaling to regulate NC differentiation and cartilage morphogenesis. In addition, we also identify a role for neural adhesion molecule 1.1 (nadl1.1), a known interacting partner of Alcama expressed in neural crest, in NC differentiation. Our data shows that nadl1.1 is required for alcama rescue of NC differentiation in edn1-/- mutants, and that Alcama interacts with Nadl1.1 during chondrogenesis. Collectively our results support a model by which Alcama on the endoderm interacts with Nadl1.1 on NC to mediate Edn1 signaling and NC differentiation during chondrogenesis.
Keywords: Alcama, Edn1, cartilage, endoderm, neural crest
Introduction
Formation of the pharyngeal apparatus is a crucial part of vertebrate development because it gives rise to the cartilage, connective tissue, sensory neurons, thyroid, parathyroid and thymus. Defects in this process results in human birth defects such as DiGeorge and Treacher-Collins syndromes. Generation of this tissue is highly complex, involving extensive cell migration and signaling between cells derived from all three germ layers. NC cells migrate from the dorsal neural tube in three distinct streams into a series of pharyngeal arches and eventually give rise to cartilages and bones of the head. Each pharyngeal arch is composed of a cylinder of NC surrounding a mesodermal core. The NC is covered by ectoderm on the outside and endoderm on the inside. Between the arches, endoderm meets ectoderm forming the pharyngeal pouches, which later develop into gill clefts and the epithelial lining of the pharynx, thyroid, parathyroid and thymus (Graham, 2003).
Though NC cells carry intrinsic cues for patterning (Noden, 1983), they receive extrinsic cues from the surrounding cells and extracellular matrix as they migrate. Recently, the endoderm has been found to contribute significantly to NC development. Ablation and extirpation experiments in chicken have revealed that the endoderm carries patterning information for the NC in segments along the antero-posterior and medio-lateral axis (Couly et al., 2002; Ruhin et al., 2003). In addition, genetic mutants in zebrafish have also revealed the requirement of endoderm in formation of cartilage. The sox32-deficient casanova mutant lacks endodermal pouches and cartilages that are rescued by wild-type endodermal transplants (David et al., 2002). Likewise, the tbx1-deficient van gogh mutant fails to form segmented endodermal pouches resulting in fusion of the pharyngeal cartilages (Piotrowski and Nusslein-Volhard, 2000). Similarly, the zebrafish mutant for integrin α5 lacks the first endodermal pouch and the anterior part of the hyoid cartilage (Crump et al., 2004). Although these data demonstrate that endoderm is essential for cartilage development, the cellular and molecular interactions between the NC and endodermal cells are not fully understood.
One major signaling factor that provides an extrinsic cue regulating NC differentiation is endothelin-1 (edn1). edn1 is expressed in the mesodermal cores, ectoderm and endoderm of the pharyngeal arches, but not in NC. Edn1 signals the NC and induces ventralization of pharyngeal arch cartilage (Miller et al., 2000). Mutations in edn1, Edn1 cleaving enzymes and other genes in the Edn1 signaling cascade (sucker, schmerle, hoover and sturgeon) cause similar cartilage defects, and have been placed in the same class (Kimmel et al., 2001; Piotrowski et al., 1996). Typically, the ventral domains of the first two arches are reduced in size, changed in orientation, and fused with the dorsal domains, while the posterior arches are mostly unaffected. Hence, Edn1 is an important signaling factor that is required non-autonomously for NC differentiation into cartilage.
Other molecular players that may signal from endoderm to NC remain elusive. While Alcama is commonly used as a marker for pharyngeal endoderm in zebrafish (Crump et al., 2004; Piotrowski and Nusslein-Volhard, 2000), its role in this tissue has not been elucidated thus far. In zebrafish, Alcama has been studied primarily for its role in neurogenesis (Diekmann and Stuermer, 2009; Fashena and Westerfield, 1999). Initially identified in chicken for its role in neurite extension (Burns et al., 1991), ALCAMA has now been shown to be involved in axonal pathfinding and axonal fasciculation (Diekmann and Stuermer, 2009; Weiner et al., 2004). Its non-neuronal roles include T-cell activation (Bowen et al., 2000; Fashena and Westerfield, 1999; Ofori-Acquah and King, 2008; Zimmerman et al., 2006), metastasis (Degen et al., 1998; Ofori-Acquah and King, 2008) and cell migration (Heffron and Golden, 2000). Human ALCAM is a transmembrane glycoprotein having five Ig domains, a transmembrane domain and a short cytoplasmic tail. It mediates cell-cell clustering through homophilic (ALCAM-ALCAM) as well as heterophilic (ALCAM-NgCAM and ALCAM-CD6) interactions (DeBernardo and Chang, 1996; Degen et al., 1998). While ALCAM can activate signal transduction pathways in neighbouring cells through heterophilic interactions (Ibanez et al., 2006), a non-autonomous role of ALCAM has not been defined thus far. In this paper we demonstrate that zebrafish Alcama, expressed in the pharyngeal endoderm, is an important non-autonomous molecule for NC differentiation. In addition, we provide evidence that Alcama mediates Edn1 signaling from the endoderm to the NC by interacting with Nadl1.1 (NgCAM in chicken) on the NC cells. These data for the first time link Alcama to Edn1 signaling and identify a role for the molecular interaction between Alcama and Nadl1.1 in cartilage formation.
Materials and Methods
Fish stocks and maintenance
Fish were maintained at 28.5 °C under standard conditions (Westerfield, 2000) and were staged as described (Kimmel et al., 1995). The suckertf216b mutant (edn1-/-) line was obtained from Zebrafish International Resource Center (Miller et al., 2000). Homozygous mutants were obtained by inbreeding of heterozygous carriers. Tg(fli1:EGFP) fish have been previously described (Lawson and Weinstein, 2002). Alcian Blue stained larvae of furina-/- mutants at 5 days post fertilization (dpf) were a kind gift from Chuck Kimmel (Institute of Neuroscience, University of Oregon).
Identification and genotyping of edn1-/- mutants
edn1-/- mutants have an A-to-T missense mutation (Miller et al., 2000). The mutants were identified by Derived Cleaved Amplified Polymorphic Sequences assay (Neff et al., 1998). DNA was extracted from the tails of stained embryos and PCR was conducted using the primers 5′AGATGCTCCTGCGCAAGTTTTCTAG3′ and 5′CTGACTTACTCTGGTGTGTTCACCC3′ The mismatch in the primer which introduces a XbaI site in the wild-type (WT) but not in the mutant is underlined. The 93 bp PCR product, when digested with XbaI, gives a 68 bp product in WT. The 93 and 68 bp products were resolved on a 4% Metaphor agarose gel (Lonza). All the identified WT and mutants were included in the analysis.
Cloning and RNA transcription
RNA extracted from 48 hours post fertilization (hpf) Tü larvae was used for cDNA synthesis. PCR for alcama was performed using the forward primer 5′-ggatccgccaccATGCATTCGGTTATCTGCCTTTTCG-3′ with a BamHI and Kozak overhang and the reverse primer 5′-ctcgagTTAGACATCTGCTTTATGATTGTTCTCCTCC-3′with a XhoI overhang. The overhangs are shown in lower case. The PCR product was cloned into pCMV-Script using TOPO TA kit (Invitrogen). The edn1 cDNA clone in pBK-CMV was obtained from ZIRC.
To make sense RNA for injection, the edn1 and alcama plasmids were cut with KpnI and in vitro transcription was driven from the T3 promoter using mMessage Machine kit (Ambion). ednrb1 was cloned from cDNA into TOPO TA using the primers 5′-ATGCGTTTCCAAATTATTATGGAAACAAGATGCG-3′(forward) and 5′-TCAGTGCCTAATTTGAAGTATACTTGTTGGAGAC-3′ (reverse) and this plasmid was used to make ISH probe.
Morpholino antisense oligonucleotide and RNA injections
Translation blocking (TB) and splice site blocking (SB) Morpholinos (MOs) were designed to bind 143 bp upstream of the transcription start site and at the donor site of exon 6 alcama mRNA, respectively. alcama blocking and control MO were purchased from Gene Tools, Inc. The sequences are TB MO: 5′ GTTCTCCTTTATACAGTCCGGCGAC3′; SB MO: 5′ GCAGTCCCTCACCTTAATGTTAAAG3′; control MO: 5′TGATCACCTGCAGATGGACGCTGCG3′. The optimal doses were determined to be 1.1 ng for the TB MO and 2.2 ng for the SB MO. The control MO was injected at 1.1 ng per embryo. The TB MO for nadl1.1: 5′CAGGCTGACTCTGCACTGGAGGCAT3′ has been previously described (Wolman et al., 2007) and was injected at 4.4 ng per embryo. 26 pg of alcama or edn1 RNA was injected per embryo. MOs and RNA were dissolved in molecular biology grade water and pressure injected into 1-4 cell zebrafish embryos. For suboptimal doses, the alcama TB MO was used at 0.5 ng and the nadl MO at 1.1 ng per embryo.
