Abstract
This neuroimaging study examines the development of cognitive flexibility using the Change task in a sample of youths and adults. The Change task requires subjects to inhibit a prepotent response and substitute an alternate response, and the task incorporates an algorithm that adjusts task difficulty in response to subject performance. Data from both groups combined show a network of prefrontal and parietal areas that are active during the task. For adults vs. youths, a distributed network was more active for successful change trials versus go, baseline, or unsuccessful change trials. This network included areas involved in rule representation, retrieval (lateral PFC), and switching (medial PFC and parietal regions). These results are consistent with data from previous task-switching experiments and inform developmental understandings of cognitive flexibility.
Keywords: change task, development, strategy, executive function, cognitive control
Introduction
One key component of behavior regulation is cognitive flexibility, the ability to modify behavior in response to changing environmental contingencies. Cognitive flexibility enables adaptive decision making and behavioral flexibility (Payne, Bettman, & Johnson, 1993). Adult humans, compared to youths, manifest increased cognitive flexibility and thus are better able to regulate their behavior (Davidson, Amso, Anderson, & Diamond, 2006; Diamond, 2002; Zelazo & Muller, 2002).
Cognitive flexibility is a complicated psychological process that involves multiple executive functions, such as working memory and response inhibition; therefore, it is not surprising that cognitive flexibility shows a longer developmental progression than these other two processes, in isolation (Davidson et al., 2006). Performance on executive function tasks such as anti-saccade tasks, the Wisconsin Card Sort Test (WCST), the Stroop and span tasks show marked improvement through childhood (Diamond, 2006). Work with task-switching paradigms indicates that there is a greater speed and accuracy cost in children vs. adults when switching task blocks; this is thought to indicate that cognitive flexibility remains immature until the early 20s (Cepeda, Kramer, & Gonzalez de Sather, 2001; Cohen, Bixenman, Meiran, & Diamond, 2001). The current developmental study of cognitive flexibility relies on the Change task. In the next sections, we first describe the task and then review the neural and developmental correlates of cognitive flexibility.
The Change task used here is an adapted version of the Stop-Change task first described by Logan and colleagues (Logan, 1982, 1983; Logan & Burkell, 1986). The main differences between our task and the original stop-change task are that our task is visual, not auditory. Our task also includes an algorithm that adjusts the time between the Go and Change signals based on subject performance. Subjects are instructed to inhibit their response to a prepotent go signal when a change signal is presented, and to instead execute an alternative response (McClure et al., 2005; Nelson et al., 2007). The inhibition of one initiated response and substitution of another simulates a situation that people encounter frequently in everyday life, such as a sudden change in plans. The Change task incorporates an algorithm that adjusts task difficulty in response to subject performance. This is a potentially important feature in research on development because it tends to equalize task performance, as indexed by accuracy, across adults and children. Due to this algorithm, the Change task has a relatively high error rate in comparison with the much lower error rates (approximately 1–16%) in many other frequently-employed rule-switching paradigms (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Crone, Bunge, van der Molen, & Ridderinkhof, 2006).
As mentioned, the algorithm in the Change task is designed to minimize between-groups differences in accuracy. In neuroimaging studies that compare groups, there are both advantages and disadvantages to minimizing between-group differences in performance. On the one hand, it is important to dissociate activation differences associated with performance disparities from those associated with the independent variable; in our case, age. If behavioral performance is not equated between groups, it is difficult to determine whether activation differences are due to differences in age or performance (Casey, Tottenham, Liston, & Durston, 2005). On the other hand, it is typically impossible to completely equate behavior between groups (e.g., here, the groups differed in a reaction-time variable). Moreover, even if this was possible, such a design might in essence “control away” the very phenomenon that one wants to study (i.e., group differences in behavior). Therefore, in the Change paradigm, we employed an algorithm to minimize between-group differences in change trial accuracy, but this algorithm was associated with between-group differences in reaction time. To account for this factor, we controlled for between-group differences in reaction time in our analysis. Nevertheless, neuroimaging data must always be interpreted in light of the behavioral data obtained in the scanner, and the presence of this reaction-time between-group difference must be noted.
Behavioral studies identify three important components of cognitive flexibility: a) rule representation and retrieval, b) response inhibition, and c) rule switching (Davidson et al., 2006; Diamond, 2006). Neuroimaging studies in adults and, to a lesser extent, in children dissociate neural correlates of these three components. As detailed below, several areas important for successful rule retrieval, inhibition, and response selection continue to develop throughout adolescence (Casey et al., 2005).
Specifically, studies suggest that rule representation and retrieval is subserved by the lateral prefrontal cortex (PFC), including the ventral lateral PFC (VLPFC) and dorsolateral PFC (DLPFC) (Brass & Von Cramon, 2002, 2004; Bunge, 2004; Bunge, Kahn, Wallis, Miller, & Wagner, 2003). According to Bunge and colleagues, the DLPFC encompasses BAs 9 and 46, and the VLPFC includes BAs 44, 45, and 47 (Bunge, 2004; Bunge et al., 2002; Crone, Bunge et al., 2006). Grey matter in the DLPFC continues to mature throughout adolescence (Gogtay et al., 2004; Kanemura, Aihara, Aoki, Araki, & Nakazawa, 2003). Consistent with this slow maturity, children aged 8–12 years implement rules in a more effortful manner than adults (Crone, Donohue, Honomichl, Wendelken, & Bunge, 2006), and the ability to maintain a rule on-line continues to develop throughout adolescence (Cepeda et al., 2001; Crone, Ridderinkhof, Worm, Somsen, & van der Molen, 2004).
Successful response inhibition is viewed as a distinct function from rule representation and retrieval. Nevertheless, these functions are thought to require brain networks that encompass shared structures. Inhibition is essential when switching tasks or when switching rules within a task (Crone, Donohue et al., 2006; Crone, Wendelken, Donohue, & Bunge, 2006). A circuit encompassing the VLPFC, medial PFC, and basal ganglia has been implicated in successful response inhibition in adults (Alexander, Crutcher, & DeLong, 1990; Aron, Behrens, Smith, Frank, & Poldrack, 2007; Aron & Poldrack, 2006; Verbruggen & Logan, 2008). The VLPFC is important for coding stimulus-response and stimulus-stimulus associations and retrieving rewarded rules (Crone, Bunge et al., 2006). The DLPFC is a crucial node in a broader circuit sustaining and updating representations of current goals, thus facilitating appropriate changes in goal-directed behavior (Miller & Cohen, 2001; Zanolie, Van Leijenhorst, Rombouts, & Crone, 2008). As such, VLPFC and DLPFC have been implicated in inhibition as well as rule representation and retrieval, but these regions perform these distinct functions through their connections to partially non-overlapping circuitry. Recent brain imaging research has begun to compare functioning of these networks among youths and adults.
Behaviorally, children are slower than adults to inhibit responses on motor inhibition tasks such as the Stop paradigm (Bunge & Wright, 2007; Ridderinkhof, Band, & Logan, 1999). Developmental fMRI studies of the Stop paradigm suggest diminished recruitment of the VLPFC and functionally connected regions in children compared to adults (Rubia, Smith, Taylor, & Brammer, 2007; Rubia et al., 2001). These connected regions include the thalamus, caudate, and cerebellum (Rubia et al., 2001). Another response inhibition task, the Go/NoGo task, also relies on the VLPFC for accurate response inhibition (Kawashima et al., 1996; Konishi, Nakajima, Uchida, Sekihara, & Miyashita, 1998; Swick, Ashley, & Turken, 2008). Behaviorally, children make significantly more errors in a Go/No-Go task than do adults (Casey, Castellanos et al., 1997), and neuroimaging studies show that children have less DLPFC activity than adult in these tasks (Bunge et al., 2002; Casey, Trainor et al., 1997).
