Abstract
Background
Weak and inconsistent correlations between measurements of asthma health status suggest that the disease is composed of non-overlapping components.
Objective
Factor analysis was used to explore the relationships between measures of asthma morbidity and to identify heterogeneous components of asthma health status in 5 to 12 year old children. Results were compared across time (baseline and 48 month visit) and treatment arms.
Methods
Analyses were conducted in 7 different study windows in a database from a large clinical trial of children with mild to moderate asthma (n=1041). Measurements of lung function, symptoms and health care utilizations from daily diary cards, serum IgE levels, total eosinophil count, skin test positivity, and airway hyper-responsiveness were included. Data on fractional exhaled nitric oxide (FENO) and sputum eosinophil cationic protein (ECP) was included in a subgroup of patients.
Results
In each of the study windows, factor analysis identified 5 factors which explained between 50% and 60% of the common variance. Factors identified included: (1) inflammatory markers, (2) symptoms/medication use, (3) asthma exacerbations, and measures of lung function which subdivided into (4) Forced Expiratory Volume (FEV1 ) and Forced Vital Capacity (FVC), (5) Bronchodilator response and the FEV1/FVC ratio. Exploratory analyses suggest that FENO comprise the atopy/inflammatory marker factor, and sputum measurements comprise a 6th, separate factor.
Conclusion
The consistent identification of a five factor structure across time and treatment arms suggests that each of these factors provides independent information in the assessment of asthma.
Clinical Implications
This study confirms the importance of a multidimensional approach to asthma in the clinical setting.
Keywords: factor analysis, asthma health status, components, associations, morbidity
INTRODUCTION
Asthma is a disease characterized by reversible airway obstruction, episodes of dyspnea with wheezing, bronchial hyper-responsiveness, and atopy (1). Traditionally, measurements of airway function, symptoms/activity limitation, medication use and morbidity are used as outcome measures in the assessment of asthma, and there is growing interest in the use of inflammatory and atopic markers (2). However, correlations between measurements of asthma health status, the overall burden or impairment of health related to asthma, are weak and inconsistent (3–6), suggesting that these outcomes actually comprise independent components of asthma health status. Each outcome or group of outcomes potentially give clinicians unique information in the assessment of disease activity.
Factor analysis is a statistical technique which allows for the extraction of latent, non-overlapping dimensions (factors) which account for the inter-correlations between a larger number of variables. This technique has been widely applied as a validation technique for tools measuring asthma symptoms and quality of life (7–10). Fewer studies have incorporated clinical data into factor analyses in order to identify the major domains of asthma (11–14). In a study of 199 patients with asthma, Bailey et al found that pulmonary function (FEV1%) asthma symptoms, and asthma management (medications, side effects, and asthma exacerbations) separated into different domains (11). This finding is supported by Grazzini et al’s study of 69 asthmatic patients in which airway obstruction (FEV1% and FVC%) appeared as a factor independent from measures of dyspnea(12). Juniper’s analysis of 763 patients participating in 3 clinical trials shows that, airway caliber (FEV1%, FVC, FEF and Peak Flow), daytime symptoms and β2-agonist use, night time symptoms and β2-agonist use, and asthma specific quality of life make up distinct components of asthma (13). Other studies have shown evidence that when inflammatory markers (eosinophils and FENO) are included in the factor analysis, they comprise factors distinctly separate from symptoms and lung function (15–17).
Though previous studies have used clinical data to identify some of the non-overlapping dimensions in asthma health status, no one factor analysis has yet combined symptom information, lung function measurements, inflammatory markers and atopic measures in a large population of asthmatic children. The purpose of this study is to provide a comprehensive picture of the heterogeneous factors which comprise asthma health status in children using a large number of variables not previously analyzed together. Multiple factor analyses are performed to explore differences across time periods and treatment arms in a database from a large, standardized clinical trial (n=1040). The results of this analysis will support the validity of using routine, multi-factorial assessment of asthma in children.
METHODS
Data for this study was taken from the Childhood Asthma Management Program (CAMP), a double blind, controlled study designed to evaluate whether the long-term treatment with budesonide or nedocromil produced improvements in lung growth compared with placebo over a 5 to 6½ year period. Eligible patients were between 5 and 12 years of age with mild or moderate asthma as defined by NAEPP criteria. The protocol for this study was approved by the Institutional Review Board of each of the participating clinical sites. CAMP study design and methods have been published elsewhere (18–20).
Outcome Measures
Prebronchodilator FEV1, Forced vital capacity (FVC), peak expiratory flow (PEF), and bronchodilator responsiveness [(Postbronchodilator FEV1 − Prebronchodilator FEV1/Prebronchodilator FEV1)*100] were measured at each study visit in accordance with American Thoracic Society Standards as previously reported (18, 19). Airway responsiveness to methacholine (PC20) was measured at the end of the screening period, then yearly until the end of treatment visit.
