Abstract
Background
Menstrual cups have been available for decades, but their use is limited by bulky design and the need for multiple sizes. The Softcup® (Instead, Inc., San Diego, CA) is a simple single-size disposable over-the-counter (OTC) menstrual cup that compresses to tampon shape to facilitate insertion and can be worn during coitus. This report describes preclinical evaluation, clinical testing, and postmarketing monitoring of the Softcup.
Methods
Preclinical testing complied with U.S. Food and Drug Administration (FDA) guidelines and used standard United States Pharmacopoeia methodologies for assessment of potential toxicity. Clinical testing enrolled 406 women in seven U.S. centers. A detailed written questionnaire assessed safety, acceptability, and effectiveness for menstrual collection. Study safety parameters included pelvic examinations, Pap smears, colposcopy, urinalysis, vaginal pH, wet mounts, gram stain, and vaginal microflora cultures. Postmarketing surveillance of over 100 million Softcups has been conducted by the manufacturer and by the FDA Medwatch system.
Results
No toxicity or mutagenicity was observed in preclinical evaluations. In clinical testing, after three cycles of cup use, 37% of subjects rated the cup as better than, 29% as worse than, and 34% as equal to pads or tampons. The cup was preferred for comfort, dryness, and less odor. Cups received lower ratings for disposal and convenience. Eighty-one percent of enrolled women were able to insert and remove their first cup using only written instructions. Use difficulties resulting in study discontinuations included cramping (1%), leakage (1%), and improper fit (3%). No safety parameters were adversely affected. No significant health risks were reported during postmarketing surveillance.
Conclusions
These results demonstrate that a single-size vaginal device has no significant health risks and is acceptable to many women without the need for fitting or other medical services.
Introduction
Vaginal devices have a long and intriguing history. In the United States, the first device was patented in 1867.1 These devices have been intended for menstrual collection, contraception, uterine/pelvic support, drug delivery, and as a conception aid for retention of semen over the cervix. In the developing world, menstrual management is not only difficult; it also may have serious adverse effects on the lives of women. The taboos associated with menstruation are not just of historical interest. It is still common for menstruating women to be confined at home or even in menstrual huts or to be considered unclean and shunned.2 Even when taboos are not a major problem, convenient or inexpensive menstrual collection materials may simply not be available. Efforts to produce inexpensive materials are sorely lacking.3 In many cultures, women resort to the use of rags, which must be reused, but washing them may be limited by lack of water or the privacy needed to wash and reuse pads, resulting in forced use of damp or even wet contaminated rags.2–4
These restrictions reduce quality of life in general and can have measurable consequences, particularly for women's education. Not only do schoolage adolescent girls lack convenient menstrual supplies, but also their school experience is strongly tainted by lack of adequate school sanitary facilities and by overt sexual harassment (euphemistically labeled “gender insensitivity”). As a consequence, some girls may simply avoid school altogether during menses.5 Fortunately, these issues are slowly being recognized by multiple agencies and nongovernmental agencies (NGOs) worldwide, and solutions are beginning to emerge.3
In developed countries, menstrual management is still a source of social embarrassment, with such euphemisms as “time of the month,” “period,” or “on the rag” still in common use. Fortunately, disposable menstrual products are now widely available in the developed world. In the United States, the first disposable pads (“Lister's Towels—for Ladies Use”) were manufactured around 1896, but their use was limited by the societal constraints that prevented significant advertising. Kotex pads, introduced in the 1920s, finally crept through advertising barriers to make disposable pads become widely available. Tampons followed in 1933.4 Even today, advertisers are still reluctant to talk directly about menstruation; they continue to discuss “feminine hygiene” and urge women to “stay fresh.” This persistent discomfort with the reality of menstruation—aptly called the “culture of concealment” by Karen Houppert6—has allowed the menstrual products industry to bypass careful premarketing evaluation of the health risks of menstrual products.6 In the United States, safety requirements have also been lax because the preponderance of these devices predates the medical device regulatory amendments, which became effective in 1976. A 2003 publication claims to be the first published study to combine “gynecological, microbiological, and diary recall methodologies to assess in more detail safety-related endpoints associated with tampon use.”7
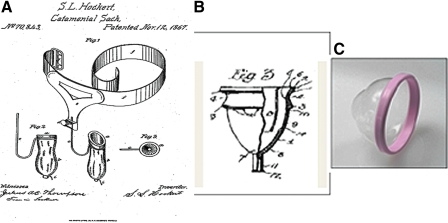
Early vaginal cups used specifically for menstrual collection have never achieved widespread use, even though they have been available in developed countries for many decades. In the United States, the first menstrual cup was the Hockert Catamenial Sack. It was patented in 1867 but was obviously not commercially viable (Fig. 1A).1 The first usable commercial cup (Fig. 1B) was patented by Leona Chalmers in 1937.8 This bell-shaped prototype has been used with little variation in more than 12 brands of reusable menstrual cups available today. Popular use of these cups may be limited by several factors. Insertion can be difficult. These cups do not collapse or compress easily; for insertion, the cup must be tightly held in a folded position, but even folded it remains bulky.9 Once inserted, it may still need to be oriented properly in the vaginal canal.