Cognitive flexibility tasks involving rule switching necessitate retrieval of currently-relevant stimulus-response associations (Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003; Cools, Clark, & Robbins, 2004; Sohn, Ursu, Anderson, Stenger, & Carter, 2000). Accordingly, it is not surprising that rule switching, like rule representation, depends to some extent on engagement of the lateral PFC (Rushworth, Hadland, Paus, & Sipila, 2002; Rushworth, Paus, & Silipa, 2001; Wager, Jonides, & Reading, 2004). Adult patients with damage to the lateral PFC perseverate in the Wisconsin Card Sorting Task (Milner, 1963), a task requiring rule representation and switching. Moreover, fMRI studies in healthy adults show that this region is more active in task-switching conditions than during task-repetition (Dove, Pollmann, Schubert, Wiggins, & von Cramon, 2000; Dreher & Berman, 2002; Sohn et al., 2000; Sylvester et al., 2003). On the WCST, children aged 6–12 perform similar to adults with lateral PFC damage, consistent with PFC immaturity in this age group (Huizinga, Dolan, & van der Molen, 2006; Welsh, Pennington, & Groisser, 1991).
In addition to the lateral PFC, switching also relies on the medial PFC, ACC, superior parietal cortex, and basal ganglia (Cools et al., 2004; Paus, 2001; Rushworth et al., 2002; Wager et al., 2004). Like other PFC sub-regions, grey matter of the ACC and medial PFC matures throughout adolescence (Sowell et al., 2004). Indeed, an fMRI study found less ACC activation in adolescents than adults during a task-switching experiment (Crone, Zanolie, Van Leijenhorst, Westenberg, & Rombouts, 2008). Regarding parietal regions, these are activated during switches in stimulus-response mappings (Crone, Bunge et al., 2006; Wager et al., 2004), and adults show more superior and inferior parietal activation in response flexibility tasks than do children (Casey et al., 2004; Rubia et al., 2006). Indeed, along with the DLPFC, superior parietal cortex is amongst the last grey matter to mature (Gogtay et al., 2004; Kanemura et al., 2003). The basal ganglia is especially important for overriding inappropriate responses when task or rule switching (Cools et al., 2004), and adults again show more striatal activity versus children in response control tasks (Casey et al., 2004; Rubia et al., 2006).
In sum, during cognitive flexibility tasks, children appear to show less activity than do adults in a number of regions mediating component processes of such tasks. Specifically, children may have less neural activity than adults in: 1) areas important for inhibition or for rule representation and retrieval, such as the DLPFC and VLPFC; 2), a partially-overlapping fronto-striatal circuit involved in response inhibition; and 3) areas involved in task switching, such as parietal regions and the ACC.
As previously described, the Change task involves two types of trials: go and change, with the latter involving inhibiting a response and making an alternate response. Our primary focus is on between-group differences during successful response substitution (successful ‘change’ trials) relative to successful execution of the prepotent response (successful ‘go’ trials). This contrast controls for successful task performance, presence of a motor response, and the presence of a go signal (although in change trials the go signal is followed by a change signal). The contrast of successful change vs. go trials assesses response flexibility directly while controlling for task demands and potential behavioral performance differences between the groups. We additionally compared successful change and go trials versus a fixation baseline. Finally, in an exploratory analysis, we compared successful change vs. unsuccessful change. We examined these contrasts for both adults and youths combined, and we compared the two age groups.
Based on neurodevelopmental studies reviewed above, we hypothesized that for successful change vs. go, adults will show greater activation in i) lateral PFC (important for rule representation and retrieval), ii) the fronto-basal ganglia circuit (important for response inhibition), and iii) parietal and ACC regions (important for task-switching).
Methods
Participants
This study was approved by the Institutional Review Board at the National Institute of Mental Health. Informed consent was obtained from adult subjects. For youths, parents or legal guardians gave consent while youths gave assent.
Inclusion criteria were ages 10 to 16 years for youths, ages 22 to 40 years for adults, and an IQ above 70. Exclusion criteria included: any current or past psychiatric diagnosis, the use of any medication, substance use within the past 3 months, and chronic medical illness. Youths were also excluded if their parent reported that any of the child’s first degree relatives had psychiatric illness. After scanning, subjects’ data were also excluded from group level analysis for excessive movement during scanning (>3.5mm in any direction), scanner malfunction, or accuracy on go trials below 65% (Nelson et al., 2007).
Subjects were recruited through flyers and advertisements in newspapers. To confirm that youths were psychiatrically healthy, youths and their parents or guardians were administered standardized, semi-structured diagnostic interviews: the Schedule for Affective Disorders and Schizophrenia for School Aged Children-Present and Lifetime version (Kaufman, Birmaher, Brent, Ryan, & Rao, 2000). The KSADS-PL was administered by clinicians, master’s level or above, with established inter-rater reliability (kappa > 0.9). The Structured Clinical Interview for DSM Disorders (SCID; (First, Spitzer, Biggon, & Williams, 1996) was administered to adult subjects.
The final sample included in the group level analysis consisted of 21 youths aged 10–16 (9 female, Mage= 13.9 ± 1.9 yrs, and 21 adults aged 22–40 (10 female, Mage= 28.7 ± 5.8 yrs). Sixteen participants in the youth sample were included in the control group in Nelson et al. (2007). The Wechsler Abbreviated Scale of Intelligence (WASI; (Wechsler, 1999) was used to measure IQ in both groups. Adults and youths did not differ significantly in IQ (p = 0.23, adult mean = 117.53 ± 11.53 range 100–134, youth mean = 112.29 ± 13.45 range 87–136).
Design and Procedure
The current task was based on previous published work (Logan, 1994; McClure et al., 2005; Nelson et al., 2007), and consists of go and change trials. During change trials subjects must inhibit a prepotent response and execute an alternative one. On go trials subjects were instructed to press “1” if they see “X” or “2” if they see “O”. However, if the background around the “X” or “O” changed from black to blue (change trials), subjects were to press “3”. Subjects were instructed to respond before the X or O disappeared from the screen (within 1000 ms). To establish the prepotency of the go response, there were more go than change trials (176 go trials and 80 change trials). There were also 80 fixation trials which served as a baseline. Subjects completed four 3.5 minute runs, each including 44 go, 20 change and 22 fixation trials, which were randomized.
The difficulty level of each change trial was adjusted using an algorithm based on the subject’s performance on the previous change trial. On the first change trial, the change signal (blue background) occurred 250 ms after the go signal. If the subject was successful (i.e., pressed “3”), on the next change trial the change signal was delayed by an additional 50 ms. In this way the tracking procedure influenced the race between the execution of the first response and the inhibition process. If the subject was unsuccessful on a change trial, the change signal occurred 50 ms sooner on the subsequent change trial, making the task easier. Since this timing adjustment occurred throughout the task, change accuracy should approximate 50%. Prior to entering the scanner subjects were trained to proficiency, including being trained to adhere to the requirement to respond within 1000 ms. At the end of each block subjects received feedback on average response time (RT) for “go” trials (Go-RT). Thus, in total, this version of the change task generates approximately 140 “correct-go” trials, 40 “incorrect-change” trials, and 40 “correct-change” trials for each individual subject.