Treatment with proteasome inhibitor MG-132
MG-132 was dissolved in DMSO at a stock concentration of 500mM. Embryos were dechorionated at 5 hpf and transferred to E2 with 50 μM MG-132 (Bretaud et al., 2007) or with 1 % dimethyl sulfoxide (DMSO). MG-132- and DMSO-treated control larvae were fixed at 30 hpf and stained.
Tissue labeling procedures
Alcian Blue cartilage staining and dissection were performed as described (Kimmel et al., 1998). Whole mount RNA in situ hybridization (ISH) with digoxigenin was performed as described (Miller et al., 2000). The plasmids for dlx2a, dlx3b, dlx5a were a kind gift from Gage DeKoeyer Crump (Keck School of Medicine, University of Southern California). The plasmid for nadl1.1 was a gift from Gavin J Wright (Cell Surface Signalling Laboratory, Wellcome Trust Sanger Institute). Alcama protein was stained using Zn-5 antibody from ZIRC at 1:500 dilution. A goat anti-mouse secondary antibody conjugated with Alexa 555 (Invitrogen) was used for fluorescence quantification purposes. DAPI staining was used for counting the number of cells in the pouches.
Imaging and Quantification
Skeletons and ISH embryos were photographed on a Nikon Y-IDP microscope at 20× zoom using Spot software. Confocal images of antibody stained embryos were taken on Olympus FV1000 microscope. Images of all larvae from the same experiment were taken using the same settings and exposure. Cell numbers were counted using Imaris software and fluorescence intensity of each z-section was measured using ImageJ. For immunohistochemistry coupled to ISH, the secondary antibody conjugated to alkaline phosphatase (Bio-Rad) was used with Fast Red for detection (Sigma-Aldrich). Fluorescent ISH was performed following immunohistochemistry as described (Welten et al., 2006).
Results
alcama is necessary for ventral cartilage formation
We tested whether alcama plays a role in cartilage morphogenesis by injection of two different MOs: one blocking translation (TB MO), and the other blocking splicing of exon 6 (SB MO; Fig. 1A). Staining with Zn-5 antibody, which recognizes Alcama, revealed effective knockdown of Alcama protein by both MOs (Fig. 1C-E). 5 dpf alcama morphants are characterized by a protruding lower jaw, cardiac edema and absence of swim bladder (Fig. 1G). The jaw is shorter in the antero-posterior (A-P) direction and the ventral elements of the anterior arches, Meckels (mc) and ceratohyal (ch), are bent ventrally leading to the protruding lower jaw (Fig. 1I). Injection of the TB MO consistently leads to a more pronounced phenotype than the SB MO. Therefore, in subsequent experiments we use the TB MO exclusively and refer to it as alcama MO.
Fig. 1. alcama morphants have defects in facial skeletal patterning.
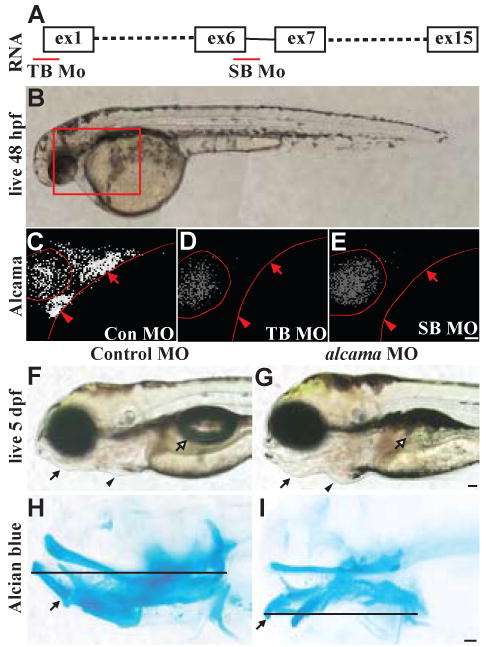
(A) Graphic representation of alcama RNA illustrating the position of TB and SB MOs. (B) Nomarski image of a 48 hpf WT larva with the box indicating the region shown in (C-E). 48 hpf Control (C), alcama TB (D), and alcama SB (E) morphants stained with Zn-5 antibody. Both MOs effectively knock down alcama expression in the heart (arrowheads) and pouches (arrows). (F, G) Lateral views of 5 dpf larvae injected with control and alcama TB MOs. alcama morphants have a protruding jaw (arrows), cardiac edema (arrowheads) and an absent swim bladder (open arrows). Lateral views of Alcian blue stained Control (H) and alcama (I) morphants at 5 dpf. The line indicates the length of the pharyngeal cartilage, which is shortened in alcama morphants. The arrow points to Meckels cartilage, which is bent ventrally in alcama morphants. Scale bars: 50 μm.
To eliminate the possibility that the jaw defect is caused by non-specific MO-induced p53-activation (Robu et al., 2007), alcama MO was injected into the p53 (I166T) mutant line (Parant et al., 2010). Alcian blue staining of 5 dpf larvae revealed that the percentage of individuals with affected cartilage was equivalent in wild-type (WT) and the p53-/-mutant lines (Fig. S1). The specificity of the phenotype was also tested by co-injection of alcama MO and alcama RNA. alcama RNA can rescue the ventral cartilage defect caused by alcama knockdown (Fig. S1). This indicates that the cartilage defect is caused by alcama knockdown and not by off-target MO effects and that alcama is required for the proper formation of anterior ventral cartilages in zebrafish.
Cartilage defects in alcama morphants are reminiscent of the edn1-class of mutants
In alcama morphants the pharyngeal skeleton is shorter in the antero-posterior direction and the anterior arch ventral cartilages (mc and ch) point ventrally rather than anteriorly (Fig. 1H, I). In addition, mc and ch are fused to their respective dorsal elements, palatoquadrate (pq) and hyosymplectic (hm) (Fig. 2A,C). The posterior arches are shorter in the antero-posterior direction, but are otherwise unaffected (Fig. 1I). To identify the pathway affected in alcama morphants, we compared the jaw phenotype in alcama morphants to previously described cartilage mutants. The three characteristic phenotypes of the ventral cartilages in alcama morphants; shortening, change of orientation and fusion to dorsal cartilages, are typical of the edn1-class of mutants (Piotrowski et al., 1996; Walker et al., 2006; Walker et al., 2007). Particularly striking is the similarity of alcama morphants to furinA-/- mutants (Fig. 2C, E). This observation suggests that alcama and edn1 may be in the same genetic pathway.
Fig. 2. alcama morphants have cartilage defects similar to the edn1 class of mutants.
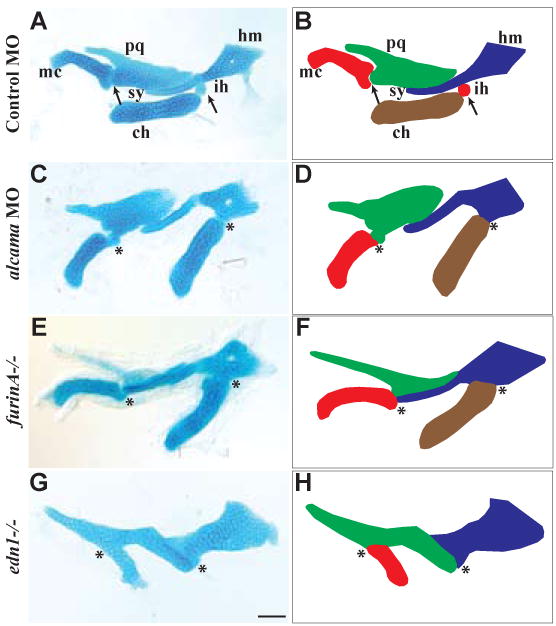
Flatmounts of mandibular and hyoid cartilage from 5 dpf alcian-blue stained larvae (A,C,E,G); corresponding schematics (B,D,F,H). The joint between Meckel's and palatoquadrate is fused in alcama morphants and furinA-/- and edn1-/- mutants. Similarly, the interhyal is absent in alcama morphants and furinA-/- mutants leading to a fusion of the ceratohyal and hyosymplectic cartilages. alcama morphants and furinA-/- mutants have misshapen Meckel's cartilage and ceratohyal, but edn1-/- mutants have the most severe defect with a lack of ceratohyal and severely misshapen Meckel's cartilage. DV joint regions are indicated with arrows in A and B. Fusions at joints are indicated with asterisks in (C-H). Cartilages are labeled as followed: pq (palatoquadrate), mc (Meckel's cartilage), hm (hyomandibula), ch (ceratohyal), sy (symplectic), and ih (interhyal). Scale bar: 50 μm.
edn1 regulates Alcama protein levels in the pharyngeal pouches
In order to test if an epistatic relationship exists between alcama and edn1, we analyzed edn1 expression in alcama morphants. While alcama is also expressed in the heart, retina and the brain (Fig. S2A), expression of edn1 and alcama coincides in the ventral pharyngeal area from 18 to 36 hpf (Thisse, 2004; Thisse, 2005). By 30 hpf edn1 expression is clearly demarcated in the mesodermal cores and surface ectoderm of pharyngeal arches in addition to the pharyngeal pouches (Miller et al., 2000). At that stage, alcama is expressed in pharyngeal pouch endoderm (Kopinke et al., 2006). edn1 expression is unchanged between control and alcama morphants, suggesting that edn1 may act upstream of alcama (Fig. 3A,B).