Symptoms, medication use, and asthma exacerbation information were recorded on daily diary cards by patients or parents. For the purposes of this study, diary data was taken from the 28 day screening period prior to randomization, the 28 day period prior to the 48 month visit, and the 28 day period prior to the end of treatment visit. Patients recorded daily symptom codes describing their asthma, and the overall symptom code for the day was defined as follows: 0 = no asthma episodes; 1 = 1–3 mild asthma episodes of ≤ 2 hours; 2 = ≥ 4 mild asthma episodes or ≥1 episode interfering with activity, play, school, or sleep; 3 = ≥ 1 episode of more than 2 hours or resulting in shortening normal activity, seeing a doctor, or going to a hospital. A mean symptom score for each patient was calculated for the 28 day period leading up to the visit signifying the start of that study window. Patients also recorded the following information on their diary cards: number of albuterol puffs per day for asthma signs, number of prednisone pills taken per day, number of night awakenings, number of absences from school due to asthma, and number of doctor contacts for asthma.
Sensitivity to environmental allergens (measured by 10 standard skin prick tests), total serum IgE levels, and total numbers of blood eosinophil were taken from the end of the screening period and the 44 month follow-up visit. Fractional exhaled nitric oxide (FENO) at a constant expiratory flow rate of 50 mL/s, sputum eosinophilic cationic protein, and sputum eosinophil percentage were measured at the end of treatment visit on a subset of patients at one of the clinical sites. Detailed descriptions of measurement methods are published elsewhere (19, 21, 22).
Statistical Analysis
Principal Factor analysis with varimax rotation was used to identify the distinctive factors from the available outcome variables. Principal or “common” factor analysis is a statistical technique, which seeks the least number of latent variables (factors) which can explain the common variance and correlation of a larger set of variables. This technique condenses the information contained in a larger number of original variables and identifies factors which account for inter-relationships between these variables (23, 24). Variables are clustered into subgroups, and then a rotation is performed which simplifies the factor structure to a more interpretable structure. Varimax orthogonal rotation was chosen as the primary rotation for this analysis, because it does not allow correlations to exist between factors. An exploratory oblique rotation which allows for correlation between factors was also performed on the data.
A scree plot of eigen values vs. each identified factor was created and examined to determine the appropriate number of factors to retain. It was decided a priori that the number of factors retained in the varimax rotation would be chosen by both the break in the scree plot and by the number of factors with eigenvalues (the amount of common variance explained by the factor) at or above the mean eigenvalue. Based on a common rule of thumb used in previous analyses, significant factor loading was considered to be greater than 0.40 (14, 16, 17, 24). Serum IgE, total peripheral eosinophils, and PC20 FEV1, were logarithimically transformed for the analysis.
Factor analysis was performed in 7 different study windows in order to explore differences by time and treatment arm. Analyse included subjects with non-missing values on all variables. The study windows were: 1) all subjects at the screening period two weeks prior to randomization (n=990), 2) all subjects (data from each of the 3 treatment arms was combined) at the 48 month visit (n=676), and subjects ion each of the separate treatment arms at the 48 month visit: 3) placebo (n=277), 4) Nedocromil, (n=196), and 5) Budesonide (n=203). In addition, two exploratory analyses including FENO (n=79) and sputum (n=55) variables were performed on a smaller subset at the end of treatment visit, which fell approximately 7 months after a patient’s 48 month study visit.
RESULTS
Clinical, laboratory, and demographic characteristics of the study population during the screening period prior to randomization are shown in Table 1. Of the 1041 patients enrolled in the trial, 990 had non-missing values and were used in the analysis at the randomization window. The majority of patients were white and male, and the mean age of this population at baseline was 9.0 ± 2.1. During the randomization window, 40.6% of patients had at least one night awakening due to symptoms, and 12.0% of patients reported at least one physician contact due to asthma.
Table 1.