Scanning Parameters
Scanning occurred in a General Electric Signa 3T magnet (Milwaukee, WI). Images were presented through Avotec Silent Vision Glasses (Stuart, FL). After sagittal localization and a manual shim procedure, single-shot gradient echo T2* weighting images were acquired (matrix 64 × 64, TR = 2000 ms, TE = 40 ms, field of view = 240 mm, voxels = 3.75 × 3.75 × 5 mm). These images were 23 contiguous 5-mm axial slices parallel to the anterior commissure-posterior commissure line. A high-resolution T1-weighted anatomical image with a standardized magnetization-preparation gradient echo sequence (124 2-mm sagittal slices, FOV = 256, NEX = 1, TE = 4.4 ms, matrix = 256 × 256, T1 = 300 ms, bandwidth = 130 Hz/pixel, 33 kHz/256 pixels) was acquired for spatial normalization.
The task consisted of four 3.5 min runs inside the scanner, for a total of 14 minutes of experimental testing. Each one of the four blocks contained 44 go, 20 change, and 22 baseline fixation trials distributed randomly throughout the block, for a total of 176 go trials, 80 change trials, and 88 baseline fixation trials. Based on the ‘rapid event-related’ paradigm (Friston et al., 1998; Zarahn & Slifstein, 2001) the task included randomly occurring ‘blank’ fixation trials which allowed us to deconvolve unique events occurring relatively close in time. As in Zarahn and Slifstein (2001) these blank trials were not modeled and provide an implicit statistical baseline.
Behavioral Data Analysis
Outcome measures recorded during scanning included go and change accuracy, as well as reaction time (RT) for the correct go and change trials. Go RT reflects the time it took for a participant to correctly press “1” or “2” in response to the “X” or “O” on the screen. Change RT equals the time it took for a participant to correctly press “3” after the background screen changed to blue. The inhibit delay time, the interval between the onsets of the go and change signals, was also recorded and used to calculate the change-signal RT (CSRT), or the speed of the change response. We use the same metric to calculate CSRT as employed in our prior report with the Change Task (Nelson et al., 2007). As in this prior report, the CSRT is calculated as the mean change RT minus the mean inhibit delay, at the point where a subject inhibits successful task performance on 50% of change trials. Since the subject’s accuracy on change trials may deviate somewhat from 50%, an interpolation algorithm was used to calculate CSRT: the mean change signal delay was subtracted from the go RT at the Xth percentile, where X is the subject’s percent accuracy on change trials. Since mean RT during go trials differed significantly between groups (see Behavioral Results), this was entered as a covariate in analyses of activation data in contrasts involving go trials.
Imaging Data Analysis
To be consistent with prior work with this task (Nelson et al., 2007), analyses were conducted using SPM99 (Wellcome Department of Imaging Neuroscience, University College, London). Preprocessing included slice time correction, motion correction, and spatial normalization. At the subject level, event-related response amplitudes were estimated using the general linear model. Event types included successful change, unsuccessful change, and go trials. Incorrect-go trials were extremely rare and excluded from the analysis.
A rectangular pulse the duration of each event (2250 ms) was used to model each event, convolved with the hemodynamic response function provided by statistical parametric mapping. A random-effects model was used, a high-pass filter of 0.024 Hz was applied, and the data were smoothed (smoothing kernel FWHM = 8 mm). Subject contrasts were then entered into a second order group level analysis.
When we conduct a whole-brain analysis at p <.05 multiple-comparison corrected, no between-group differences survive. Therefore, we also conducted a whole brain analysis at a statistical threshold of p <.001 uncorrected with a spatial extent of at least 10 contiguous voxels, a commonly-used threshold in studies such as the current one conducting whole-brain between-group contrasts (Dollfus et al., 2007; Nelson et al., 2007). Indeed, there are several studies of rule-learning paradigms, similar to the Change task, that have used the threshold of p <.001 uncorrected with at least 10 contiguous voxels (Bunge et al., 2003; Crone, Donohue et al., 2006; Crone, Wendelken et al., 2006; Crone et al., 2008; Zanolie et al., 2008) as well as even more liberal thresholds of 5-contiguous voxels (van Leijenhorst, Crone, & Bunge, 2006) or p <.01 uncorrected (Cone, Burman, Bitan, Bolger, & Booth, 2008). One rule-learning study that used a threshold of p<.001 uncorrected with at least 10 contiguous voxels was published this year in Developmental Science (Crone et al., 2009).
Our data analytic strategy allowed us to focus particularly on the contrast of successful change vs. go trials, while using successful change vs. baseline, go vs. baseline, and contrasts using unsuccessful change to both decompose the complex interactions represented by successful change vs. go. These additional contrasts also provide important information about other potential developmental differences in activation. Areas of activation were identified by using the Talairach Daemon atlas after translating coordinates from MNI to Talairach space (Talairach & Tournoux, 1988).
Contrast images were created for each subject using pair-wise comparisons of event-related response amplitudes. The contrasts we performed were successful change vs. go, successful change vs. unsuccessful change, successful change vs. baseline, and go vs. baseline. Since there was a significant difference between adults and youths in go RT, contrasts including go trials were covaried with go RT. Also, the age range of the youth group was somewhat wide (10–16), with known neural maturation and pruning occurring during this period (see Casey et al., 2005 for review). Therefore, we covaried the age of the youth group in our analyses so that differences between the two age groups would not be obscured by the high amount of variance amongst youths.
After reporting the behavioral data, we then report neural activity on the contrasts described above, first showing differences between adults and youths, and then with both groups combined. We additionally explored how individual differences in CSRT correlated with activation in the successful change vs. go contrast.
Results
Behavioral Results
Adults and youths differed significantly in go accuracy, go RT, and mean inhibit delay (see Table 1). Adults had significantly higher accuracy than youths on go trials, t (40) = 4.19, p <.05, and there was a trend for adults to have higher accuracy than youths on change trials, t (40) = 3.95, p = .06. Adults had a longer time between go and change signals on the change trials, measured as mean inhibit delay, t (40) = 3.05, p <.01. The use of the algorithm to adjust for change trial difficulty on a subject-by-subject basis was designed to minimize group differences in accuracy on the change trials. Importantly, the use of an event-related design allowed us to control for this between-group difference in accuracy, since only correct go trials were included in the analysis, and we binned event types so that correct change trials were separate from incorrect change trials. Adults had significantly longer RTs than youths on go trials t (40) = 6.53, p <.01, so go RT was used as a covariate in the fMRI analyses. The groups did not differ in CSRT, t (40) = .24, p > 1.
Table 1.
Behavioral performance on the Change task for adults and youths
| Adults | Youths | ||
|---|---|---|---|
| Mean ± SD | Mean ± SD | t | |
| Percent accurate go | 86 ± 10 | 79 ± 2.2 | 4.19* |
| Percent accurate change | 42 ± 12 | 35.6 ± 9.9 | 3.95◆ |
| Inhibit delay | 558 ± 84 | 461 ± 120 | 3.05** |
| Go RT | 768.9 ± 67.9 | 698.1 ± 107.1 | 6.53** |
| Change signal RT | 193.3 ± 58.6 | 185.4 ± 40.1 | 0.24 |
All RTs in ms.
p <.06
p <.05
p <.01
Neuroimaging Results
The neuroimaging analysis included successful change, go, unsuccessful change, and baseline trials. As noted above, go RT was used as a covariate since adults had significantly longer go RTs than youths, and age was covaried in the youth group to ensure that between-group differences in activation were not masked by high variance among youths.