FIG. 1.
(A) Drawing of the Hockert Catamenial Sack from patent 70,865 in 1867. (B) Drawing of first commercial menstrual cup by Leona Chalmers from patent 2,089,113 in 1937. (C) Photograph of the Instead Softcup® (Instead, Inc., San Diego, CA).
Another cup design, the Gynaeseal, was marketed in 1989 but was not commercially successful. It was designed as a diaphragm tampon, had a bulky complex dual chamber design, and required an applicator for insertion. The major complaint from Gynaeseal users was the “messy” contact with menstrual fluid during removal.10 Many women are unwilling to perform the intravaginal positioning needed because they find it unpleasant to touch their vaginal tissues or find it distasteful to handle menstrual fluid during device removal and washing.11,12 Finally, concerns about “virginity” may limit use among inexperienced women.2,12,13
There are relatively few study reports that evaluate safety and acceptability of cups with actual use during menses. In 1962, Karnaky14 evaluated 50 women using a bell-shaped cup. He obtained vaginal smears, gram stains, and basic aerobic cultures of vaginal secretions. Vaginal speculum examination was performed, and pH was measured. No significant changes were noted.
This report is the first containing extensive information on the safety and acceptability of a widely used menstrual cup that includes both preclinical and clinical testing and over 10 years of postmarketing surveillance.
Device description
The Softcup® (Instead, Inc., San Diego, CA) is an internally worn device with a pliable rim 70 mm in diameter and a thin-walled reservoir to collect and hold the menstrual fluid (Fig. 1C). It was designed to minimize bulk in order to facilitate insertion and removal. The compression strength of the rim (450 ± 50 g) is less than that of most other vaginal cups, caps, and diaphragms, which allows the user to squeeze the rim into a cylindrical tampon shape to facilitate insertion. Once inserted, it opens to an oval shape, positioned between the posterior fornix and the notch behind the pubic bone, covering the cervix. Because the Softcup is aligned along the long axis of the vagina, intercourse can take place below the cup, avoiding contact with the rim and minimizing risk of displacement. Removal is accomplished by hooking a finger over the rim behind the pubic bone. It is composed of a proprietary blend of soft biocompatible polymer compounds in conformance with the United States Pharmacopoeia XIX Class VI criteria for plastics. The Softcup was first marketed in the United States as an approved 510K device in 1996.
Materials and Methods
Preclinical toxicity and microbiological testing
The preclinical toxicity testing program assessed what effects, if any, might occur from exposure of vaginal tissues to the proprietary blend of polymer materials used in the Softcup. The selected tests (Table 1) complied with U.S. FDA guidelines15 and used standard United States Pharmacopoeia methodologies for extraction of polymer compounds using aqueous and nonpolar extraction vehicles in the assessment of potential toxicity.16 None of the tests performed showed any irritation, mutagenicity, or toxicity (Table 1).
Table 1.