Characteristics of the study sample in the randomization window
| Characteristic | Results |
|---|---|
| Number of patients included in analysis | 990 |
| Ethnicity, n(%) | |
| Non-Hispanic White | 677 (68.4%) |
| Non-Hispanic Black | 131 (13.2%) |
| Hispanic | 93 (9.4%) |
| Other | 89 (9.0%) |
| Age, yrs (mean ± SD) | 9.0 ± 2.1 |
| Female Gender, n (%) | 398 (40.2 %) |
| BMI, kg/m2 (mean ± SD) | 18.2 ± 3.5 |
| Lung Function (median (IQR)) | |
| Pre-BD FEV1/FVC ratio, % | 80.0 (75.0–86.0) |
| Pre-BD FEV1, % predicted | 94.0 (85.0–103.0) |
| Pre-BD FVC % predicted | 104.0 (96.0–112.0) |
| Pre-BD FVC, L | 2.0 (1.6–2.5) |
| Pre-BD FEV1, L | 1.6 (1.3–1.9) |
| Pre-BD Peak Flow, L/min | 270.0(225.0–320.0) |
| FEV1 PC20 methacholine, mg/ml* | 1.1 (0.5–2.7) |
| FEV1 BD Responsiveness | 8.3 (4.3–14.4) |
| Atopic/Inflammatory Markers (median (IQR)) | |
| Serum IgE level, ng/ml | 445.5 (174.0–1240.0) |
| Total Eosinophil Count, mm3 | 400.0 (200.0–636.0) |
| No. of positive core skin tests | 3.0(1.0–5.0) |
| Symptoms† | |
| No. of diary entries (mean ± SD) | 38.3 ± 13.4 |
| No. of episode free days‡ (mean ± SD) | 12.8 ± 11.7 |
| No. of days with albuterol use (mean ± SD) | 17.4 ± 13.6 |
| Mean symptom score ≥1, n(%) | 172 (17.4%) |
| At least 1 night awakening, n(%) | 402 (40.6%) |
| At least 1 school absence, n(%) | 111 (11.2%) |
| At least 1 call to doctor, n(%) | 120 (12.0%) |
FEV1 BD Responsiveness = [(Postbronchodilator FEV1 − Prebronchodilator FEV1/Prebronchodilator FEV)*100]
Each study window contained approximately 4 weeks (28 days) of diary entries. The average number of entries is higher for the randomizationscreening period because some children took 2 or more tries to get through the period without a flare. Children could not use prednisone during the 28-day screening period.
Episode Free Day: a day with no night awakenings, am peak flow ≥80% personal best, no albuterol use for symptoms, no prednisone use, no absence from school due to asthma, no physician contact due to asthma, or symptom code equal to 0
Examination of the scree plot shows that 5 factors have eigenvalues greater than the average eigenvalue and factor 5 corresponds with a break in the scree plot (Figure 1). Thus, 5 factors were retained in the varimax orthogonal rotation for the primary analysis. Correlations of the variables with each factor (factor loadings) are shown in tables 2 and 3. Each factor was comprised of the variables that were most highly correlated with that factor. The clinical description of the five factors identified in the varimax rotation includes: (1) inflammatory/atopic markers, (2) symptoms/medication use, and (3) asthma exacerbations, and measures of lung function which subdivided into (4) lung function FEV and FVC, (5) BD response and the FEV/FVC ratio. The general factor structure and % of variance accounted for remained highly consistent over a 4 year period: the results of the 5 factor model explained 58.9% and 58.4% of the variance in the randomization and 48 month study windows, respectively. Factor loadings and scree plot structures did not differ substantially by treatment arm at the 48 month visit. Results from the exploratory oblique rotation did not differ qualitatively from the orthogonal (varimax) rotation, suggesting that there is minimal correlation between the factors.
Figure 1.
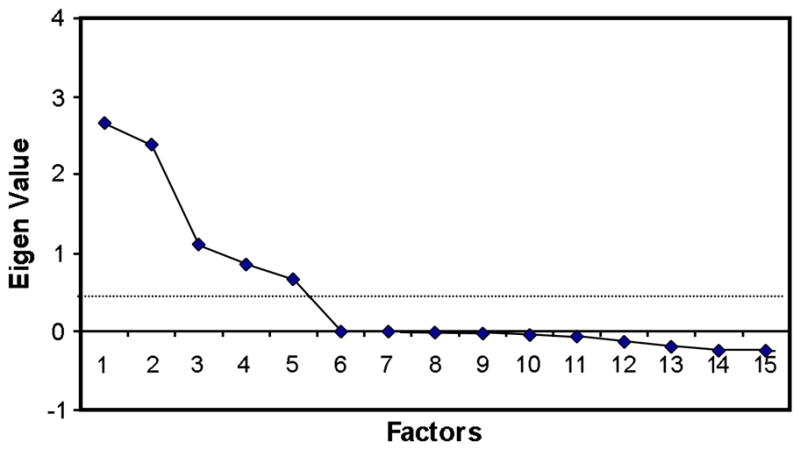
Scree Plot of Eigen Values from Principal Factor Analysis (Randomization window). Five factors had Eigen Values greater than the average Eigen Value (0.45).
Table 2.