Youth and Adults Combined
Successful Change vs. Go
At a threshold of p <.05 whole-brain corrected, the analysis for adults and youths combined on successful change vs. go trials revealed activation in bilateral inferior parietal lobules (BAs 40, 7), the left inferior frontal gyrus (BA 9), and bilateral culmen (all p’s <.0001; see Table 2 for number of voxels, peak activation location, and t value). We also generated results for this contrast using the lower threshold, p <.001 uncorrected. This revealed several additional areas of activation, in bilateral inferior frontal gyrus (BAs 9, 47) bilateral middle frontal gyrus (BAs 9, 10) left inferior parietal lobule (BA 40), thalamus/pulvinar nucleus, and the right insula (all p’s <.0001; see Table 3 for number of voxels, peak activation location, and t value).
Table 2.
Significant areas of activation in adults and youths combined at p<.05, whole-brain corrected.
| Region | ~ BA | # Voxels | x | y | z | t | |
|---|---|---|---|---|---|---|---|
| Successful change vs. Successful go | L Inferior Parietal Lobule | 40 | 1805 | −46 | −50 | 40 | 7.49 |
| R Inferior Parietal Lobule | 7 | 804 | 35 | −59 | 50 | 6.64 | |
| R Culmen | 65 | 23 | −54 | −24 | 5.41 | ||
| L Culmen | 63 | −31 | −52 | −21 | 5.13 | ||
| L Inferior Frontal Gyrus | 9 | 14 | −49 | 5 | 29 | 4.66 | |
| Successful change vs. Unsuccessful change | L Culmen | 12 | −17 | −33 | −21 | 5.61 | |
| Successful change vs. Baseline | L Precentral Gyrus | 3 | 7915 | −37 | −32 | 47 | 10.03 |
| L Lentiform Nucleus | 5117 | −25 | −6 | 7 | 9.91 | ||
| R Inferior Parietal Lobule | 40 | 1488 | 37 | −57 | 46 | 9.06 | |
| R Dentate | 663 | 21 | −54 | −24 | 9.03 | ||
| R Lentiform Nucleus | 2089 | 19 | 0 | 6 | 7.37 | ||
| L Culmen | 165 | −30 | −55 | −23 | 6.42 | ||
| R Middle Frontal Gyrus | 9 | 180 | 38 | 33 | 33 | 5.65 | |
| R Middle Frontal Gyrus | 6 | 141 | 26 | −12 | 50 | 5.61 | |
| L Middle Occipital Gyrus | 37 | 130 | −42 | −64 | −12 | 5.44 | |
| R Middle Frontal Gyrus | 6 | 40 | 49 | 6 | 42 | 5.38 | |
| L Middle Frontal Gyrus | 10 | 33 | −34 | 44 | 20 | 5.26 | |
| R Precuneus | 19 | 34 | 8 | −76 | 35 | 4.99 | |
| Successful go vs. Baseline | L Postcentral Gyrus | 40 | 5475 | −46 | −34 | 49 | 11.97 |
| L Lentiform Nucleus | 2460 | −23 | −4 | 5 | 9.71 | ||
| R Lentiform Nucleus | 1347 | 21 | 0 | 5 | 8.76 | ||
| R Dentate | 472 | 16 | −54 | −24 | 8.59 | ||
| R Supramarginal Gyrus | 40 | 164 | 35 | −44 | 33 | 5.32 | |
BA = Brodmann Area
p values are all <.0001
Table 3.
Significant areas of activation in both adults and youths at p<.001 uncorrected.*
| Region | ~ BA | # Voxels | x | y | z | t | |
|---|---|---|---|---|---|---|---|
| Successful change vs. Successful go | L Inferior Parietal Lobule | 40 | 8886 | −46 | −50 | 40 | 7.49 |
| L Inferior Frontal Gyrus | 9 | 1236 | −49 | 5 | 29 | 4.66 | |
| L Culmen | 718 | −31 | −52 | −21 | 5.13 | ||
| R Culmen | 549 | 23 | −54 | −24 | 5.41 | ||
| L Middle Frontal Gyrus | 10 | 373 | −36 | 41 | 13 | 4.79 | |
| R Precentral Gyrus | 6 | 275 | 28 | −12 | 50 | 4.01 | |
| L Pulvinar Nucleus | 275 | −5 | −27 | 12 | 3.83 | ||
| R Middle Frontal Gyrus | 9 | 270 | 49 | 6 | 40 | 4.45 | |
| R Inferior Frontal Gyrus | 47 | 150 | 36 | 18 | −6 | 4.78 | |
| R Insula | 44 | 123 | 43 | 7 | 9 | 3.81 | |
| R Inferior Temporal Gyrus | 21 | 85 | 56 | −48 | −5 | 4.11 | |
| L Lentiform Nucleus | 44 | −12 | −4 | 9 | 3.46* | ||
| R Middle Frontal Gyrus | 10 | 32 | 42 | 40 | 20 | 3.62 | |
| Successful change vs. Unsuccessful change | L Precuneus | 7 | 2580 | −28 | −49 | 50 | 4.47 |
| L Lentiform Nucleus | 1064 | −29 | −6 | 12 | 4.61 | ||
| L Culmen | 151 | −17 | −33 | −21 | 5.61 | ||
| R Parahippocampal Gyrus | 28 | 118 | 16 | −15 | −15 | 4.28 | |
| R Superior Parietal Lobule | 7 | 119 | 31 | −57 | 52 | 3.94 | |
| L Precuneus | 7 | 44 | −17 | −65 | 52 | 3.68 | |
| R Dentate | 18 | 20 | −50 | −24 | 3.58 | ||
| R Precentral Gyrus | 6 | 33 | 28 | −18 | 50 | 3.55* | |
| Successful change vs. Baseline | L Postcentral Gyrus | 3 | 23264 | −37 | −32 | 47 | 10.03 |
| R Inferior Parietal Lobule | 40 | 3027 | 37 | −57 | 46 | 9.06 | |
| R Dentate | 1441 | 21 | −54 | −24 | 9.03 | ||
| L Culmen | 1180 | −30 | −55 | −23 | 6.42 | ||
| L Middle Frontal Gyrus | 10 | 448 | −34 | 44 | 20 | 5.26 | |
| L Culmen | 196 | −6 | −35 | −23 | 4.37 | ||
| R Inferior Temporal Gyrus | 127 | 41 | −67 | −3 | 3.93 | ||
| Successful go vs. Baseline | L Inferior Parietal Lobule | 40 | 9081 | −46 | −34 | 49 | 11.97 |
| L Lentiform Nucleus | 4718 | −23 | −4 | 5 | 9.71 | ||
| R Lentiform Nucleus | 3486 | 21 | 0 | 5 | 8.76 | ||
| R Dentate | 1003 | 16 | −54 | −24 | 8.59 | ||
| R Supramarginal Gyrus | 40 | 998 | 35 | −44 | 33 | 5.32 | |
| L Declive | 116 | −34 | −73 | −16 | 3.98 | ||
| R Middle Frontal Gyrus | 9 | 68 | 38 | 33 | 33 | 4.03 | |
| R Middle Occipital Gyrus | 19 | 22 | 38 | −72 | −9 | 3.6 | |
BA = Brodmann Area
p values are all <.0001, except where noted by an *, where p <.001.
Cluster size at least 10 contiguous voxels.