Summary of Preclinical Biocompatibility Testing Performed on the Softcup® Material
| Toxicology | |
| 1. USP toxicity assaysa | |
| Acute systemic injection with both saline and cottonseed oil as vehicles—mice | No evidence of systemic toxicity |
| Intracutaneous reactivity—rabbit | No evidence of irritation or toxicity |
| Intramuscular implant with pathology—rabbit | No significant tissue reaction |
| Pyrogen test—rabbit | No pyrogen detected |
| 2. Cellular toxicityb | |
| Cytotoxicity—MRC-5 human embryonic lung cells | No toxicity or cell lysis |
| Hemolysis of red blood cells | No hemolysis detected |
| 3. Dermal toxicityb | |
| Vaginal irritation with histopathology—rabbit | Not an irritant to vaginal mucosal tissue |
| Delayed contact sensitization—guinea pig | No evidence of delayed dermal sensitization and no visible reaction |
| 4. Ames Salmonella/Microsome Plate Testb | Not mutagenic |
| Microbiologyb | |
| 1. Effect on vaginal flora—20 strains | Saline and alcohol extracts showed no stimulation or inhibition of growth |
| 2. Effect on TSS toxin production of Staphylococcus aureus | None |
United States Pharmacopeia, Classification of Plastics (Class VI) testing, USP XXII, 1990:1497–1500.
Testing performed in accordance with the FDA, Tripartite Biocompatibility Guidance for Medical Devices, September 1986.
TSS, toxic shock syndrome.
Premarket human clinical testing
A multicenter clinical study was conducted to establish the safety, effectiveness, and acceptability of this device. Four hundred six women were enrolled. The study was conducted in seven U.S. centers that encompassed a mix of private practices, Planned Parenthood centers, and a university health service (Table 2). Each center used its own IRB and obtained written informed consent on their respective consent forms. Study participants between the ages of 18 and 55 with regular menstrual cycles (either from normal menstruation or from use of cyclical hormone therapy) and in general good health were enrolled. Potential subjects were recruited from each center's patient population via posters, leaflets, and word of mouth. Participants were compensated ($50 per monthly visit for a maximum of seven visits) and received study-related gynecological examinations and testing at no charge. Participants also received reminder telephone calls from clinical coordinators before monthly visits. Most women in the study were between 20 and 40 years of age and nulligravid (Table 3). Because all study participants were English speaking, written study materials were English language only. On admission, participants completed a detailed written questionnaire about menstrual history and practices (Appendix A). In a practice session, each woman was given a written instruction sheet (Appendix B), then asked to insert and remove a cup without further assistance. Correct or incorrect positioning of the cup was then assessed by pelvic examination. Subjects were asked to use the cup for at least three menstrual cycles and to return to the clinic between menses for examination, testing, and additional questionnaires (Appendix C) to assess acceptability and ease of use and to compare their cup experience with their current menstrual protection method. They were also asked to report the experience of the male sexual partner if the cup was worn during intercourse. (Male partners received no compensation.) Vulvovaginal health was monitored via physical examination, Pap smear, urinalysis, vaginal pH, whiff test of vaginal secretion for amine odor, and microscopic examination (wet mounts).
Table 2.
Summary of Study Centers and Evaluations Performed
| |
|
|
|
Type of evaluation |
||
|---|---|---|---|---|---|---|
| Center No. | Location | Type of center | Number of subjects enrolled | Clinical | Colposcopy | Vaginal flora |
| 1 | Laguna Beach, CA | Private practice | 96 | X | Xa | Xa |
| 2 | Missoula, MT | Private practice | 75 | X | ||
| 3 | Seattle, WA | Planned Parenthood | 50 | X | Xb | Xb |
| 4 | Seattle, WA | Private practice | 24 | X | ||
| 5 | Corvallis, OR | University health center | 75 | X | ||
| 6 | San Diego and Riverside, CA | Planned Parenthood | 49 | X | ||
| 7 | San Juan, Puerto Rico | Private practice | 37 | X | ||
n = 24.
n = 20.
Table 3.