Fig. 3. Edn1 regulates Alcama levels.
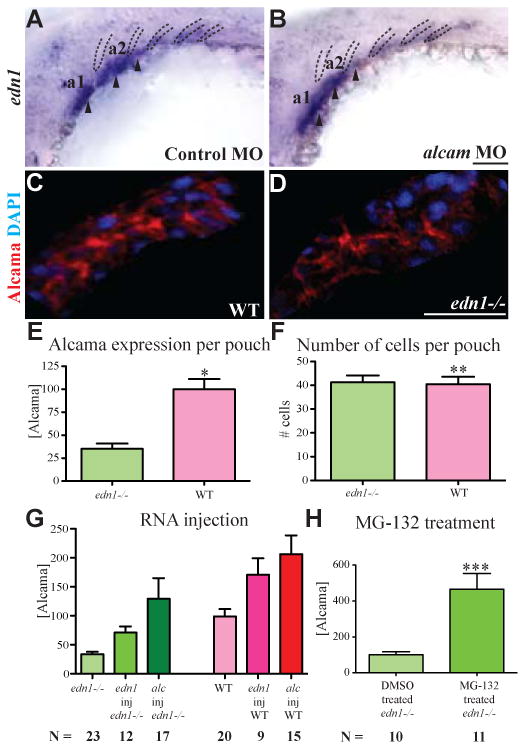
edn1 is expressed in the mesodermal cores (arrowheads) of the first 3 arches and in pharyngeal pouches 2-4 (dotted lines) (A). Its expression is unchanged in alcama morphants (B). Cropped image of a single pharyngeal pouch from 30 hpf WT sibling (C) and edn1-/- mutant (D) stained with Zn-5 (anti-Alcama) antibody in red and DAPI in blue. (E) Bar graph showing the difference in total Alcama protein in a pouch (second or third pouch), represented as measured fluorescence intensity normalized to WT; *p-value <0.0001 by a two-tailed t-test. The bar graph in (F) shows the total number of cells per pouch is unchanged in WT and edn1-/- mutants; **pvalue = 0.08495 by a two-tailed t-test. n=14 for WT; n=11 for edn1-/- mutants for this experiment which was repeated with similar results. (G) edn1 regulates the Alcama levels in pouches. The bar graph shows the sum of Alcama intensity in the first three pouches of 30 hpf edn1-/- (green) and WT (red) larvae after the indicated injections (p-value <0.0001 by one way analysis of variance). (H) Bar graph showing the sum of Alcama intensity in the first three pouches of 30 hpf edn1-/- larvae after treatment with DMSO or the proteasome inhibitor MG-132; ***p-value = 0.0009 by a two-tailed t-test. N is the number of larvae in a single experiment, which was repeated with similar results. a1 and a2 label pharyngeal arches 1 and 2. Scale bars: 50 μm.
To test this possibility, we analyzed Alcama protein expression in pharyngeal pouches of edn1-/- mutants. 30 hpf larvae were co-stained with Zn-5 Alcama antibody and DAPI (Fig. 3C, D). Confocal images were cropped to individual endodermal pouches and analyzed. Alcama intensity in individual pouches of edn1-/- mutants was down-regulated to less than 40% of the WT individuals (Fig. 3E). The total number of Alcama-expressing cells in each pouch (determined by DAPI staining) is comparable between WT and edn1-/- mutants (Fig. 3F), indicating that endodermal pouch tissue is present in edn1-/- mutants, but it expresses reduced amounts of Alcama. This observation suggests that edn1 possibly regulates Alcama levels in pharyngeal pouches.
Edn1 is produced as a proprotein, which is cleaved twice to produce a diffusible 21-amino acid peptide. The peptide acts by binding to endothelin receptors (Ednr) coupled to G proteins. In zebrafish, two Ednrs exist; EdnrA and EdnrB. Previous ISH studies have revealed that ednrA1 and ednrA2 are expressed almost exclusively in the NC of pharyngeal arches (Nair et al., 2007). We cloned ednrb1 and validated that this receptor is expressed diffusely throughout the endoderm and NC (Fig. S2B). ednrb1 expression in the endoderm is compatible with our hypothesis that Edn1 signals to the endoderm to regulate Alcam levels.
To corroborate our hypothesis that edn1 regulates Alcama levels, we tested whether edn1 over-expression could rescue Alcama levels in edn1-/- mutants. edn1 RNA was injected into edn1-/- embryos at the 1-cell stage and Alcama protein levels were quantified at 30 hpf as discussed previously with one modification. The fluorescence intensity of Zn-5 staining in the pharyngeal area from the first to the third pouch, instead of single pouches, was measured and normalized to uninjected WT (Fig. S3). alcama RNA was injected as a positive control. edn1 RNA injection results in more than 50% increase in Alcama protein in WT and edn1-/- mutants (Fig. 3G), suggesting that edn1 regulates Alcama levels in the endoderm.
Additionally, we investigated the mechanism by which Edn1 regulates Alcama. ISH studies revealed that alcama RNA levels, while slightly down-regulated in edn1-/-mutants (Fig. S2C, D), do not explain the severe down-regulation observed in Alcama protein levels (Fig. 3D,E). This observation suggests that edn1 does not regulate Alcama levels via regulating transcription. Previous studies have indicated that edn1 may lead to Snail and β-catenin protein stabilization by regulating the proteasome pathway (Rosano et al., 2005; Sato-Jin et al., 2008). To test whether Edn1 stabilizes Alcama protein by interfering with its degradation by the proteasome, we treated edn1-/- larvae with the proteasome inhibitor MG132 and measured Alcama levels. The fluorescence intensity was normalized to DMSO-treated edn1-/- mutants. Alcama levels in the pharyngeal pouches increased by more than 300% upon treatment with MG-132 (Fig. 3H), indicating that inhibition of proteasomal degradation is involved in Edn1-induced regulation of Alcama. While edn1 RNA injection increases Alcama levels in the pharyngeal pouches, Alcama levels are unchanged in the heart and retina, where Edn1 signaling is not active (data not shown). These experiments indicate that Edn1 stabilizes Alcama levels specifically in the pharyngeal endodermal pouches by inhibiting some component of the proteasome pathway.
alcama is required for end1-dependent differentiation of NC
We have determined that alcama morphants have defects in ventral cartilages. Since cartilage is formed from NC in the pharyngeal arches, we investigated whether alcama knockdown caused a defect in the patterning or specification of NC. We injected alcama MO into Tg(fli:EGFP) line which labels NC cells. Analysis of larvae at 30 hpf, a stage at which the cranial NC has already populated the arches, revealed that NC migration is unaffected in alcama morphants (Fig. S4B). This observation was corroborated by ISH with the NC marker distalless 2a (dlx2a) at the same developmental stage, showing normal patterning of NC (Fig. S4D). In addition nkx2.3 expression in endoderm (Fig. S4F), and edn1 expression in mesodermal cores and endodermal pouches (Fig. 3B) is unaffected in alcama morphants, suggesting that NC, endoderm and mesoderm are all patterned and specified correctly in the pharyngeal region of alcama morphants. This result implies that similarly to genes in the Edn1 pathway, Alcama is not required for NC migration or specification, but is involved in a later stage of cartilage morphogenesis.
Our data suggest that Alcama functions downstream of Edn1 in cartilage formation. To assess the functional importance of alcama in the Edn1 signaling pathway, we analyzed the expression of genes downstream of edn1 in alcama morphants. Edn1 signaling is required for the expression of Dlx and Hand2 transcription factors in NC (Miller et al., 2000; Walker et al., 2006). Like Edn1, Hand2 is required for ventral cartilage formation and Dlx factors pattern the dorso-ventral axis of pharyngeal arches (Depew et al., 2002; Miller et al., 2003). In alcama morphants, the expression of hand2, dlx3b, dlx5a and dlx6a is strongly reduced in the first and the posterior arches, and mildly reduced in the second arch at 30 hpf (Fig. 4B, E, H, K). Although hand2 expression recovers by 48 hpf, dlx genes continue to be down-regulated (Fig. S5). At 30 hpf, hand2 expression is down-regulated in NC, but unaffected in the heart of alcama morphants (Fig. 4B) and edn1-/-mutants (Fig. 4C), indicating that Alcama is required specifically for edn1-dependent expression of NC genes.