Successful Change vs. Unsuccessful Change
At a threshold of p <.05 whole-brain corrected, the analysis for adults and youths combined on successful change vs. unsuccessful change trials revealed only left culmen activity (p <.0001; see Table 2 for number of voxels, peak activation location, and t value). We also generated results for this contrast using the lower threshold, p <.001 uncorrected. This revealed several additional areas of activation: right middle frontal gyrus (BA 4), bilateral inferior parietal lobule (BA 40), and bilateral superior parietal lobule (BA 7). There was also activation in the left lentiform nucleus and the right parahippocampal gyrus (BA 28) (all p’s <.0001, see Table 3 for number of voxels, peak activation location, and t value).
Successful Change vs. Baseline
At a threshold of p <.05 whole-brain corrected, the analysis for adults and youths combined on successful change vs. baseline trials revealed several areas of shared activation in the lateral PFC, middle frontal gyrus (BAs 6, 9, 10), middle occipital gyrus (BA 37), parietal lobule (BA 40), and precentral gyrus (BA 3). There was also activation in the right dentate, right lentiform nucleus, and left culmen (all p’s <.0001, see Table 2 for number of voxels, peak activation location, and t value). We also generated results for this contrast using the lower threshold, p <.001 uncorrected. This revealed several additional areas of activation in the right inferior temporal gyrus (all p’s <.0001; see Table 3 for number of voxels, peak activation location, and t value).
Go vs. Baseline
At a threshold of p <.05 whole-brain corrected, the analysis for adults and youths combined on the go vs. baseline contrast revealed areas of shared activation in the right supramarginal gyrus, left postcentral gyrus, and bilateral lentiform nucleus (all p’s <.0001, see Table 2 for number of voxels, peak activation location, and t value). We also generated results for this contrast using the lower threshold, p <.001 uncorrected. This revealed additional areas of left pariental activation, in the left inferior parietal lobule (BA 40) and right middle frontal gyrus (BA 9). In addition, there was activation in the right dentate, left declive, and the right middle occipital gyrus (BA 19) (all p’s <.0001; see Table 3 for number of voxels, peak activation location, and t value).
Adults vs. Youths
Successful Change vs. Go
Direct comparison of whole brain activity for adults vs. youths on the successful change vs. go contrast revealed that adults had significantly more activity than youths in the lateral prefrontal regions of the bilateral precentral gyrus (BA 6) p <.0001, and the middle prefrontal region of the right precentral gyrus (BA 6) p <.0001. There were several parietal regions that were significant as well: right postcentral gyrus (BA 40) p <.0001, left precuneus (BA 7) p <.001, right superior parietal lobule (BA 7) p <.0001, and right paracentral lobule (BA 7) p <.0001. See Table 2 for number of voxels, peak activation location, and t value.
Successful Change vs. Unsuccessful Change
Direct comparison of whole brain activity between adults and youths yielded no suprathreshold clusters.
Successful Change vs. Baseline
Direct comparison of whole brain activity for adults vs. youths on successful change vs. baseline trials revealed that adults had significantly more activity than youths in the lateral prefrontal region of the left precentral gyrus (BA 6), and the right inferior frontal junction (IFJ; BA 9). Parietal regions also showed greater activation in the adults vs. youths: right superior parietal lobule (BA 7), right inferior parietal lobule (BA 7), and left precuneus (BA 7). The left cerebellar fastigial nucleus was also more active in adults vs. youths in this contrast (all ps <.0001). See Table 2 for number of voxels, peak activation location, and t value.
Go vs. Baseline
Direct comparison of whole brain activity for adults vs. youths on go vs. baseline trials revealed that adults had significantly more activity than youths in lateral PFC regions including the bilateral middle frontal gyrus (BA 6) p <.0001. Several parietal regions also showed greater activation in the adults vs. youths: the right inferior parietal lobule (BA 40) p <.0001, right precuneus, right paracentral lobule (BA 5) p <.0001, and left superior parietal lobule (BA 7) p <.001. In addition, the right lentiform nucleus p <.0001 and right cingulate gyrus (BA 24) p <.0001 were more active in adults vs. youths on go vs. baseline trials. The right cingulate gyrus just met our minimum cluster size requirement of 10 contiguous voxels p <.0001. See Table 2 for number of voxels, peak activation location, and t value.
Youths vs. Adults
Successful change vs. go; successful change vs. unsuccessful change; successful change vs. baseline; go vs. baseline
Direct comparison of whole brain activity for youths vs. adults on each of these contrasts yielded no suprathreshold clusters.
Correlations of CSRT with successful change vs. go activation
None of the 13 regions that were significant for this contrast across groups correlated significantly with CSRT when both adults and youths were included in the analysis (all r’s <.24, all p’s >.12). Examining adults only also yielded no areas of significant correlation with CSRT (all r’s <.35, all p’s >.09). However, among the youths there was a significant correlation between activation in the right middle frontal gyrus (BA 9) and CSRT in the successful change vs. go contrast (r = .45, p <.05).
Discussion
The current neuroimaging study examined a group of 21 youths (Mage = 13.9 ± 1.9 yrs) and 21 adults (Mage = 28.7 ± 5.8 yrs) while they performed the Change task, which requires inhibition of an already-initiated pre-potent motor response and substitution of an alternate response. The Change task includes an algorithm that adjusts change trial difficulty on a trial-by-trial basis in response to each subject’s performance. This yields an approximately 50% accuracy rate on change trials, thus allowing us to examine mechanisms mediating cognitive flexibility in the context of a cognitively demanding task. The ability to adapt behavior flexibly in response to changes occurring in the environment is a key component of behavior regulation, and enables behavioral flexibility, which is an adaptive trait for appropriate decision making (Payne et al., 1993).
Due to the neurodevelopmental changes taking place during late childhood and adolescence, we hypothesized that brain regions important for cognitive flexibility (lateral PFC, parietal, and striatal regions) would be more active in adults than youths on the successful change vs. go, successful change vs. baseline, and go vs. baseline contrasts. We covaried go RT because adults were slower than youths on correct go trials, and we covaried age of the youths, since the major developmental changes that occur between ages 10 and 16 could lead to high variance in neural activation in this group, thereby potentially masking informative findings.
Behaviorally, we found that, on go trials, adults took longer than youths to respond correctly, and they had higher accuracy. With regard to change trials, adults and youths did not differ on CSRT (a measure of the time needed for a subject to execute the change response and the major index of cognitive flexibility). Our finding of between-group differences in activation in the absence of between-group differences in CSRT is consistent with previous work using this paradigm in youths with bipolar disorder and controls (Nelson et al., 2007). However, in the current study, adults and adolescents did differ on accuracy, despite our use of an algorithm designed to equate accuracy between groups. In addition, the mean inhibit delay, or time between the go and change signal on change trials, was longer for adults vs. youths. The lengthened inhibit delay in adults vs. youth indicates that the algorithm led adults to experience change trials that were, on average, more difficult than the change trials experienced by youth. As with the between-group difference in accuracy, this provides behavioral evidence that adults may be more cognitively flexible than youths although to an insufficient degree as to be reflected in the CSRT measure. Thus, while adults and youth did not differ in CSRT, there are suggestions in the behavioral data that adults may have greater cognitive flexibility than youths, at least to a subtle degree.
In partial concordance with our hypotheses, for successful change vs. go there was increased activation in adults vs. youths in frontal and parietal areas (bilateral precentral gyri, left precuneus, right inferior parietal lobule, bilateral superior parietal lobules, and right paracentral lobule). For successful change vs. baseline, adults showed increased activation in DLPFC (right IFJ) and the left precentral gyrus, and parietal areas (right superior and inferior parietal lobules and left precuneus) compared to youths.