Varimax rotated factor pattern: randomization window (all subjects) n = 990
| Factor |
|||||
|---|---|---|---|---|---|
| Lung Function | Ratio/BD response | Inflammatory/Atopy | Symptoms | Exacerbations | |
| Pre-BD FEV1 | 0.979 | 0.161 | 0.024 | −0.044 | −0.056 |
| Pre-BD FVC | 0.975 | −0.188 | 0.067 | −0.005 | −0.054 |
| Pre-BD Peak Flow | 0.750 | 0.120 | 0.123 | −0.057 | −0.030 |
| Pre-BD FEV1/FVC ratio | −0.085 | 0.942 | −0.133 | −0.106 | 0.012 |
| FEV1 BD Responsiveness | −0.178 | −0.644 | 0.240 | 0.157 | 0.023 |
| Serum IgE level (ng/ml)* | 0.100 | −0.078 | 0.692 | 0.049 | −0.037 |
| No. of positive core skin tests | 0.132 | −0.017 | 0.583 | 0.109 | −0.002 |
| Total Eosinophil Count (mm3)* | 0.007 | −0.122 | 0.520 | 0.003 | 0.027 |
| FEV1 PC20 methacholine, mg/ml* | 0.109 | 0.292 | −0.428 | −0.193 | −0.066 |
| Mean Symptom Score | 0.055 | −0.082 | 0.093 | 0.708 | 0.179 |
| No. of days with albuterol puffs | −0.050 | −0.077 | 0.078 | 0.502 | 0.247 |
| No. of episode-free days | 0.081 | 0.096 | −0.066 | −0.661 | 0.110 |
| No. of doctor contacts | −0.014 | 0.013 | 0.020 | 0.085 | 0.571 |
| No. of school absences | −0.037 | 0.021 | −0.072 | −0.025 | 0.541 |
| No. of nights awakened | −0.034 | −0.067 | 0.095 | 0.218 | 0.359 |
| No. of days with any prednisone use** | NA | NA | NA | NA | NA |
| % Variance Explained† | 17.1% | 10.1% | 9.3% | 8.9% | 5.8% |
Data was logarithimically transformed for factor analysis
Participants were taken off of Prednisone during the 28 day screening period but could use it afterwards
Common variance explained by all 5 factors: 58.9%
Table 3.
Varimax rotated factor pattern: 48-month visit (all subjects) n=676
| Factor |
|||||
|---|---|---|---|---|---|
| Lung Function | Ratio/BD response | Inflammatory/Atopy | Symptoms | Exacerbations | |
| Pre-BD FVC | 0.982 | −0.164 | −0.001 | −0.002 | 0.002 |
| Pre-BD FEV1 | 0.967 | 0.220 | −0.045 | −0.058 | 0.009 |
| Pre-BD Peak Flow | 0.795 | 0.118 | 0.041 | −0.090 | 0.020 |
| Pre-BD FEV1/FVC ratio | 0.018 | 0.938 | −0.153 | −0.165 | 0.005 |
| FEV1 BD Responsiveness | −0.159 | −0.630 | 0.322 | 0.223 | −0.035 |
| Serum IgE level (ng/ml)* | 0.023 | −0.067 | 0.687 | 0.092 | −0.019 |
| No. of positive core skin tests | 0.121 | −0.045 | 0.583 | 0.059 | 0.022 |
| Total Eosinophil Count (mm3)* | −0.051 | −0.114 | 0.525 | 0.108 | −0.044 |
| FEV1 PC20 methacholine, mg/ml* | 0.198 | 0.223 | −0.445 | −0.225 | 0.055 |
| Mean Symptom Score | 0.022 | −0.075 | 0.141 | 0.791 | 0.207 |
| No. of days with albuterol puffs | −0.035 | −0.092 | 0.078 | 0.575 | 0.161 |
| No. of nights awakened | −0.029 | −0.053 | 0.104 | 0.336 | 0.133 |
| No. of episode-free days | 0.092 | 0.132 | −0.106 | −0.728 | −0.047 |
| No. of doctor contacts | 0.002 | 0.026 | −0.065 | 0.121 | 0.893 |
| No. of school absences | 0.053 | 0.044 | −0.039 | 0.221 | 0.748 |
| No. of days with any prednisone use | −0.016 | −0.023 | 0.002 | 0.186 | 0.679 |
| % Variance explained* | 16.4% | 9.2% | 9.2% | 11.6% | 12.0% |
Data was logarithimically transformed for factor analysis
Common variance explained by all 5 factors: 51.2%
Exploratory analyses were done on a subset of 79 patients with FENO measurements, and on a subset of 55 patients with FENO and sputum measurements (ECP and eosinophil %). In the first analysis, FENO loaded with serum IgE level, skin test positivity, and total eosinophil count, and the rest of the 5 factor loading structure remained almost identical to the other analysis windows. The only difference in the exploratory FENO analysis was that prednisone use loaded with symptoms variables rather than exacerbation variables. When sputum ECP and sputum eosinophil % variables were added in the second exploratory analysis, the overall loading structure for the original variables remained the same with the sputum variables separating out as a clearly defined sixth factor.
DISCUSSION
Our inclusive analysis has identified a clinically sensible, 5 factor structure explaining between 50% and 60% of the common variance from an inclusive list of variables not previously analyzed together. The factor loading structure remains consistent over the 48-month treatment period and among treatment arms. We believe that this analysis provides further evidence that asthma requires a multi-component assessment: measurement of one or two variables alone will not give an adequate picture of the disease. According to our results, clinically assessing one variable from each identified factor would be more informative then assessing 5 variables which fall in the same one or two factors. For example, assessment of doctor contacts, mean symptom score, IgE, FEV1, and BD responsiveness would provide a more accurate picture of a child’s asthma status than doctor contacts, nights awakened, prednisone use, albuterol puffs, episode free days, and mean symptom score (all which fall under the same 2 factors.)