Fig. 4. alcama is required for edn1-dependent gene expression.
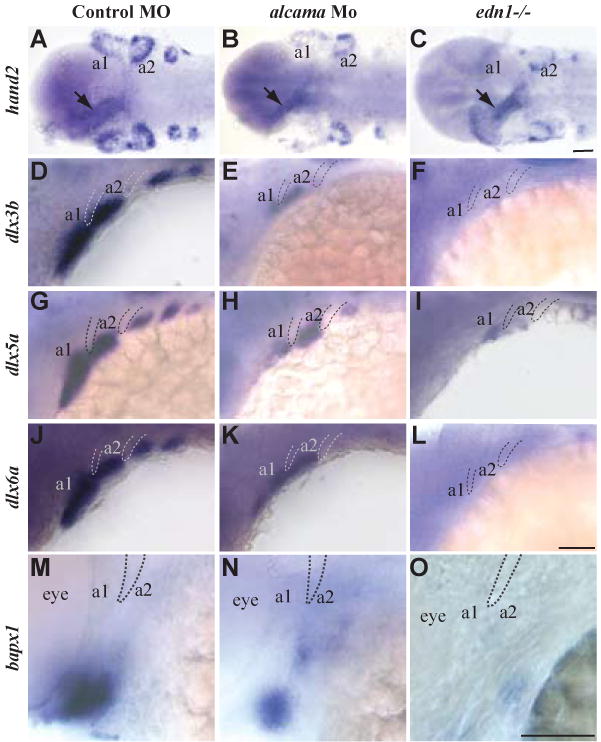
Ventral views of larvae at 30 hpf (A-C), and lateral views at 30 hpf (D-L) and at 48 hpf (M-O) respectively. ISH for hand2 (A-C), dlx3b (D-F), dlx5a (G-I), dlx6a (J-L), and bapx1 (M-O) in control and alcama morphants and in edn1-/- mutants. hand2, dlx3b, dlx5a and dlx6a expression is strongly reduced in the first and branchial arches and moderately reduced in the second arch in alcama morphants. Their expression is strongly reduced in all arches in the edn1-/-mutants. bapx1 expression in the first arch intermediate domain is moderately reduced in alcama morphants and strongly reduced in edn1-/- mutants. a1 and a2 label pharyngeal arches 1 and 2. Dotted lines highlight pharyngeal pouches 1 and 2. Scale bars: 50 μm.
edn1-dependent bapx1 expression in the intermediate region of the precartilage arch is required for cartilage joint formation (Miller et al., 2003). Consistent with the joint fusion observed in alcama morphants, bapx1 expression in the intermediate region of arch1 is down-regulated in alcama morphants (Fig. 4N). Down-regulation of Edn1-dependent genes in alcama morphants is similar to, but less severe than that observed in edn1 mutants (Fig. 4 C, F, I, L, O). This observation is consistent with the less severe jaw defect observed in alcama morphants when compared to edn1 mutants. Taken together these data suggest that Alcama is required for Edn1-dependent NC differentiation and the proper formation of ventral cartilage and jaw joint.
Our data suggests that NC is patterned and specified correctly in alcama morphants (Fig. S3). Hence, the failure of NC to express edn1-dependent genes that come on later in development, suggests that alcama knockdown causes a defect in NC differentiation. In subsequent experiments, dlx5a expression is used as a marker for NC differentiation due to its robust expression. The specificity of MO-induced NC differentiation defect was tested by co-injection of alcama MO with alcama RNA, and by alcama MO injection in p53-/- mutants (Robu et al., 2007). dlx5a expression was categorized as strong (all arches stained), partial (all arches stained but weaker) and absent (most arches missing staining and some with very weak staining) (Fig. S6A-C). Co-injection of alcama RNA with alcama MO decreases the number of affected individuals (absent dlx5a staining) to WT levels (Fig. S6D), suggesting that the NC differentiation defect is caused specifically by alcama knockdown. The percentage of affected larvae is similar in WT and p53-/-mutants (Fig. S6D), indicating that similarly to the cartilage defect, the NC differentiation defect is caused by alcama knockdown and is not dependent on off-target MO effects.
alcama over-expression can rescue NC differentiation defect in edn1-/- mutants
Our data suggests that edn1 regulates Alcama levels and knockdown of either gene results in a NC differentiation defect. We tested our hypothesis that edn1 affects NC differentiation via Alcama, by over-expressing alcama in edn1-/- mutants followed by analysis of NC differentiation. We injected alcama RNA into edn1-/- mutants at the one-cell stage and assessed dlx5a expression at 30 hpf as a marker for NC differentiation. dlx5a staining was classified as absent, partial and strong (Fig. 5A-C) and the number of larvae in each category were counted. edn1 over-expression was used as a positive control, resulting in a 300% increase in the number of edn1-/- mutants with dlx5a staining (sum of strong and partial) (Fig. 5D). alcama RNA induces similar increases of rescued edn1-/- mutants as edn1 RNA, although full rescue of dlx5a expression was not achieved. This indicates that Alcama functions downstream of Edn1 to regulate NC differentiation. To bolster this hypothesis, we asked if edn1 rescue of edn1-/- mutants could be abrogated by alcama knockdown. Indeed, co-injection of alcama MO abrogates the ability of edn1 RNA to rescue dlx5a staining in edn1-/- mutants (Fig. 5D), supporting our previous finding that alcama expression is necessary for Edn1 function. Injection of either edn1 or alcama RNA was unable to rescue the cartilage defect of edn mutants at 5 dpf, possibly due to dilution of RNA in rapidly dividing cells.
Fig. 5. alcama rescues NC differentiation defect in edn1-/- mutants.
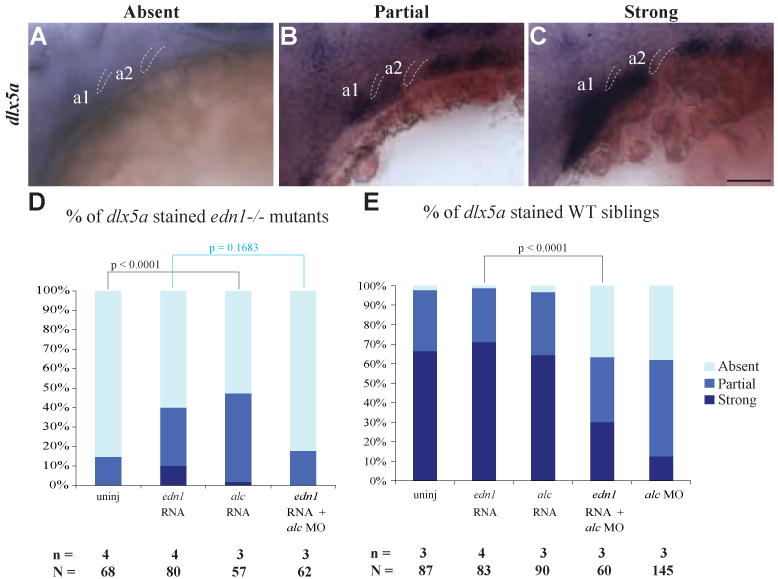
Lateral views of 30 hpf larvae with representative absent (A), partial (B) or strong (C) staining for dlx5a. Bar graphs show the percentage dlx5a expressing larvae in edn1-/- mutants (D) and WT (E) following the stated injections. edn1 or alcama RNA was injected with or without alcama MO. alcama RNA decreases the percentage of affected individuals (absent dlx5a staining) by nearly 30% in edn1-/- mutants and co-injection of alcam Mo abrogates the ability of edn1 RNA to rescue dlx5a expression in edn1-/- mutants. Indicated p-values are calculated by Fishers exact test, n depicts the number of experiments and N depicts the total number of larvae represented in the columns of the plot (p-value < 0.0001 for both D and E by χ2 analysis). a1 and a2 label pharyngeal arches 1 and 2. Dotted lines highlight pharyngeal pouches 1 and 2. Scale bar: 50 μm.
As opposed to edn1-/- mutants, less than 5% of WT larvae have absent dlx5a expression. alcama or edn1 RNA injections into WT embryos do not affect dlx5a expression (Fig. 5E), demonstrating that these genes do not negatively affect NC differentiation when they are over-expressed. Co-injection of edn1 RNA with alcama MO results in a similar percentage of larvae with absent dlx5a staining to that observed in alcama morphants (Fig. 5E). However, there are more larvae with strong dlx5a expression, suggesting that edn1 may regulate NC differentiation by another parallel pathway independent of alcama. In aggregate, these data provide evidence that Alcama functions downstream of Edn1 in regulation of NC differentiation.