Successfully changing responses involves inhibition (stopping) of the prepotent response as well as the execution of a new response. Previous neuroimaging work with the stop signal paradigm has shown activation of areas similar to ones we find in correct change trials, such as the IFG (Rubia et al., 2007; Rubia et al., 2001) and IFJ (Brass & Von Cramon, 2002, 2004), suggesting that the activation of inhibition networks is important in the current change task. There is some debate about the role of pre-SMA vs. IFG in response inhibition (Verbruggen & Logan, 2008). Our results suggest that the IFG in involved in the case of inhibiting one response and initiating another across both adults and youths.
This pattern of results was also true for go vs. baseline: adults had significantly more lateral PFC (bilateral middle frontal gyrus) and parietal activity (right supramarginal gyrus, bilateral precuneus, and left superior parietal lobule) than did youths. Thus, overall, we found that adults engaged prefrontal and parietal regions to a greater extent than youths in a task that examines cognitive flexibility.
The lateral PFC is one of the latest regions of the brain to develop, continuing its maturation throughout adolescence (Casey, Giedd, & Thomas, 2000; Casey et al., 2005; Giedd et al., 1999; Sowell, Trauner, Gamst, & Jernigan, 2002). Hence, grey matter in the lateral PFC does not reach adult volumes until the third decade (Giedd, 2004; Reiss, Abrams, Singer, Ross, & Denckla, 1996). Consistent with the majority of prior studies of behavior (Crone, Bunge et al., 2006; Crone et al., 2004; Crone et al., 2008), and neurodevelopment (Casey et al., 2000; Casey et al., 2005; Giedd, 2004; Giedd et al., 1999; Reiss et al., 1996; Rubia et al., 2006; Sowell et al., 2002) we found that adults showed significantly more activation than youths in the lateral PFC and parietal regions during this cognitive flexibility task.
Common areas of activation in adults and youths were in lateral PFC, parietal, and striatal areas for successful change vs. go, successful change vs. baseline, and go vs. baseline. Thus, the results from adults and youths combined suggest that areas important for the three main components of cognitive flexibility mediate performance of the Change task: rule representation and retrieval (lateral PFC), response inhibition (basal ganglia), and task-switching (parietal areas).
The results from both our between-group analysis and from the two groups combined mesh with recent work on a fronto-parietal network for top-down cognitive control. This network includes the lateral PFC and is involved in initiating and adapting control on a trial-by-trial basis (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Dosenbach et al., 2007). The fronto-parietal network has been hypothesized to support response initiation and flexibility by adjusting behavior to feedback, since it integrates information from one trial to the next (Miller & Cohen, 2001). These aspects of cognitive control are imperative for successful performance on the change task. In addition, inhibition of irrelevant stimulus-response associations in a rule or task-switching paradigm has been shown to rely strongly on the lateral PFC as well as the parietal cortex (DiGirolamo et al., 2001; Konishi et al., 2002; Konishi et al., 1999; Smith, Taylor, Brammer, & Rubia, 2004). As previously reviewed, we not only found lateral PFC and parietal activity for successful change trials (vs. go or baseline trials) in both groups combined, but activity in both these regions was greater in adults than youths, suggesting that the fronto-parietal network is still developing throughout adolescence. In addition to our primary contrast of successful change vs. go, we also examined activation on the successful change vs. baseline and go vs. baseline contrasts. It should be noted that, in the successful change vs. baseline and go vs. baseline contrasts, the results could be contaminated by the fact that a motoric response was required for go and successful change trials, but not for the fixation baseline. A cleaner baseline would be a simple button press.
In addition to a motoric response, go vs. baseline also necessitates rule retrieval in order to make a correct response, and in that sense could be seen as a working memory test. Therefore, the most meaningful contrast for the current discussion of cognitive flexibility is successful change vs. go. We did indeed find areas in the lateral PFC important for rule retrieval active for both successful change vs. baseline and go vs. baseline (Bunge et al., 2002; Crone, Bunge et al., 2006), as well as parietal activation and areas of the postcentral gyrus (BAs 2 and 3) that are involved in somatosensory experience. We should note that our areas of frontal activation along the middle frontal gyrus are more posterior than some other neuroimaging studies of rule-retrieval tasks (Bunge et al., 2003; Rubia et al., 2006).
We additionally investigated correlations of the behavioral measure of cognitive flexibility, CSRT, on which the groups did not differ, within both groups combined and in each separately on the successful change vs. go contrast. For adults and youths combined, and for adults alone, there were no significant correlations of CSRT with activation. However, in the youth sample there was a significant correlation of activation in the right middle frontal gyrus (BA 9) with CSRT on the successful change vs. go contrast. This finding suggests that, in our younger sample, subjects for whom the task was more difficult (longer CSRT) had more activity in this dorsal prefrontal area, which is important for rule maintenance and maintenance of the goal of overriding a strong response tendency (Bunge, 2004).
The most notable limitation in our study is the relatively wide age range amongst the youth, without sufficient power to allow us to examine developmental differences within the group of youths. There is not enough power at each of the ages between 10 and 16 to be able to draw conclusions about the neurodevelopment of cognitive flexibility at specific ages in late childhood. Future studies should include a larger sample covering the age range from late childhood until early adulthood, in order to examine more closely the developmental trajectory of the fronto-parietal network (as in Rubia et al., 2006). Further neurodevelopmental research using ecologically valid cognitive flexibility tasks will increase our understanding of how the fronto-parietal network matures throughout childhood and adolescence. It would be valuable for future investigations of cognitive flexibility to have a more robust sample across this wide age range in order to have enough power for developmental analyses within a youth sample.
A second limitation relates to the inability of the current task to probe differential strategies employed during the Change task. Adults in the current study exhibited both higher go accuracy and longer “go” RT. This suggests that adults were more likely than youths to utilize a so-called “speed-accuracy-trade-off (SAT)” strategy, waiting longer in order to avoid responding before the onset of a change signal. Youths, on the other hand, being less likely to utilize such a tradeoff, instead responded more quickly and less accurately. While adults might be expected to utilize this strategy more often than youth, one study which attempted to detect such developmental correlates of SAT did not actually demonstrate that strategy behaviorally, with adults performing both better and faster than youth (Smits-Englesman, Sugden, & Duysens, 2006). To most effectively evaluate this possibility, a future study might differentially reinforce accurate responses in some task blocks and fast responses in others. This future study could then examine the degree to which encouraging either speed or accuracy influences engagement of the fronto-parietal network, both in subjects as a whole as well as in specific age groups.
In sum, compared to adults, youths showed less activation of lateral prefrontal and parietal regions in our main contrast of interest, successful change vs. go, as well as successful change vs. baseline and go vs. baseline, suggesting that, on a challenging cognitive flexibility task, youths do not engage lateral PFC and parietal areas to the same extent as do adults. In both adults and youth, this task activated areas involved in rule representation and retrieval (lateral PFC), response inhibition (striatum), and rule switching (medial PFC, basal ganglia). These areas were more active for successful change trials versus go, baseline, or unsuccessful change trials.
The Change task models a skill needed to function in everyday life: namely, to adapt behavior flexibly in response to changes occurring in the environment. This skill has been shown to be deficient in some patient populations, such as bipolar disorder and antisocial personality disorder. Future work incorporating not only patient populations but also multiple age groups within patient populations will help us better understand the neurodevelopment of cognitive flexibility.
Figure 1.