The results from our factor analyses both support those from earlier studies and add novel findings to the literature. As in previous factor analyses, lung function and symptoms measures separate out clearly as independent factors. In each analysis window, measurements of airway function (FEV1, FVC, PEF ) emerge as the most prominent factor, consistently explaining around 17% of the variation across study windows. Symptoms measures (symptom score, episode free days, days with albuterol use for symptoms) separated out distinctly from symptoms denoting asthma exacerbations (school absences, calls to doctor, prednisone use). This finding is consistent with the findings of Schatz et al, in which acute exacerbations (steroids use and hospital visits) comprise a factor distinct from both symptom frequency and symptom bother in asthmatic adults(14). Night awakenings did not load strongly with other symptoms measures though its loading coefficient (approximately 0.35) can still be considered significant (24).
Our analysis also showed that FEV1/FVC % and BD response loaded together forming a separate factor from FEV1, FVC, and PEF. The reason for this is unclear. While bronchodilator response is strongly correlated with the absolute level of FEV, it is a measure of existing tone within the airways than the absolute value of FEV1 alone. Similarly, since the ratio of FEV1/FVC also more clearly delineates the level of airflow obstruction, both these values may be giving subtly different but independent information.
Spirometry and symptoms measurements are widely viewed as effective diagnostic tools for childhood asthma, and previous studies have validated their use as independent predictors of asthma health status. FEV1% has been documented as an independent predictor of subsequent asthma attacks in children(25, 26), asthma symptoms have been identified as risk factors for asthma hospitalizations(27), bronchodilator response has been identified as an independent predictor of future lung function improvements in response to inhaled corticosteroids (28), and greater airflow obstruction (as measured by FEV1/FVC ratio) has been identified as an independent risk factor for prior hospitalization for asthma (29).
Few factor analyses have included atopic and inflammatory markers, and none have combined atopic, inflammatory, lung function, and symptoms measurements in the same analysis. In a factor analysis performed by Leung et al, plasma IgE, blood eosinophil percentage and FENO loaded together on the same factor, separate from disease severity score and other inflammatory markers (16). Similarly, in our analysis, IgE and skin test positivity loaded together with other inflammatory markers (eosinophil count and bronchial hyper-responsiveness). When we added FENO and sputum measurements to the analysis, FENO loaded on the atopy/inflammatory marker factor, and sputum measurements separated into a 6th, separate factor. These results are similar to Rosi et al’s finding that inflammatory outcomes (sputum ECP’s and sputum eosinophils percentage) loaded together on their own factor, separate from both lung function and baseline bronchial hyper-responsiveness (17). Similar results have also been shown in COPD patients: in a recent factor analysis, sputum markers, lung function, and IgE/PC20 comprise non-overlapping factors (15).
The use of atopic and inflammatory markers as reliable measurements in asthma diagnosis has been supported in a number of studies (30–32), and there is growing evidence that these measures of asthma health status are effective management strategies in improving clinical outcomes (31, 33, 34). In our study, peripheral eosinophils, atopic markers and PC20 consistently loaded on the same factor. This finding is not surprising due to evidence that total IgE is highly correlated with both prevalence of asthma and airway hyper-responsiveness in asthmatic children, and peripheral blood eosinophilia is associated with increased airway responsiveness and higher total serum IgE levels (35–37).
Research has also shown FENO tests have relatively high diagnostic values in assessing asthma and are reproducible and well accepted by patients. Sputum induction has been identified as a relatively noninvasive procedure which can provide useful information for treatment response (21, 34, 38, 39). The lack of correlation of these parameters with other outcome measures suggests that they may be useful as complementary diagnostic tools (17, 40). The results from our analysis add to the evidence showing the value gained by using sputum markers and FENO in asthma diagnosis. However, due to the small sample size of our analysis, these results should be confirmed in a larger population.
The 5 factor model chosen for this analysis was based both upon commonly used rules of thumbs (eigenvalues and scree plot examination) as well as the identification of a clinically sensible factor structure (23). In order to test the robustness of these findings, we also conducted rotations retaining 4 and 6 factors in the randomization window. In each of these exploratory models, lung function consistently separated out as the most prominent factor, while the other factors rearranged only slightly. For example, in the 4 factor model, airway function loadings remained prominent, and all symptom and exacerbation variables loaded together as a single factor. These results as well as the fact that neither time nor treatment affected the structure of the factor analysis demonstrate the stability of our findings.