Nadl1.1 mediates Alcama differentiation signal to the NC
Since Alcama is expressed in the endoderm, we investigated the possibility that it interacts with another protein to mediate Edn1-signaling to the adjacent NC. Nadl1 (Ng-CAM in chick) has been shown to interact with Alcama in the chick brain to promote neurite extension (DeBernardo and Chang, 1996). Zebrafish have two orthologs of Ng-CAM, nadl1.1 and nadl1.2, but only nadl1.1 is expressed in the NC (Fig. 6A, 7A). To test if nadl1.1 is involved in cartilage development, we used a previously described TB MO targeting nadl1.1 (Wolman et al., 2007). Phenotypically, nadl1.1 morphants are similar to alcama morphants: by 5 dpf they have a protruding lower jaw, cardiac edema and absent swim bladder (data not shown). Similarly to alcama MO, nadl1.1 MO affects edn1-dependent gene expression: hand2 and dlx5a expression is strongly reduced in the arches with a more pronounced effect on the posterior arches (Fig. 6B-E). Alcian blue staining reveals cartilage defects reminiscent of alcama morphants: mc and ch are changed in orientation and are fused to pq and hm respectively (Fig. 6G-H′). Injection of nadl1.1 MO into p53-/- mutants yields similar percentages of larvae with dlx5a down-regulation and jaw abnormalities (Figure 6F, I), indicating that the observed effect is specific to nadl1.1 knockdown. These data suggest that nadl1.1 may be another player in the Edn1 signaling pathway that regulates ventral cartilage formation.
Fig. 6. nadl1.1 morphants phenocopy alcama morphant cartilage defects.
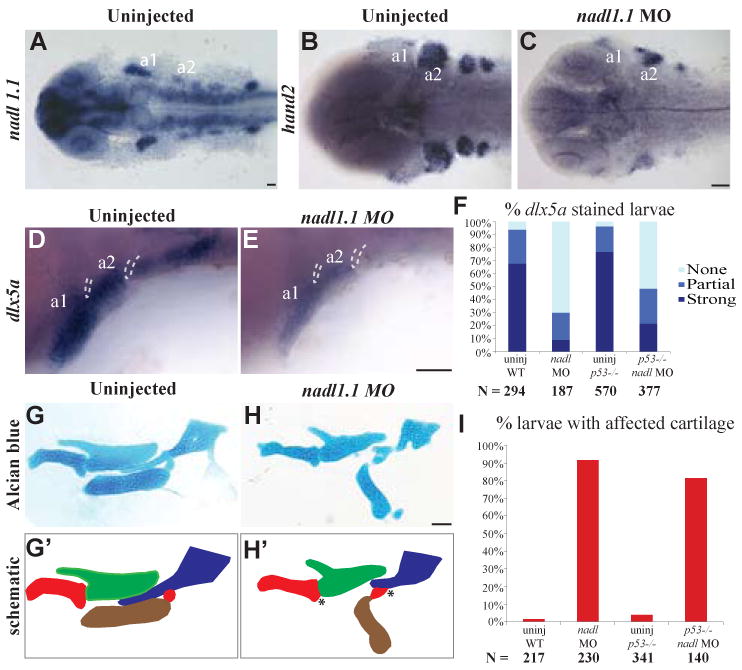
Dorsal views (A-C) and lateral views (D, E) of 30 hpf larvae. nadl1.1 is expressed in the pharyngeal arches, diencephalon, telencephalon, hindbrain neurons, neural tube and pectoral fin in 30 hpf WT larvae (A). 30 hpf nadl 1.1 morphants have down-regulated hand2 (C) and dlx5a (E) expression as compared to uninjected WT (B, D). (F) Bar graph showing that the percentage of larvae with down-regulated (absent) dlx5a expression remains unchanged in the p53-/- background (p-value = 1.000 by Fishers exact test, indicating that WT and p53-/- mutants are not significantly different). Dissected mandibular and hyoid elements from 5 dpf WT and nadl1.1 morphant larvae stained with Alcian blue (G, H) and their corresponding schematics (G′, H′). The joint fusions in nadl1.1 morphants (H, H′) marked by * resemble those seen in alcama morphants. (I) Bar graph comparing the percentage of larvae with affected cartilage in alcama morphants in WT versus p53-/-mutants (p-value = 0.1941 by Fishers exact test, indicating that WT and p53-/- mutants are not significantly different). N is the total number of larvae from three experiments. Scale bars: 50 μm.
Fig. 7. alcama and nadl1.1 interact during cartilage formation.
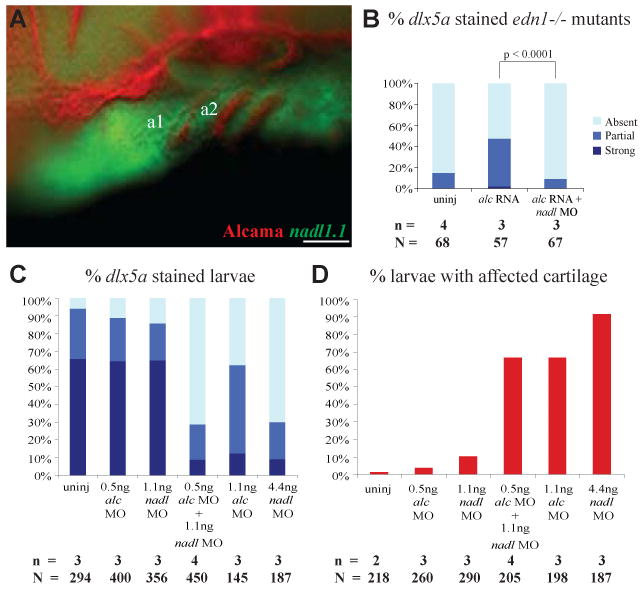
(A) Lateral view of a 30 hpf WT larva stained with Zn-5 antibody (Fast Red) and anti-sense nadl1.1 RNA (Fluorescein), revealing that nadl1.1 is expressed in the NC adjacent to alcama in the endoderm. The bar graphs display the percentage of 30 hpf edn1-/- (B) and WT (C) larvae with dlx5a staining after the indicated injections. (B) shows that nadl1.1 is required for alcama rescue of dlx5a expression in edn1-/- mutants (p-value < 0.0001 by Fishers exact test). (C) and (D) show that alcama and nadl1.1 interact during dlx5a expression and cartilage morphogenesis. (D) Bar graph displaying the percentage of 5 dpf larvae with affected cartilage after the indicated injections (p-value < 0.0001 by χ2 analysis). n indicates the number of experiments and N the total number of larvae. Scale bar: 50 μm.
We investigated the possibility that nadl1.1 is another downstream effector of Edn1-mediated NC differentiation and cartilage morphogenesis that functions independently of Alcama. nadl1.1 expression is not down-regulated in edn1-/- mutants as compared to WT at 30 hpf (Fig. S2E, F), indicating that edn1 does not regulate nadl1.1 at the transcriptional level. Hence, it is unlikely that nadl1.1 functions in the edn1-pathway independently of Alcama. These data show that loss of alcama and nadl1.1 causes very similar defects in cartilage morphogenesis and NC differentiation, supporting our hypothesis that the two adhesion molecules might interact.
alcama and nadl1.1 are expressed adjacent to each other in the endoderm and NC respectively (Fig. 7A), supporting our hypothesis that Nadl1.1 may propagate Alcama differentiation signal from the endoderm to NC. To bolster this hypothesis, we first asked if alcama-mediated rescue of dlx5a expression in edn1-/- mutants (Fig 5) is abrogated by nadl1.1 knockdown. Co-injection of nadl1.1 MO with alcama RNA indeed blocked rescue of dlx5a expression in edn1-/- mutants (Fig. 7B), suggesting that Nadl1.1 expression is necessary for transmission of Edn1 signal through Alcama to NC.
To corroborate this observation we next tested possible interaction of Alcama and Nadl1.1 in synergy experiments by co-injecting suboptimal doses of alcama MO and nadl1.1 MO into WT embryos. The read-out consisted of assessment of NC differentiation (dlx5a expression) and cartilage morphogenesis. While suboptimal doses of alcama and nadl1.1 MOs alone do not cause significant changes in dlx5a expression or cartilage shape, co-injection resulted in synergistic increase in the number of larvae with down-regulated dlx5a expression and cartilage defects (Fig. 7C, D). This indicates that Alcama and Nadl1.1 interact during cartilage morphogenesis. Taken together with the ability of nadl1.1 MO to block rescue of NC differentiation by Alcama over-expression in edn1-/- mutants, these data suggest that Alcama affects cartilage and NC differentiation by interacting with Nadl1.1.