Behavioral paradigm of the Change task. Each trial begins with 500 ms fixation, which is then replaced by an X or an O. Subjects are instructed to press ‘1’ if an X appears and ‘2’ if and O appears. These constitute go trials. If a blue square appears after the X or O, subjects are to press a ‘3’ instead of a ‘1’ or ‘2’. These constitute change trials. The onset of the change signal varies from trial to trial. If subjects respond correctly on a change trial, the inhibit delay (the interval between the onsets of the go and change signals) on the next change trial increases by 50 ms, making it more difficult for the subject to change successfully. If subjects respond incorrectly on a change trial, inhibit delay on the next change trial decreases by 50 ms. In total there were 176 go, 80 change, and 80 fixation trials.
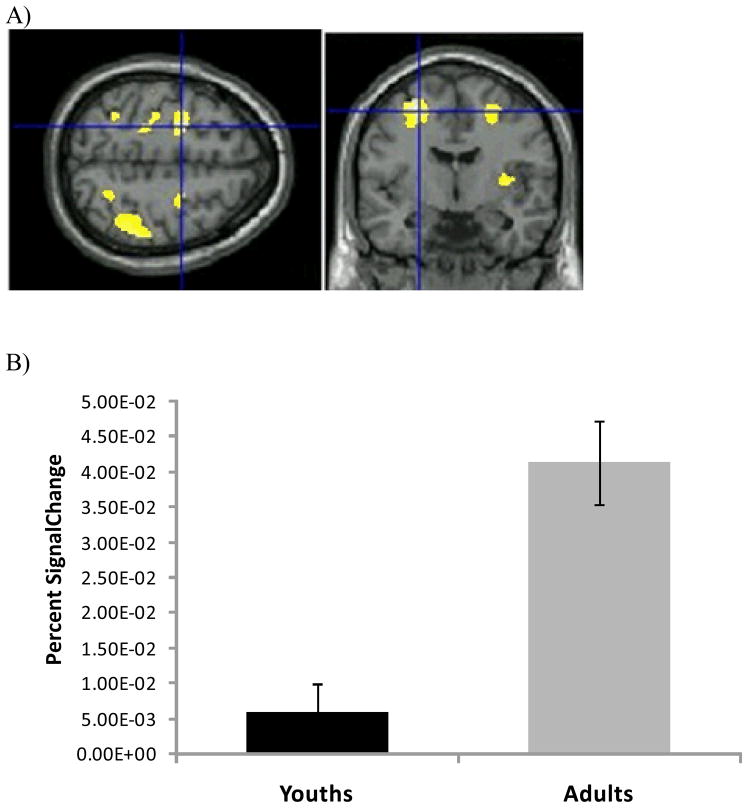
Figure 2.
A) Activation for Adults vs. Youths, Successful change vs. go, left middle frontal gyrus (−26,−−6, 52). Data shown at p < 0.001, whole-brain uncorrected. B) Percent change of the blood oxygen level-dependent signal at −26,−6, 52 for the youths (black bar) and adults (gray bar). Error bars depict standard error of the mean.
Figure 3.
A) Activation for Adults vs. Youths. Successful change vs. baseline, right superior parietal lobule (32,−60, 60). Data shown at p < 0.001, whole-brain uncorrected. B) Percent change of the blood oxygen level-dependent signal at 32,−60, 60 for the youths (black bar) and adults (gray bar). Error bars depict standard error of the mean.
Figure 4.
A) Activation for Adults vs. Youths, Go vs. Baseline, bilateral middle frontal gyrus (centered on −26, −6, 54). Data shown at p < 0.001, whole-brain uncorrected. B) Percent change of the blood oxygen level-dependent signal at −26, −6, 54 for the youths (black bar) and adults (gray bar). Error bars depict standard error of the mean.
Figure 5.

Overall activation for both adults and youths combined on: A) successful change vs. go; B) successful change vs. unsuccessful change C) successful change vs. baseline; and D) go vs. baseline. Data shown at p < 0.001, whole-brain uncorrected.
Table 4.
Significant between group differences for Adults vs. Youths
| Region | ~ BA | # Voxels | x | y | z | t | |
|---|---|---|---|---|---|---|---|
| Sucessful change vs. Sucessful Go | L Precentral Gyrus | 6 | 179 | −26 | −12 | 50 | 4.51 |
| L Precuneus | 7 | 210 | −4 | −67 | 41 | 4.49 | |
| R Inferior Parietal Lobule | 40 | 159 | 33 | −39 | 52 | 4.13 | |
| R Superior Parietal Lobule | 7 | 35 | 26 | −61 | 49 | 3.92 | |
| R Paracentral Lobule | 7 | 44 | 4 | −38 | 44 | 3.79 | |
| R Precentral Gyrus | 19 | 43 | −17 | 29 | 3.58 | ||
| L Superior Parietal Lobule | 7 | 13 | −24 | −62 | 48 | 3.55* | |
| Sucessful change vs. Baseline | R Inferior Parietal Lobule | 40 | 908 | 33 | −42 | 51 | 4.28 |
| L Precuneus | 7 | 602 | −2 | −67 | 41 | 4.2 | |
| L Precentral Gyrus | 6 | 602 | −26 | −12 | 50 | 4.57 | |
| R Superior Parietal Lobule | 7 | 229 | 28 | −63 | 53 | 4.44 | |
| R Inferior Frontal Gyrus | 9 | 159 | 43 | 9 | 26 | 3.87 | |
| L Precentral Gyrus | 6 | 89 | −42 | 2 | 29 | 3.62 | |
| L Fastigial Nucleus | 86 | −8 | −48 | −26 | 3.67 | ||
| Successful go vs. Baseline | R Inferior Parietal Lobule | 40 | 1320 | 35 | −39 | 35 | 5.42 |
| L Middle Frontal Gyrus | 6 | 532 | −24 | −12 | 51 | 4.95 | |
| R Lentiform Nucleus | 64 | 30 | −6 | 10 | 4.09 | ||
| R Middle Frontal Gyrus | 6 | 95 | 22 | −12 | 49 | 3.9 | |
| R Precuneus | 7 | 28 | 20 | −59 | 48 | 3.62 | |
| R Cingulate Gyrus | 24 | 10 | 13 | −7 | 42 | 3.61 | |
| R Paracentral Lobule | 5 | 11 | 17 | −39 | 59 | 3.58 | |
| L Superior Parietal Lobule | 7 | 13 | −30 | −55 | 47 | 3.54* | |
| L Superior Parietal Lobule | 7 | 15 | −20 | −61 | 52 | 3.53* | |
BA = Brodmann Area
p values are all <.0001, except where noted by an * p<0.001
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health. The authors have no conflicts to disclose.