Demographics of the 406 Enrolled Subjects
| |
Center number |
|
||||||
|---|---|---|---|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Total |
| Race | b | d | e | |||||
| White | 91 | 68 | 42 | 23 | 70 | 42 | 26 | 362 |
| Black | 1 | 0 | 3 | 0 | 0 | 4 | 7 | 15 |
| Asian | 4 | 0 | 3 | 1 | 2 | 0 | 0 | 10 |
| Other | 0 | 0 | 2 | 0 | 3 | 2 | 0 | 7 |
| Age | b | f | ||||||
| 18–29 | 32 | 18 | 35 | 6 | 70 | 31 | 10 | 202 |
| 30–39 | 39 | 41 | 10 | 12 | 4 | 12 | 12 | 130 |
| 40–55 | 25 | 9 | 5 | 6 | 1 | 6 | 12 | 64 |
| Marital status | b | f | ||||||
| Single | 34 | 8 | 37 | 12 | 66 | 31 | 7 | 195 |
| Married | 36 | 52 | 12 | 9 | 5 | 11 | 23 | 148 |
| Divorced/widowed | 26 | 8 | 1 | 3 | 4 | 7 | 4 | 53 |
| Pregnancies | b | a | ||||||
| 0 | 53 | 18 | 43 | 19 | 74 | 38 | 16 | 261 |
| 1–2 | 38 | 35 | 7 | 4 | 0 | 10 | 8 | 102 |
| ≥3 | 5 | 15 | 0 | 1 | 1 | 1 | 10 | 33 |
| Sexually active | b | f | ||||||
| No | 8 | 3 | 16 | 2 | 13 | 10 | 1 | 53 |
| Rarely | 14 | 6 | 9 | 4 | 15 | 7 | 3 | 58 |
| Frequently | 74 | 59 | 25 | 18 | 47 | 32 | 30 | 285 |
| Oral contraceptives | b | f | ||||||
| No | 74 | 48 | 27 | 16 | 36 | 18 | 27 | 246 |
| Yes | 22 | 20 | 23 | 8 | 39 | 31 | 7 | 150 |
| Household Income ($1000) | a | b | c | e,g | ||||
| <20 | 20 | 12 | 31 | 8 | 28 | 25 | 18 | 142 |
| 20–30 | 21 | 11 | 6 | 3 | 9 | 9 | 3 | 62 |
| 30–49 | 28 | 29 | 8 | 9 | 13 | 11 | 6 | 104 |
| 50–69 | 9 | 13 | 2 | 3 | 12 | 2 | 2 | 43 |
| ≥70 | 15 | 3 | 3 | 1 | 11 | 2 | 3 | 38 |
Three subjects did not provide an answer.
Seven patient case report forms were lost.
Two subjects did not provide an answer.
One patient case report form was lost.
Four patient case report forms were lost.
Three patient case report forms were lost.
One subject did not provide an answer.
Two of the centers (Table 2) were asked to conduct more detailed evaluations of cup use in a subset of their study participants who volunteered for additional evaluation. Colposcopic evaluation of the vaginal vault and cervix and analysis of vaginal flora were performed on this subset of 44 women. These additional tests were performed at entry into the study (before use of the cup) and at monthly intervals up to 6 months. Indicators of cervical neoplasia, mechanical tissue injury, and infection were monitored, including cervical and vaginal topography, inflammation, abrasion, acetowhite changes, punctuation, mosaicism, abnormal vessels, and the transition zone. A single physician evaluated all colposcopy data at each of two centers.
Vaginal flora was examined in the same subset after 1, 2, and 3 months of cup use. Women were then followed for up to 3 additional months after returning to their normal method of menstrual protection (pads or tampons). Vaginal flora (Staphylococcus aureus, group B Streptococcus, Enterococcus, Escherichia coli, Candida spp., Gardnerella vaginalis, Bacteroides spp., Lactobacillus) were collected using (1) vaginal swabs placed in transport media and with (2) air-dried vaginal smears. Both specimens were transported within 24 hours to the Research Microbiology Laboratory at the University of Washington, Seattle. Sharon Hillier, Ph.D., conducted the vaginal microbiology assessment.
Results
At the time they entered the study, 31% (125) of women regularly used tampons for menstrual protection, 18% (73) used pads, and 51% (208) used both. Only 31% (125) were “extremely satisfied” with their current method of sanitary protection. They were particularly dissatisfied with the performance of their current method during overnight wear (45%, 183), when exercising (23%, 93) and swimming (14%, 57), in certain clothes (14%, 57), and when busy at work (21%, 85). Subjects' menstrual periods averaged 5 days in length. They reported using an average of 11 pads or 16 tampons per cycle. Most women had prior experience with some type of intravaginal device, primarily with the Today® contraceptive sponge (26%, 106) or the contraceptive diaphragm (32%, 130). Only 9 women had previously used any kind of menstrual cup.