Though this study includes a more comprehensive list of variables than previous analyses, the five factors identified in our analysis do not necessarily include all of the factors which comprise asthma health status. In Juniper’s analysis, asthma-specific quality of life was identified as an independent component of health status (13). Asthma-specific quality of life outcomes were not available for our analysis; however, it is possible that the “school absences” variable from our analysis captures similar information. Further, it is important to emphasize that this study population is comprised of mild to moderate asthmatic children, thus a different factor structure may have emerged if the analysis had been performed in an adult population.
In summary, this study has identified the latent, non-overlapping components of asthma health status in children from a comprehensive list of clinical, atopic, and inflammatory outcome measures. The consistent identification of a five factor structure across time and treatment arms supports application of the separate assessment of each of these factors in the clinical setting. It is possible that the factors are driven by different mechanisms, supporting the separate assessment of each of the factors identified in gaining an overall picture of asthma health. Though additional measurements may require more time and resources, the evaluation of asthma disease status utilizing any one individual component of disease may lead to inadequate treatment of asthma.
Table 4.
Factor loadings (varimax rotation) across study windows
| Lung Function | BD Response/Ratio | Inflammatory/Atopic | Symptoms | Exacerbation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| screening | 48 month | screening | 48 month | screening | 48 month | screening | 48 month | screening | 48 month | |
| Pre-BD FEV1 | √ | √ | ||||||||
| Pre-BD FVC | √ | √ | ||||||||
| Pre-BD Peak Flow | √ | √ | ||||||||
| Pre-BD FEV1/FVC ratio | √ | √ | ||||||||
| FEV1 BD Responsiveness | √ | √ | ||||||||
| Total serum IgE level (ng/ml)* | √ | √ | ||||||||
| No. of positive core skin tests | √ | √ | ||||||||
| Total Eosinophil Count (mm3)* | √ | √ | ||||||||
| FEV1 PC20 methacholine, mg/ml* | √ | √ | ||||||||
| Mean Symptom Score | √ | √ | ||||||||
| No. of days with albuterol puffs | √ | √ | ||||||||
| No. of episode-free days | √ | √ | ||||||||
| No. of doctor contacts | √ | √ | ||||||||
| No. of school absences | √ | √ | ||||||||
| No. of nights awakened | √ | √ | ||||||||
| No. of days w/prednisone use** | n/a | n/a | n/a | n/a | n/a | √ | ||||
| % of variance explained | 17.10% | 16.40% | 10.10% | 9.20% | 9.30% | 9.20% | 8.90% | 11.60% | 5.80% | 12.00% |
Data was logarithimically transformed for this analysis
Participants were taken off of Prednisone during the 28 day screening period but could use it afterwards
Acknowledgments
Funding Source: NHLBI NO1-HR-16044 - 16052 and GlaxoSmithKline
Abbreviations
- FEV1
Forced Expiratory Volume in 1 Second
- FEF
Forced Expiratory Flow 25–75%
- FVC
Forced Vital Capacity
- FENO
Fractional Exhaled Nitric Oxide
- ECP
Eosinophil Cationic Protein
- NAEPP
National Asthma Education and Prevention Program
- PEF
Peak Expiratory Flow
- PC20
Provocative concentration of methacholine required to produce a 20% fall in FEV1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987 Jul;136(1):225–44. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 2.National Asthma Education and Prevention Program Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Maryland: National Institutes of Health; 1997. Pub. No. 97–4051. [Google Scholar]
- 3.Apter AJ, ZuWallack RL, Clive J. Common measures of asthma severity lack association for describing its clinical course. J Allergy Clin Immunol. 1994 Oct;94(4):732–7. doi: 10.1016/0091-6749(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 4.Sharek PJ, Mayer ML, Loewy L, Robinson TN, Shames RS, Umetsu DT, et al. Agreement among measures of asthma status: a prospective study of low-income children with moderate to severe asthma. Pediatrics. 2002 Oct;110(4):797–804. doi: 10.1542/peds.110.4.797. [DOI] [PubMed] [Google Scholar]
- 5.Osborne ML, Vollmer WM, Pedula KL, Wilkins J, Buist AS, O’Hollaren M. Lack of correlation of symptoms with specialist-assessed long-term asthma severity. Chest. 1999 Jan;115(1):85–91. doi: 10.1378/chest.115.1.85. [DOI] [PubMed] [Google Scholar]