Discussion
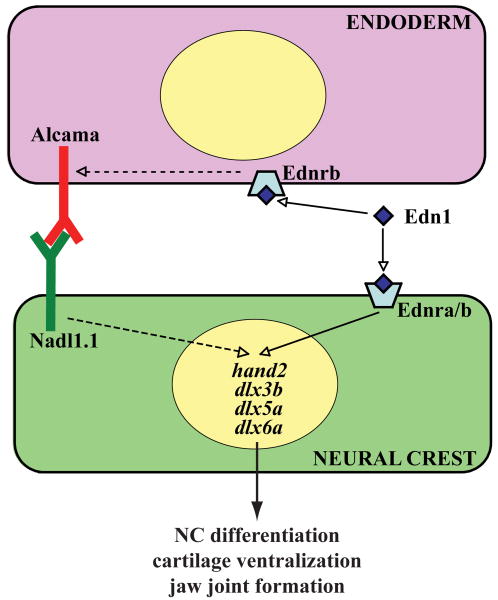
Our data shows for the first time that endodermally expressed Alcama is required for differentiation of NC and ventral pharyngeal cartilage morphogenesis. The alcama-deficient cartilage defect resembles that seen in edn1-class of mutants. In addition, edn1 regulates Alcama protein levels in the pharyngeal endoderm. Moreover, similarly to genes involved in Edn1 signaling, alcama is dispensible for NC patterning and migration, but is required for NC differentiation. Additionally, alcama over-expression partially rescues the NC differentiation defect in edn1-/- mutants, indicating that Alcama mediates Edn1 signaling to NC. Finally, we identify that Nadl1.1, an interacting partner of Alcama, is crucial for transmitting Alcama differentiation signal to NC and promoting ventral cartilage morphogenesis. These data lead us to propose a model (Fig. 8), whereby Edn1 signaling turns on dlx genes in the NC by two independent pathways. Edn1 binds to its receptor on NC to activate NC genes directly. In parallel, Edn1 binds to its receptor on the endoderm to stabilize Alcama protein, which in turn binds to Nadl1.1 on NC and further activates NC genes. Activation of both pathways is required for normal differentiation of NC and cartilage morphogenesis.
Fig. 8. Model demonstrating how Alcama mediates Edn1 signaling and NC differentiation.
Previous data has supported the model that Edn1 peptide binds to its G-protein coupled receptor on NC to turn on transcription of NC genes. We propose a parallel pathway by which Edn1 signaling stabilizes Alcama protein in endodermal cells. Alcama, in turn, binds to Nadl1.1 in NC and regulates NC differentiation.
edn1 signals to NC by two parallel pathways
Edn1 is synthesized as a proprotein, which is cleaved twice by Furin and endothelin-converting enzyme resulting in a short, active Edn1 peptide. This short, active Edn1 peptide binds to the G protein-coupled Edn1 receptor. Phospholipase C enzyme further transmits the signal by producing inositol trisphosphate and diacylglycerol. Mouse mutants for Edn1, Ednra and the G-protein share skeletal defects with zebrafish mutants for edn1, furinA and phospholipase C beta 3 (plcβ3); the ventral domains of the lower jaw are reduced in size and fused to the dorsal domains in both species (Clouthier et al., 1998; Clouthier et al., 2000; Dettlaff-Swiercz et al., 2005; Kurihara et al., 1994; Miller et al., 2000; Walker et al., 2006; Walker et al., 2007). Thus Edn1 signaling has been conserved during evolution to regulate ventral cartilage morphology.
Zebrafish edn1-/-and plcβ3-/- mutants have more severe ventral cartilage reductions and joint fusions than alcama morphants. The milder cartilage defect in alcama morphants correlates with the less pronounced reduction of dlx, hand2 and bapx1 gene expression in NC. We propose that this milder defect is due to two concomitant, additive pathways for edn1 signaling; the first acts directly in the NC through the Edn1 receptor and the second activates Alcama signaling from the endoderm to the NC (Fig. 8). While both pathways are affected in edn1 and plcβ3 mutants, in alcama morphants direct signaling from the Edn1 receptor is still intact and can partially activate dlx gene expression in the NC, resulting in a milder phenotype.
Edn1 regulates Alcama protein levels in pharyngeal endoderm
Previous data has implicated Edn1 as a regulator of the proteasome pathway (Rosano et al., 2005; Sato-Jin et al., 2008). Our data suggest that edn1 stabilizes Alcama protein by inhibiting the proteasome pathway because inhibiting the proteasome pathway in edn1-/-mutants results in increased Alcama levels. It is still unknown whether Edn1 stabilizes Alcama by inducing a structural change that prevents polyubiquitination, or indirectly by regulating transcription of proteasome subunits or by activation of de-ubiquitinases. Alternatively, Alcama may not be a direct target of the proteasome pathway; instead a chaperone protein that stabilizes Alcama might be the target of proteasome pathway. An alternative possibility is that Edn1 signals to NC and thus induces Alcama stabilization indirectly. Further investigation into this mechanism will broaden our understanding of the process of NC differentiation.
Further inquiry is also needed into other proteins/signals regulating alcama expression. Although edn1 RNA injections and MG132 treatment led to higher Alcama expression, in both cases expression was mostly restricted to the endoderm and not the NC. This data points to an endoderm-specific role of Alcama in cartilage formation. Similarly, edn1 and alcama RNA injections into the one-cell stage induced rescue of dlx5a expression, specifically in NC. Further investigation of these mechanisms, that restrict Alcama expression to endoderm and dlx genes to NC, is needed to gain a more complete understanding of the process of chondrogenesis.
Alcama interacts with Nadl1.1 on the NC to mediate differentiation signals
We established that Nadl1.1, a known interacting partner of Alcama expressed in NC, is important for cartilage morphogenesis and its knockdown results in similar cartilage defects to alcama knockdown. We also demonstrated that nadl1.1 is necessary for Alcama-mediated rescue of NC differentiation in edn1-/- mutants and that Alcama and Nadl1.1 interact during cartilage formation, either directly or indirectly. Hence we concluded that Nadl1.1 possibly transmits Alcama-mediated Edn1 signaling to the NC. Future studies will focus on how Nadl1.1 activates dlx genes in the NC.
One possible function for Alcama and Nadl1.1 interaction during NC differentiation and cartilage morphogenesis may be to simply maintain cell-cell contacts between the endoderm and NC. Support for this hypothesis is provided by the integrin α5 mutant and the Eph-ephrin system. Integrins are required for cell adhesion and migration in many tissues (Benoit et al., 2009; Brakebusch and Fassler, 2005). The zebrafish integrin α5 mutant has defects in formation of the first pouch, resulting in defective compaction and survival of NC cells adjacent to the first pouch, giving rise to a deformed hyoid cartilage (Crump et al., 2004). Eph genes encode tyrosine kinase receptors for ephrin ligands. Eph-ephrin mediated repulsion establishes polarity and boundaries during development (Holder and Klein, 1999; Robinson et al., 1997). Since EphA3 is expressed in ventral NC, it is also postulated that Eph-ephrin repulsion may play a role in separating the dorsal and ventral cartilages, giving rise to the joint between them (Kimmel et al., 2001). Hence, both adhesive (integrins) and repulsive forces (ephrins) play a role in NC differentiation. By analogy it is conceivable that Alcama-Nadl1.1 mediated adhesion between the endoderm and NC may play a similar role in chondrogenesis.
Conclusion
In summary, this is the first demonstration that Alcama, a commonly used marker for endoderm, plays a critical role in NC differentiation and cartilage morphogenesis. We show that Edn1 regulates Alcama levels in vivo and that Alcama functions downstream of Edn1 during cartilage formation. In addition, we provide support for a mechanism by which Alcama mediates Edn1-signaling by interacting with Nadl1.1 on NC cells.
Supplementary Material
Fig. S1. Jaw defects in alcama morphants are not due to off-target MO effects. Bar graph showing the percentage of 5 dpf larvae with affected cartilage after the indicated injections. Co-injection of alcama RNA with alcama MO decreases the percentage of individuals with affected cartilage significantly (p-value < 0.0001 by Fishers exact test). Percentage of alcama morphants with affected cartilage is similar in WT and p53-/-mutants (p-value = 0.1948 by Fishers exact test, indicating that WT and p53-/- mutants are not significantly different).
Fig. S2. alcama and nadl1.1 ISH in edn1-/- mutants. (A, B) 30 hpf WT larvae showing expression of alcama and ednrb1 respectively. (C, D) alcama ISH showing expression in pharyngeal pouches of WT and edn1-/- larvae at 30hpf (E, F) 30hpf larvae stained with Alcama antibody (Fast Red) and nadl1.1 RNA (Fluorescein). nadl1.1 expression in the NC is unchanged while Alcama is down-regulated in edn1-/- mutants (F). Arrows point to alcama expression in the pharyngeal pouches. Scale bars: 50 μm.