During the trial, a total of 13,963 cups were used in 4,750 days of menstruation. Women used an average of 13 cups in the first cycle, 14 in the second, and 13 in the third cycle. Of the 405 subjects who participated in the practice session using only written instructions, 328 (81%) required only 1 cup, 40 (9.8%) required 2 cups, 13 (3.2%) required 3 cups, 2 (0.5%) required more than 3 cups, and 4 (1.0%) could not insert the cup even after repeated attempts. Twenty (4.9%) women reported difficulty with removal during the practice session. This distribution was consistent among all seven centers. Five (1.2%) women did not indicate how many cups were required for correct placement, 12 (3.0%) women lost practice session forms, and 1 woman was discontinued because of Pap test results.
Ease of insertion, removal, wearing comfort, and “messiness” of the cup were evaluated by subjects after each menstrual period. The cup received an average score of 8 (standard deviation [SD] 1) for comfort, ease of insertion, and removal, on a scale of 1 (worst) to 10 (best). The cup was used during intercourse by 67 subjects during their first menstrual cycle, 61 subjects during their second menstrual cycle, and 58 during their third menstrual cycle. In this group, 9 women reported discomfort while wearing the cup; thirteen women reported that their male partner had experienced some discomfort (one man described discomfort during two different cycles). The remaining women stated that their partner either was unable to detect its presence or was aware of its presence but did not find it uncomfortable.
“Messiness” was a greater concern, with a score of around 5 (SD 1). About half of the study participants reported no (or rare) leakage during the use of the cup. Another third reported occasional leakage. Those who did experience leakage rated it as equivalent to leakage with their prior method. There was no change in the use of a backup method as women gained experience with the cup over three cycles of use. Slightly over half the subjects (56%) continued to use a backup method after three cycles of use, compared to 58% who used both pads and tampon at baseline. Significant cramping (greater than normally experienced during menses) was reported by 4 women. Pain resolved in all cases within a few hours of cup removal. Nine women experienced mild or transient cramping, which in many cases they considered normal for them.
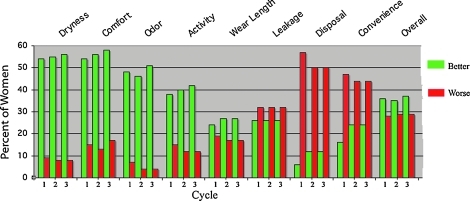
Women preferred the cup to their usual method of sanitary protection in comfort, dryness/irritation, odor, length of wear, and interference with various activities. In addition, they were more satisfied with cup performance on light flow days. The cup received a lower rating than their current method in convenience and disposal; leakage was rated as slightly lower. However, women preferred the cup overall (Fig. 2).
FIG. 2.
Cup performance vs. other methods after each cycle of use, with percent of women rating cup better or worse than current method; the rest of the women rated it the same.
Monthly monitoring of gynecological health via urinalysis, pelvic examination with visual evaluation of tissues, vaginal pH, and microscopic wet mount showed no adverse effects of cup use (Table 4). The Softcup caused no alteration or disruption in vaginal or cervical epithelium, as assessed by colposcopy and cervical cytology (Table 5).
Table 4.
Vulvovaginal Evaluation Through Three Cycles of Cup Use
| Parameter evaluated | Baseline (n = 406) | First cycle (n = 368) | Second cycle (n = 329) | Third cycle (n = 308) |
|---|---|---|---|---|
| Vulva | ||||
| Normal | 389a | 357b | 320b | 300b |
| Abnormalc | 4 | 8 | 6 | 5 |
| Mean pHd | 4.6 | 4.6 | 4.6 | 4.5 |
| Cervix | ||||
| Normal | 367a,e | 344b,f | 320g | 296b,g |
| Ectopy | 16 | 9 | 4 | 4 |
| Friable | 6 | 0 | 0 | 0 |
| Abnormal Pap testh | 1 | 1 | 2 | 0 |
| Wet mount | ||||
| Candida | 6 | 6 | 3 | 6 |
| Clue cells | 6 | 6 | 5 | 4 |
| Trichomonas | 0 | 0 | 0 | 0 |
13 sets of missing forms.