References
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progressive Brain Research. 1990;85:119–146. [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27(14):3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6(2):115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26(9):2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Von Cramon DY. The role of the frontal cortex in task preparation. Cereb Cortex. 2002;12(9):908–914. doi: 10.1093/cercor/12.9.908. [DOI] [PubMed] [Google Scholar]
- Brass M, Von Cramon DY. Selection for cognitive control: a functional magnetic resonance imaging study on the selection of task-relevant information. J Neurosci. 2004;24(40):8847–8852. doi: 10.1523/JNEUROSCI.2513-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA. How we use rules to select actions: A review of evidence from cognitive neuroscience. Cognitive, Affective & Behavioral Neuroscience. 2004;4(4):564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol. 2003;90(5):3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol. 2007;17(2):243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(3):374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Davidson MC, Hara Y, Thomas KM, Martinez A, Galvan A, et al. Early development of subcortical regions involved in non-cued attention switching. Dev Sci. 2004;7(5):534–542. doi: 10.1111/j.1467-7687.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, et al. The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Dev Psychobiol. 1997;30(1):61–69. [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JC. Changes in executive control across the life span: examination of task-switching performance. Dev Psychol. 2001;37(5):715–730. [PubMed] [Google Scholar]
- Cohen S, Bixenman M, Meiran N, Diamond A. Task switching in children. Paper presented at the South Carolina Bicentennial Symposium on Attention.2001. [Google Scholar]
- Cone NE, Burman DD, Bitan T, Bolger DJ, Booth JR. Developmental changes in brain regions involved in phonological and orthographic processing during spoken language processing. Neuroimage. 2008;41(2):623–635. doi: 10.1016/j.neuroimage.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24(5):1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Bunge SA, van der Molen MW, Ridderinkhof KR. Switching between tasks and responses: a developmental study. Dev Sci. 2006;9(3):278–287. doi: 10.1111/j.1467-7687.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- Crone EA, Donohue SE, Honomichl R, Wendelken C, Bunge SA. Brain regions mediating flexible rule use during development. J Neurosci. 2006;26(43):11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Ridderinkhof KR, Worm M, Somsen RJ, van der Molen MW. Switching between spatial stimulus-response mappings: a developmental study of cognitive flexibility. Dev Sci. 2004;7(4):443–455. doi: 10.1111/j.1467-7687.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16(4):475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, van Leijenhorst L, Honomichl RD, Christoff K, Bunge SA. Neurocognitive development of relational reasoning. Dev Sci. 2009;12(1):55–66. doi: 10.1111/j.1467-7687.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Zanolie K, Van Leijenhorst L, Westenberg PM, Rombouts SA. Neural mechanisms supporting flexible performance adjustment during development. Cogn Affect Behav Neurosci. 2008;8(2):165–177. doi: 10.3758/cabn.8.2.165. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44(11):2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In: Stuss D, Knight R, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002. pp. 466–503. [Google Scholar]
- Diamond A. The Early Development of Executive Functions. In: Bialystok E, Craik FIM, editors. Lifespan Cognition: Mechanisms of Change. Oxford: Oxford University Press; 2006. pp. 70–95. [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, et al. General and task-specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task-switching. Neuroreport. 2001;12(9):2065–2071. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- Dollfus S, Razafimandimby A, Maiza O, Lebain P, Brazo P, Beaucousin V, et al. Functional deficit in the medial prefrontal cortex during a language comprehension task in patients with schizophrenia. Schizophrenia Research. 2007;99(1–3):304–311. doi: 10.1016/j.schres.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res. 2000;9(1):103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Berman KF. Fractionating the neural substrate of cognitive control processes. Proc Natl Acad Sci U S A. 2002;99(22):14595–14600. doi: 10.1073/pnas.222193299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Biggon J, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington D.C: American Psychiatric Press, Inc; 1996. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7(1):30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijendbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia. 2006;44(11):2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Kanemura H, Aihara M, Aoki S, Araki T, Nakazawa S. Development of the prefrontal lobe in infants and children: a three-dimensional magnetic resonance volumetric study. Brain Dev. 2003;25(3):195–199. doi: 10.1016/s0387-7604(02)00214-0. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-Sads-Pl. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R, et al. Functional anatomy of GO/NO-GO discrimination and response selection--a PET study in man. Brain Res. 1996;728(1):79–89. [PubMed] [Google Scholar]
- Konishi S, Hayashi T, Uchida I, Kikyo H, Takahashi E, Miyashita Y. Hemispheric asymmetry in human lateral prefrontal cortex during cognitive set shifting. Proc Natl Acad Sci U S A. 2002;99(11):7803–7808. doi: 10.1073/pnas.122644899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Kawazu M, Uchida I, Kikyo H, Asakura I, Miyashita Y. Contribution of working memory to transient activation in human inferior prefrontal cortex during performance of the Wisconsin Card Sorting Test. Cereb Cortex. 1999;9(7):745–753. doi: 10.1093/cercor/9.7.745. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci. 1998;10(3):1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit complex movements: a stop-signal study of typewriting. J Exp Psychol Hum Percept Perform. 1982;8:778–792. [Google Scholar]
- Logan GD. On the ability to inhibit simple thoughts and actions: I. Stop-signal studies of decision and memory. J Exp Psychol Hum Percept Perform. 1983;9:585–606. [Google Scholar]
- Logan GD. Spatial attention and the apprehension of spatial relations. J Exp Psyhol Hum Percept Perform. 1994;20(5):1015–1036. doi: 10.1037//0096-1523.20.5.1015. [DOI] [PubMed] [Google Scholar]
- Logan GD, Burkell J. Dependence and independence in responding to double stimulation: A comparison of stop, change, and dual-task paradigms. Journal of Experimental Psychology: Human Perception and Performance. 1986;12(4):549–563. [Google Scholar]
- McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, et al. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry. 2005;162(9):1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. Arch Neurol. 1963:90–100. [Google Scholar]
- Nelson EE, Vinton DT, Berghorst L, Towbin KE, Hommer RE, Dickstein DP, et al. Brain systems underlying response flexibility in healthy and bipolar adolescents: an event-related fMRI study. Bipolar Disord. 2007;9(8):810–819. doi: 10.1111/j.1399-5618.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2(6):417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Payne JW, Bettman JR, Johnson EJ. The adaptive decision maker. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119(Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Band GPH, Logan GD. A study of adaptive behavior: Effects of age and irrelevant information on the ability to inhibit one’s actions. Apr 1999. Acta Psychologica. 1999;101(2–3) [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28(11):1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Taylor E, Smith AB, Oksanen H, Overmeyer S, Newman S. Neuropsychological analyses of impulsiveness in childhood hyperactivity. Br J Psychiatry. 2001;179:138–143. doi: 10.1192/bjp.179.2.138. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002;87(5):2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Paus T, Silipa PK. Attention systems and the organization of the human parietal cortex. J Neurosci. 2001;21(14):5262–5271. doi: 10.1523/JNEUROSCI.21-14-05262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Rubia K. Neural correlates of switching set as measured in fast, event-related functional magnetic resonance imaging. Hum Brain Mapp. 2004;21(4):247–256. doi: 10.1002/hbm.20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits-Englesman BCM, Sugden D, Duysens J. Developmental trends in speed accuracy trade-off in 6–10-year-old children performing rapid reciprocal and discrete aiming movements. Human movement science. 2006;25(1):37–49. doi: 10.1016/j.humov.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. Inaugural article: the role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci U S A. 2000;97(24):13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, et al. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41(3):357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brian. New York: Thieme; 1988. [Google Scholar]
- van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44(11):2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cogn Sci. 2008;12(11):418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22(4):1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- Welsh MC, Pennington BF, Groisser DB. A normative-developmental study of executive function: A window of prefrontal function in children. Developmental Neuropsychology. 1991;7(2):131–149. [Google Scholar]
- Zanolie K, Van Leijenhorst L, Rombouts SA, Crone EA. Separable neural mechanisms contribute to feedback processing in a rule-learning task. Neuropsychologia. 2008;46(1):117–126. doi: 10.1016/j.neuropsychologia.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Slifstein M. A reference effect approach for power analysis in fMRI. Neuroimage. 2001;14(3):768–779. doi: 10.1006/nimg.2001.0852. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Muller U. Executive function in typical and atypical development. In: Goswami U, editor. Handbook of childhood cognitive development. 445–469. Oxford: Blackwell; 2002. [Google Scholar]