Fig. S3. edn1-mediated rescue of Alcama expression. Representative images of the data shown in Fig. 3G. Cropped images of pharyngeal pouches 1-3 from 30 hpf larvae stained with Zn-5 antibody and photographed at the same exposure. Alcama expression in edn1-/-mutants (A) is rescued to near WT levels (C) upon injection of edn1 RNA (B). Scale bar: 50 μm.
Fig. S4. NC and endoderm are patterned and specified correctly in alcama morphants. 30 hpf control (A,C,E) and alcama morphants (B,D,F). Tg(fli1:EGFP) larvae stained with Zn5 antibody in red (A,B) show that NC is patterned correctly in absence of alcama. dlx2a (C,D) and nkx2.3 (E,F) expression in NC and endoderm, respectively, reveals correct specification of NC and endoderm. a1 and a2 label pharyngeal arches 1 and 2. Dotted lines highlight pharyngeal pouches. Scale bars: 50 μm.
Fig. S5. hand2 expression recovers by 48 hpf in alcama morphants. Lateral views of 48 hpf control and alcama morphants and edn1-/- mutants showing hand2 (A-C), dlx3b (D-F), dlx5a (G-I), dlx6a (J-L) expression. dlx3b, dlx5a and dlx6a expression is strongly reduced in the first and branchial arches and moderately reduced in the second arch in alcama morphants. However hand2 expression recovers to WT levels in alcama morphants (B). Scale bar: 50 μm.
Fig. S6. NC differentiation defect is caused by alcama knockdown. 30 hpf larvae showing strong (A), partial (B) and absent (C) dlx5a expression. (D) Bar graph showing the percentage of 30 hpf larvae in each of the three categories of dlx5a expression. alcama RNA decreases the percentage of affected individuals (sum of absent and partial dlx5a staining) in alcama morphants to WT levels (p-value < 0.0001 by Fishers exact test). The percentage of affected individuals is similar in WT and p53-/- (p-value = 0.1683 by Fishers exact test). n indicates the number of experiments and N the total number of larvae.
Acknowledgments
The authors wish to thank Raju Kucherlapati and Len Zon for material and technical support, Sarah Hutchinson for intellectual contributions, and Gavin Wright (and his student) for technical expertise. We wish to thank Chuck Kimmel for alcian blue-stained furina-/- mutant zebrafish, and Gage Crump for dlx plasmids. This work was supported in part by R01 HD047863-01 and by the Huntsman Cancer Foundation. Huntsman Cancer Institute core facilities, supported by grant P30 CA042014, also facilitated this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Priya Choudhry, Email: priya.choudhry@hci.utah.edu.
Nikolaus Trede, Email: nikolaus.trede@hci.utah.edu.
References
- Benoit YD, Lussier C, Ducharme PA, Sivret S, Schnapp LM, Basora N, Beaulieu JF. Integrin alpha8beta1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK-dependent mechanism. Biol Cell. 2009;101:695–708. doi: 10.1042/BC20090060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MA, Aruffo AA, Bajorath J. Cell surface receptors and their ligands: in vitro analysis of CD6-CD166 interactions. Proteins. 2000;40:420–428. [PubMed] [Google Scholar]
- Brakebusch C, Fassler R. beta 1 integrin function in vivo: adhesion, migration and more. Cancer Metastasis Rev. 2005;24:403–411. doi: 10.1007/s10555-005-5132-5. [DOI] [PubMed] [Google Scholar]
- Bretaud S, Allen C, Ingham PW, Bandmann O. p53-dependent neuronal cell death in a DJ-1-deficient zebrafish model of Parkinson's disease. J Neurochem. 2007;100:1626–1635. doi: 10.1111/j.1471-4159.2006.04291.x. [DOI] [PubMed] [Google Scholar]
- Burns FR, von Kannen S, Guy L, Raper JA, Kamholz J, Chang S. DM-GRASP, a novel immunoglobulin superfamily axonal surface protein that supports neurite extension. Neuron. 1991;7:209–220. doi: 10.1016/0896-6273(91)90259-3. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Williams SC, Yanagisawa H, Wieduwilt M, Richardson JA, Yanagisawa M. Signaling pathways crucial for craniofacial development revealed by endothelin-A receptor-deficient mice. Dev Biol. 2000;217:10–24. doi: 10.1006/dbio.1999.9527. [DOI] [PubMed] [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, Vincent C, Le Douarin NM. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129:1061–1073. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- Crump JG, Swartz ME, Kimmel CB. An integrin-dependent role of pouch endoderm in hyoid cartilage development. PLoS Biol. 2004;2:E244. doi: 10.1371/journal.pbio.0020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David NB, Saint-Etienne L, Tsang M, Schilling TF, Rosa FM. Requirement for endoderm and FGF3 in ventral head skeleton formation. Development. 2002;129:4457–4468. doi: 10.1242/dev.129.19.4457. [DOI] [PubMed] [Google Scholar]
- DeBernardo AP, Chang S. Heterophilic interactions of DM-GRASP: GRASP-NgCAM interactions involved in neurite extension. J Cell Biol. 1996;133:657–666. doi: 10.1083/jcb.133.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen WG, van Kempen LC, Gijzen EG, van Groningen JJ, van Kooyk Y, Bloemers HP, Swart GW. MEMD, a new cell adhesion molecule in metastasizing human melanoma cell lines, is identical to ALCAM (activated leukocyte cell adhesion molecule) Am J Pathol. 1998;152:805–813. [PMC free article] [PubMed] [Google Scholar]
- Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002;298:381–385. doi: 10.1126/science.1075703. [DOI] [PubMed] [Google Scholar]
- Dettlaff-Swiercz DA, Wettschureck N, Moers A, Huber K, Offermanns S. Characteristic defects in neural crest cell-specific Galphaq/Galpha11- and Galpha12/Galpha13-deficient mice. Dev Biol. 2005;282:174–182. doi: 10.1016/j.ydbio.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Diekmann H, Stuermer CA. Zebrafish neurolin-a and -b, orthologs of ALCAM, are involved in retinal ganglion cell differentiation and retinal axon pathfinding. J Comp Neurol. 2009;513:38–50. doi: 10.1002/cne.21928. [DOI] [PubMed] [Google Scholar]
- Fashena D, Westerfield M. Secondary motoneuron axons localize DM-GRASP on their fasciculated segments. J Comp Neurol. 1999;406:415–424. doi: 10.1002/(sici)1096-9861(19990412)406:3<415::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Graham A. Development of the pharyngeal arches. Am J Med Genet A. 2003;119A:251–256. doi: 10.1002/ajmg.a.10980. [DOI] [PubMed] [Google Scholar]
- Heffron DS, Golden JA. DM-GRASP is necessary for nonradial cell migration during chick diencephalic development. J Neurosci. 2000;20:2287–2294. doi: 10.1523/JNEUROSCI.20-06-02287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder N, Klein R. Eph receptors and ephrins: effectors of morphogenesis. Development. 1999;126:2033–2044. doi: 10.1242/dev.126.10.2033. [DOI] [PubMed] [Google Scholar]
- Ibanez A, Sarrias MR, Farnos M, Gimferrer I, Serra-Pages C, Vives J, Lozano F. Mitogen-activated protein kinase pathway activation by the CD6 lymphocyte surface receptor. J Immunol. 2006;177:1152–1159. doi: 10.4049/jimmunol.177.2.1152. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Miller CT, Kruze G, Ullmann B, BreMiller RA, Larison KD, Snyder HC. The shaping of pharyngeal cartilages during early development of the zebrafish. Dev Biol. 1998;203:245–263. doi: 10.1006/dbio.1998.9016. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Miller CT, Moens CB. Specification and morphogenesis of the zebrafish larval head skeleton. Dev Biol. 2001;233:239–257. doi: 10.1006/dbio.2001.0201. [DOI] [PubMed] [Google Scholar]
- Kopinke D, Sasine J, Swift J, Stephens WZ, Piotrowski T. Retinoic acid is required for endodermal pouch morphogenesis and not for pharyngeal endoderm specification. Dev Dyn. 2006;235:2695–2709. doi: 10.1002/dvdy.20905. [DOI] [PubMed] [Google Scholar]
- Kurihara Y, Kurihara H, Suzuki H, Kodama T, Maemura K, Nagai R, Oda H, Kuwaki T, Cao WH, Kamada N, et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368:703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Miller CT, Schilling TF, Lee K, Parker J, Kimmel CB. sucker encodes a zebrafish Endothelin-1 required for ventral pharyngeal arch development. Development. 2000;127:3815–3828. doi: 10.1242/dev.127.17.3815. [DOI] [PubMed] [Google Scholar]