Left blank on forms for 3 subjects.
At baseline, 1 subject had folliculitis, 1 subject had a condyloma, and the other 2 were edematous/erythematous. At the first cycle, 1 subject had a nodule, 6 were edematous/erythematous, and 1 was not specified. At the second cycle, 1 subject had a mild abrasion, 1 had herpes, and 4 were edematous/erythematous. At the third cycle, 1 subject had a cyst, and 4 were edematous/erythematous.
Some values were reported as ranges corresponding to the ranges of the pH paper used to measure vaginal pH. Mean values include the high end of the range reported. For example a range of 4.4–4.7 is reported as 4.7.
Four subjects with missing data.
Left blank on forms for 12 subjects.
Left blank on forms for 5 subjects.
Pap test results were obtained after cervical examination; however, abnormal Pap test results were exclusion criteria at admission and a reason for discontinuation during the trial.
Table 5.
Frequency of Vaginal and Cervical Epithelium Conditions Throughout Study
| Condition of vaginal and cervical epithelium | Baselinea (n = 44) | 2–3 months (n = 37) | 5–6 months (n = 25) |
|---|---|---|---|
| Topography | Normal | Normal | Normal |
| Inflammation | None | None | None |
| Abrasion | 1 | 2 | None |
| Acetowhite | 7 warts | 7 warts | 6 warts |
| 6 | 2 | 0 | |
| Punctuation | No | 1 in wart | No |
| Mosaicism | 3—two rated faint and 2 mild | 2 | 1 |
| Abnormal vessels | None | None | None |
| Transition zone | 3 metaplasia | 5 metaplasia | 3 metaplasia |
Four women had significant abnormalities at baseline and were disqualified from the study and referred for treatment.
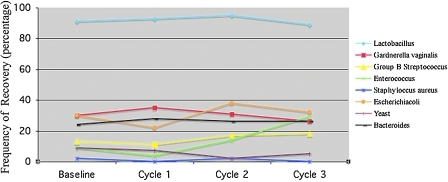
At baseline, 91% of the women harbored lactobacilli, G. vaginalis, group B Streptococcus, Enterococcus, S. aureus, E. coli, yeast, and Bacteroides spp. at frequencies considered normal and consistent with the selection of genital infection-free women. The frequencies of microorganisms recovered from vaginal flora before and after use of the Softcup (Fig. 3) were determined. Use of the cup over three successive menstrual cycles had no effect on vaginal colonization by S. aureus, the etiological agent of toxic shock syndrome (TSS). Similarly, use of the cup does not lead to increased colonization by microorganisms associated with bacterial vaginosis (G. vaginalis and Bacteroides spp.), vulvovaginitis (Candida and other yeast), or urinary tract infections (E. coli). There was a statistically significant increase in vaginal colonization by Enterococcus after 3 months of use, but this increased frequency persisted for 3 months after discontinuing use of the cup, suggesting that factors or behavior other than cup use may have influenced colonization. Finally, before, during, and after use of the cup, vaginal Lactobacillus (normal vaginal flora) was maintained at normal levels (Fig. 3). A separate safety study subsequently evaluated vaginal flora with Softcup wear times up to 24 hours. Again, no changes in normal flora were found (Appendix D).
FIG. 3.
Frequency of microorganism recovered after use of the Softcup. The only significant changes were an increase in Enterococcus from month 2 to 3 (p = 0.03) and a decrease in yeast from month 1 to 2 (p = 0.001) using Mantel Haenzel chi-square or Fisher's exact test.
The cup was used for a total of 1005 cycles by 368 women. Seventy-five percent (308) of the women admitted to the study completed at least three cycles of use. Another 5% (21) completed two cycles of use, and an additional 9.6% (39) completed one cycle of use (Table 4). Discontinuations occurred for product-related, medical, and study compliance reasons and termination of the study by the sponsor (Table 6). Of the 23 women discontinued for medical reasons, 11 were discontinued for conditions found at baseline screening. Eleven product-related discontinuations resulted from poor fit (subjects were unable to insert or retain the cup in the correct position in the vaginal vault). Fourteen (4%) women were lost to follow-up.