- 6.Gronke L, Kanniess F, Holz O, Jorres RA, Magnussen H. The relationship between airway hyper-responsiveness, markers of inflammation and lung function depends on the duration of the asthmatic disease. Clin Exp Allergy. 2002 Jan;32(1):57–63. doi: 10.1046/j.0022-0477.2001.01297.x. [DOI] [PubMed] [Google Scholar]
- 7.Schatz M, Mosen D, Kosinski M, Vollmer WM, O’Connor E, Cook EF, et al. Validation of the asthma impact survey, a brief asthma-specific quality of life tool. Qual Life Res. 2007 Mar;16(2):345–55. doi: 10.1007/s11136-006-9103-2. [DOI] [PubMed] [Google Scholar]
- 8.Schatz M, Zeiger RS, Vollmer WM, Mosen D, Apter AJ, Stibolt TB, et al. Development and validation of a medication intensity scale derived from computerized pharmacy data that predicts emergency hospital utilization for persistent asthma. Am J Manag Care. 2006 Aug;12(8):478–84. [PubMed] [Google Scholar]
- 9.Schatz M, Zeiger RS, Vollmer WM, Mosen D, Apter AJ, Stibolt TB, et al. Validation of a beta-agonist long-term asthma control scale derived from computerized pharmacy data. J Allergy Clin Immunol. 2006 May;117(5):995–1000. doi: 10.1016/j.jaci.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 10.Simeoni MC, Schmidt S, Muehlan H, Debensason D, Bullinger M. Field testing of a European quality of life instrument for children and adolescents with chronic conditions: the 37-item DISABKIDS Chronic Generic Module. Qual Life Res. 2007 Jun;16(5):881–93. doi: 10.1007/s11136-007-9188-2. [DOI] [PubMed] [Google Scholar]
- 11.Bailey WC, Higgins DM, Richards BM, Richards JM., Jr Asthma severity: a factor analytic investigation. Am J Med. 1992 Sep;93(3):263–9. doi: 10.1016/0002-9343(92)90231-y. [DOI] [PubMed] [Google Scholar]
- 12.Grazzini M, Scano G, Foglio K, Duranti R, Bianchi L, Gigliotti E, et al. Relevance of dyspnoea and respiratory function measurements in monitoring of asthma: a factor analysis. Respir Med. 2001 Apr;95(4):246–50. doi: 10.1053/rmed.2000.1017. [DOI] [PubMed] [Google Scholar]
- 13.Juniper EF, Wisniewski ME, Cox FM, Emmett AH, Nielsen KE, O’Byrne PM. Relationship between quality of life and clinical status in asthma: a factor analysis. Eur Respir J. 2004 Feb;23(2):287–91. doi: 10.1183/09031936.04.00064204. [DOI] [PubMed] [Google Scholar]
- 14.Schatz M, Mosen D, Apter AJ, Zeiger RS, Vollmer WM, Stibolt TB, et al. Relationships among quality of life, severity, and control measures in asthma: an evaluation using factor analysis. J Allergy Clin Immunol. 2005 May;115(5):1049–55. doi: 10.1016/j.jaci.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Lapperre TS, Snoeck-Stroband JB, Gosman MM, Stolk J, Sont JK, Jansen DF, et al. Dissociation of lung function and airway inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004 Sep 1;170(5):499–504. doi: 10.1164/rccm.200401-112OC. [DOI] [PubMed] [Google Scholar]
- 16.Leung TF, Wong GW, Ko FW, Lam CW, Fok TF. Clinical and atopic parameters and airway inflammatory markers in childhood asthma: a factor analysis. Thorax. 2005 Oct;60(10):822–6. doi: 10.1136/thx.2004.039321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosi E, Ronchi MC, Grazzini M, Duranti R, Scano G. Sputum analysis, bronchial hyperresponsiveness, and airway function in asthma: results of a factor analysis. J Allergy Clin Immunol. 1999 Feb;103(2 Pt 1):232–7. doi: 10.1016/s0091-6749(99)70496-3. [DOI] [PubMed] [Google Scholar]
- 18.The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000 Oct 12;343(15):1054–63. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 19.Childhood Asthma Management Program Research Group. The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Control Clin Trials. 1999 Feb;20(1):91–120. [PubMed] [Google Scholar]
- 20.Recruitment of participants in the childhood Asthma Management Program (CAMP). I. Description of methods. Childhood Asthma Management Program Research Group. J Asthma. 1999 May;36(3):217–37. [PubMed] [Google Scholar]
- 21.Covar RA, Spahn JD, Martin RJ, Silkoff PE, Sundstrom DA, Murphy J, et al. Safety and application of induced sputum analysis in childhood asthma. J Allergy Clin Immunol. 2004 Sep;114(3):575–82. doi: 10.1016/j.jaci.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 22.Covar RA, Szefler SJ, Martin RJ, Sundstrom DA, Silkoff PE, Murphy J, et al. Relations between exhaled nitric oxide and measures of disease activity among children with mild-to-moderate asthma. J Pediatr. 2003 May;142(5):469–75. doi: 10.1067/mpd.2003.187. [DOI] [PubMed] [Google Scholar]