- Miller CT, Yelon D, Stainier DY, Kimmel CB. Two endothelin 1 effectors, hand2 and bapx1, pattern ventral pharyngeal cartilage and the jaw joint. Development. 2003;130:1353–1365. doi: 10.1242/dev.00339. [DOI] [PubMed] [Google Scholar]
- Nair S, Li W, Cornell R, Schilling TF. Requirements for Endothelin type-A receptors and Endothelin-1 signaling in the facial ectoderm for the patterning of skeletogenic neural crest cells in zebrafish. Development. 2007;134:335–345. doi: 10.1242/dev.02704. [DOI] [PubMed] [Google Scholar]
- Neff MM, Neff JD, Chory J, Pepper AE. dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications in Arabidopsis thaliana genetics. Plant J. 1998;14:387–392. doi: 10.1046/j.1365-313x.1998.00124.x. [DOI] [PubMed] [Google Scholar]
- Noden DM. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. 1983;96:144–165. doi: 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- Ofori-Acquah SF, King JA. Activated leukocyte cell adhesion molecule: a new paradox in cancer. Transl Res. 2008;151:122–128. doi: 10.1016/j.trsl.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Parant JM, George SA, Holden JA, Yost HJ. Genetic modeling of Li-Fraumeni syndrome in zebrafish. Dis Model Mech. 2010;3:45–56. doi: 10.1242/dmm.003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski T, Nusslein-Volhard C. The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio) Dev Biol. 2000;225:339–356. doi: 10.1006/dbio.2000.9842. [DOI] [PubMed] [Google Scholar]
- Piotrowski T, Schilling TF, Brand M, Jiang YJ, Heisenberg CP, Beuchle D, Grandel H, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Hammerschmidt M, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Warga RM, Nusslein-Volhard C. Jaw and branchial arch mutants in zebrafish II: anterior arches and cartilage differentiation. Development. 1996;123:345–356. doi: 10.1242/dev.123.1.345. [DOI] [PubMed] [Google Scholar]
- Robinson V, Smith A, Flenniken AM, Wilkinson DG. Roles of Eph receptors and ephrins in neural crest pathfinding. Cell Tissue Res. 1997;290:265–274. doi: 10.1007/s004410050931. [DOI] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano L, Spinella F, Di Castro V, Nicotra MR, Dedhar S, de Herreros AG, Natali PG, Bagnato A. Endothelin-1 promotes epithelial-to-mesenchymal transition in human ovarian cancer cells. Cancer Res. 2005;65:11649–11657. doi: 10.1158/0008-5472.CAN-05-2123. [DOI] [PubMed] [Google Scholar]
- Ruhin B, Creuzet S, Vincent C, Benouaiche L, Le Douarin NM, Couly G. Patterning of the hyoid cartilage depends upon signals arising from the ventral foregut endoderm. Dev Dyn. 2003;228:239–246. doi: 10.1002/dvdy.10380. [DOI] [PubMed] [Google Scholar]
- Sato-Jin K, Nishimura EK, Akasaka E, Huber W, Nakano H, Miller A, Du J, Wu M, Hanada K, Sawamura D, Fisher DE, Imokawa G. Epistatic connections between microphthalmia-associated transcription factor and endothelin signaling in Waardenburg syndrome and other pigmentary disorders. FASEB J. 2008;22:1155–1168. doi: 10.1096/fj.07-9080com. [DOI] [PubMed] [Google Scholar]
- Thisse B, Thisse C. Fast Release Clones: A High Throughput Expression Analysis. 2004 ZFIN Direct Data Submission ( http://zfin.org)
- Thisse C, Thisse B. High Throughput Expression Analysis of ZF-Models Consortium Clones. 2005. [Google Scholar]
- Walker MB, Miller CT, Coffin Talbot J, Stock DW, Kimmel CB. Zebrafish furin mutants reveal intricacies in regulating Endothelin1 signaling in craniofacial patterning. Dev Biol. 2006;295:194–205. doi: 10.1016/j.ydbio.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Walker MB, Miller CT, Swartz ME, Eberhart JK, Kimmel CB. phospholipase C, beta 3 is required for Endothelin1 regulation of pharyngeal arch patterning in zebrafish. Dev Biol. 2007;304:194–207. doi: 10.1016/j.ydbio.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JA, Koo SJ, Nicolas S, Fraboulet S, Pfaff SL, Pourquie O, Sanes JR. Axon fasciculation defects and retinal dysplasias in mice lacking the immunoglobulin superfamily adhesion molecule BEN/ALCAM/SC1. Mol Cell Neurosci. 2004;27:59–69. doi: 10.1016/j.mcn.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Welten MC, de Haan SB, van den Boogert N, Noordermeer JN, Lamers GE, Spaink HP, Meijer AH, Verbeek FJ. ZebraFISH: fluorescent in situ hybridization protocol and three-dimensional imaging of gene expression patterns. Zebrafish. 2006;3:465–476. doi: 10.1089/zeb.2006.3.465. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio) 4th. University of Oregon Press; Eugene, Eugene, OR: 2000. [Google Scholar]
- Wolman MA, Regnery AM, Becker T, Becker CG, Halloran MC. Semaphorin3D regulates axon axon interactions by modulating levels of L1 cell adhesion molecule. J Neurosci. 2007;27:9653–9663. doi: 10.1523/JNEUROSCI.1741-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AW, Joosten B, Torensma R, Parnes JR, van Leeuwen FN, Figdor CG. Long-term engagement of CD6 and ALCAM is essential for T-cell proliferation induced by dendritic cells. Blood. 2006;107:3212–3220. doi: 10.1182/blood-2005-09-3881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Jaw defects in alcama morphants are not due to off-target MO effects. Bar graph showing the percentage of 5 dpf larvae with affected cartilage after the indicated injections. Co-injection of alcama RNA with alcama MO decreases the percentage of individuals with affected cartilage significantly (p-value < 0.0001 by Fishers exact test). Percentage of alcama morphants with affected cartilage is similar in WT and p53-/-mutants (p-value = 0.1948 by Fishers exact test, indicating that WT and p53-/- mutants are not significantly different).
Fig. S2. alcama and nadl1.1 ISH in edn1-/- mutants. (A, B) 30 hpf WT larvae showing expression of alcama and ednrb1 respectively. (C, D) alcama ISH showing expression in pharyngeal pouches of WT and edn1-/- larvae at 30hpf (E, F) 30hpf larvae stained with Alcama antibody (Fast Red) and nadl1.1 RNA (Fluorescein). nadl1.1 expression in the NC is unchanged while Alcama is down-regulated in edn1-/- mutants (F). Arrows point to alcama expression in the pharyngeal pouches. Scale bars: 50 μm.
Fig. S3. edn1-mediated rescue of Alcama expression. Representative images of the data shown in Fig. 3G. Cropped images of pharyngeal pouches 1-3 from 30 hpf larvae stained with Zn-5 antibody and photographed at the same exposure. Alcama expression in edn1-/-mutants (A) is rescued to near WT levels (C) upon injection of edn1 RNA (B). Scale bar: 50 μm.
Fig. S4. NC and endoderm are patterned and specified correctly in alcama morphants. 30 hpf control (A,C,E) and alcama morphants (B,D,F). Tg(fli1:EGFP) larvae stained with Zn5 antibody in red (A,B) show that NC is patterned correctly in absence of alcama. dlx2a (C,D) and nkx2.3 (E,F) expression in NC and endoderm, respectively, reveals correct specification of NC and endoderm. a1 and a2 label pharyngeal arches 1 and 2. Dotted lines highlight pharyngeal pouches. Scale bars: 50 μm.
Fig. S5. hand2 expression recovers by 48 hpf in alcama morphants. Lateral views of 48 hpf control and alcama morphants and edn1-/- mutants showing hand2 (A-C), dlx3b (D-F), dlx5a (G-I), dlx6a (J-L) expression. dlx3b, dlx5a and dlx6a expression is strongly reduced in the first and branchial arches and moderately reduced in the second arch in alcama morphants. However hand2 expression recovers to WT levels in alcama morphants (B). Scale bar: 50 μm.
Fig. S6. NC differentiation defect is caused by alcama knockdown. 30 hpf larvae showing strong (A), partial (B) and absent (C) dlx5a expression. (D) Bar graph showing the percentage of 30 hpf larvae in each of the three categories of dlx5a expression. alcama RNA decreases the percentage of affected individuals (sum of absent and partial dlx5a staining) in alcama morphants to WT levels (p-value < 0.0001 by Fishers exact test). The percentage of affected individuals is similar in WT and p53-/- (p-value = 0.1683 by Fishers exact test). n indicates the number of experiments and N the total number of larvae.