Table 6.
Summary of Total Discontinuations
| Category | Number discontinued |
|---|---|
| Product related | |
| Poor fit | 11 |
| Messy | 4 |
| Cramping | 4 |
| Difficulty removing | 2 |
| Didn't like product | 3 |
| Medical disqualification on/after admission | |
| Abnormal Pap results | 4 (1)a |
| Abnormal colposcopyb | 5 (5) |
| Subject placed on antibiotics for nonstudy-related condition | 2 (1) |
| Menses too irregular | 1 |
| Infection (Candida/bacterial vaginosis) | 11 (5) |
| Other | |
| Declined to continue | 1 |
| Lost to follow-up | 14 |
| Study closed by sponsor | 19 |
| Moved out of town | 1 |
| Complete sets of case report forms lost | 10 |
| Couldn't schedule visits | 6 |
The numbers in parentheses indicate women discontinued at admission for medical reasons.
One subject had a cyst, one subject had a cervical polyp, and the other three abnormalities were not specified.
Postmarketing surveillance
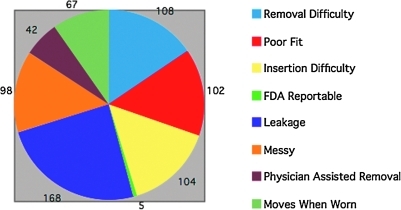
Active postmarketing surveillance within the United States has been conducted by the Softcup manufacturer using a toll-free telephone number, product labeling, print advertising, and the company's website. In recent years, e-mail has been the most frequent form of consumer feedback. This postmarketing surveillance has been conducted in compliance with applicable FDA regulations. The U.S. FDA also collects independent postmarketing surveillance information through its Manufacturers and User Facility Device Experience Database (MAUDE) program. This database was searched for complaints about the Softcup. Since initial distribution in 1996, over 100 million Softcups have been sold, according to the manufacturer. The overall complaint rate reported to the manufacturer has been remarkably consistent on a yearly basis and has averaged 1 complaint for every 47,000 cups sold. The majority of these complaints deal with the messiness, difficult insertion, difficult removal (rarely requiring physician assistance), or leakage during use (Fig. 4). Seven complaints received by the manufacturer were considered potentially serious and were submitted to the U.S. FDA. These included difficulty in removal (2), alleged allergic reactions (2), vaginal infection (1), TSS concern (1), and a complaint that the cup wore through the vaginal wall, damaging an artery that required surgical repair. None of these company-submitted complaints could be confirmed on subsequent follow-up (requested medical records were withheld by the complainants). Two additional complaints were submitted directly to the FDA by consumers; the first involved difficulty in removal, and the second was an unconfirmed case of TSS. (Neither TSS complaint fulfilled standard diagnostic criteria or was confirmed by the Centers for Disease Control and Prevention.) Thus, between 1996 and July 31, 2009, a total of 9 complaints (7 from the manufacturer and 2 from consumers) have been submitted to the FDA and listed in their MAUDE database.
FIG. 4.
Total Softcup complaints reported to the company for 2003–2008.
Discussion
Preclinical and clinical testing of the Softcup detected no adverse health effects. The Softcup was acceptable to most users in relation to comfort, ease of use, and effectiveness in menstrual collection. Messiness during removal, disposal, and inconvenience were the primary complaints. These findings are consistent with menstrual cup acceptability surveys reported elsewhere.10,12,17 These concerns about messy removal and inconvenient disposal might be minimized by including an inexpensive plastic glove with the cup packaging. This could be worn during removal and then be reversed over the cup for hygienic disposal.
Leakage was also a common complaint. There was no decrease in the use of a backup method over three cycles of use. Slightly over half the subjects continued to use a backup method, but when queried, most participants thought the leakage was similar to that experienced with tampons or pads and could be avoided by changing the cup more frequently. It may be that as women gain more experience with use of a cup, they may become less dependent on a backup method. Overall, women preferred the cup to their current method of menstrual management.
The postmarketing complaints encompassed the use difficulties expected for any vaginal device and are likely to be underreported. In the case of tampons, for example, it has been estimated that the most common use problem—physician removal of retained tampons—may be 30–400-fold more common than what is reported to the FDA.18 Like tampons, the Softcup occasionally requires physician-assisted removal, but to date, this has not been linked to any serious health risks.