- 23.Armitage P, Berry G, Matthews J, editors. Statistical methods in medical research. Oxford, UK: Blackwell Science; 2002. Multivariate methods; pp. 455–84. [Google Scholar]
- 24.Hair JFJ, Anderson RE, Tatham RL, Black WC. Multivariate Data Analysis. 5. Upper Saddle River, NJ: Prentice Hall; 1998. [Google Scholar]
- 25.Kitch BT, Paltiel AD, Kuntz KM, Dockery DW, Schouten JP, Weiss ST, et al. A single measure of FEV1 is associated with risk of asthma attacks in long-term follow-up. Chest. 2004 Dec;126(6):1875–82. doi: 10.1378/chest.126.6.1875. [DOI] [PubMed] [Google Scholar]
- 26.Fuhlbrigge AL, Kitch BT, Paltiel AD, Kuntz KM, Neumann PJ, Dockery DW, et al. FEV(1) is associated with risk of asthma attacks in a pediatric population. J Allergy Clin Immunol. 2001 Jan;107(1):61–7. doi: 10.1067/mai.2001.111590. [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, et al. Risk factors for hospital admission for asthma from childhood to young adulthood: a longitudinal population study. J Allergy Clin Immunol. 2002 Aug;110(2):220–7. doi: 10.1067/mai.2002.125295. [DOI] [PubMed] [Google Scholar]
- 28.Tantisira KG, Fuhlbrigge AL, Tonascia J, Van Natta M, Zeiger RS, Strunk RC, et al. Bronchodilation and bronchoconstriction: predictors of future lung function in childhood asthma. J Allergy Clin Immunol. 2006 Jun;117(6):1264–71. doi: 10.1016/j.jaci.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 29.Bacharier LB, Dawson C, Bloomberg GR, Bender B, Wilson L, Strunk RC. Hospitalization for asthma: atopic, pulmonary function, and psychological correlates among participants in the Childhood Asthma Management Program. Pediatrics. 2003 Aug;112(2):e85–92. doi: 10.1542/peds.112.2.e85. [DOI] [PubMed] [Google Scholar]
- 30.Van Schayck CP, Dompeling E, Van Herwaarden CL, Wever AM, Van Weel C. Interacting effects of atopy and bronchial hyperresponsiveness on the annual decline in lung function and the exacerbation rate in asthma. Am Rev Respir Dis. 1991 Dec;144(6):1297–301. doi: 10.1164/ajrccm/144.6.1297. [DOI] [PubMed] [Google Scholar]
- 31.Sont JK, Willems LN, Bel EH, van Krieken JH, Vandenbroucke JP, Sterk PJ. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. The AMPUL Study Group. Am J Respir Crit Care Med. 1999 Apr;159(4 Pt 1):1043–51. doi: 10.1164/ajrccm.159.4.9806052. [DOI] [PubMed] [Google Scholar]
- 32.Chan EY, Dundas I, Bridge PD, Healy MJ, McKenzie SA. Skin-prick testing as a diagnostic aid for childhood asthma. Pediatr Pulmonol. 2005 Jun;39(6):558–62. doi: 10.1002/ppul.20227. [DOI] [PubMed] [Google Scholar]
- 33.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002 Nov 30;360(9347):1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 34.Smith AD, Cowan JO, Brassett KP, Filsell S, McLachlan C, Monti-Sheehan G, et al. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med. 2005 Aug 15;172(4):453–9. doi: 10.1164/rccm.200411-1498OC. [DOI] [PubMed] [Google Scholar]
- 35.Burrows B, Sears MR, Flannery EM, Herbison GP, Holdaway MD. Relationships of bronchial responsiveness assessed by methacholine to serum IgE, lung function, symptoms, and diagnoses in 11-year-old New Zealand children. J Allergy Clin Immunol. 1992 Sep;90(3 Pt 1):376–85. doi: 10.1016/s0091-6749(05)80018-1. [DOI] [PubMed] [Google Scholar]
- 36.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991 Oct 10;325(15):1067–71. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 37.Durham SR, Kay AB. Eosinophils, bronchial hyperreactivity and late-phase asthmatic reactions. Clin Allergy. 1985 Sep;15(5):411–8. doi: 10.1111/j.1365-2222.1985.tb02290.x. [DOI] [PubMed] [Google Scholar]
- 38.Kharitonov SA, Gonio F, Kelly C, Meah S, Barnes PJ. Reproducibility of exhaled nitric oxide measurements in healthy and asthmatic adults and children. Eur Respir J. 2003 Mar;21(3):433–8. doi: 10.1183/09031936.03.00066903a. [DOI] [PubMed] [Google Scholar]
- 39.Smith AD, Taylor DR. Is exhaled nitric oxide measurement a useful clinical test in asthma? Curr Opin Allergy Clin Immunol. 2005 Feb;5(1):49–56. doi: 10.1097/00130832-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Ronchi MC, Piragino C, Rosi E, Stendardi L, Tanini A, Galli G, et al. Do sputum eosinophils and ECP relate to the severity of asthma? Eur Respir J. 1997 Aug;10(8):1809–13. doi: 10.1183/09031936.97.10081809. [DOI] [PubMed] [Google Scholar]