Clearly, a major barrier to menstrual cup acceptance is the requirement that the menstrual cup be manipulated into and out of the vaginal vault, necessitating contract with genital tissues and with menstrual fluid. Obviously, women who are uncomfortable with this aspect of menstrual cup use will not enroll in clinical testing, resulting in an underestimation of this deterrent to use. It is very difficult, even repugnant, for many women to touch their own genital tissues.19,20 Most women in the study had prior experience with a vaginal device. Thus, acceptability of the cup to women lacking this prior experience may be lower than that reported here. In addition, because women in this study were recruited from a narrow demographic group with respect to age, ethnicity, and nationality, these results should not be generalized to other groups.
In spite of the limitations of this study, there is growing evidence that a simple, well-designed, inexpensive menstrual cup could play an important role both in developed countries and in the developing world. Cited advantages include overall convenience, portability and easy storage, extended wear time, and greater freedom of movement.9,21 When reusable, menstrual cups are easy to clean and, therefore, more hygienic than cloth pads, and they require less water for cleansing.21 Internal placement of cups avoids the odor and discomfort of an external pad. Reusable menstrual cups have an economic advantage.3,11,12,22 A 1995 study of 52 Canadian women found a menstrual cup was acceptable to 45% after 2 to 12 cycles of use.16 A recent report describes acceptability of a menstrual cup among adolescent school girls in Nepal.21 This study reports rapid adoption of cup use, with 60% using cups by 6 months and continuing use for the length of the study. A study from Zimbabwe reported that all women surveyed (n = 43) would “definitely” try a menstrual cup, and 86% reported that using it would make a difference in their lives.23 A survey in the U.K. found that 36 of 69 women attending a menstrual disorder clinic would consider using a traditional bell-shaped cup after reading an information leaflet.12 Encouragement and assistance from healthcare providers could be expected to improve acceptance where such care is available. On a global basis, however, such services are simply not available to most women. In our study, a simple illustrated instruction sheet allowed 99% of study participants to insert the cup without assistance in the first practice session. Such instruction sheets can be tailored to the needs and concerns of diverse populations.
Thus, menstrual cups offer the advantages of simple design, low cost, ease of use, and reusability and may help women in many different social and cultural settings manage their menses more easily. We can hope that increasing global awareness of women's health issues and focused educational efforts will bring menstrual management out of the closet, so a “period” becomes no more than a simple inconvenience.
Cervical barrier devices like the Softcup have the potential to be multifunctional. The same device may provide menstrual collection, contraception, or sexually transmitted infection (STI) protection, deliver a vaginal medication, or function as a fertility aid by retaining semen close to the cervix. Use of vaginal devices in combination with a topical microbicide is the subject of current STI/HIV prevention research.22–24 The combination of a microbicide with a cervical barrier may offer several advantages: longer retention of a therapeutic agent, mechanical protection of cervical tissues, and less leakage from the vagina. Perhaps the most important aspect of these devices is that they are woman-controlled and can be used by women who cannot negotiate condom use with their sexual partners. Numerous clinical studies have suggested that cervical devices may be very acceptable to women of different ages and cultures.22,23,25–27 Thus, cervical barriers, combined with a topical microbicide could play an important future role in STI/HIV prevention, as well as vaginal drug delivery for a variety of indications.
Conclusions
The acceptability and safety of the Softcup used as a menstrual collection device have been demonstrated in both preclinical and clinical testing and further monitored by continuing postmarketing surveillance. This provides strong evidence that an intravaginal cervical barrier device can be successfully used by the majority of women without the need for multiple sizes, fitting, or other medical services.
Appendix A
Menstrual History and Practices Questionnaire. Available at http://softcup.com/download_public/1993_market_study/Appendix_A-Menstrual_History.pdf
Appendix B
Softcup use instructions. Available at http://softcup.com/download_public/1993_market_study/Appendix_B-Softcup_Use_Instructions.pdf
Appendix C
Overall cycle evaluation. Available at http://softcup.com/download_public/1993_market_study/Appendix_C-Overall_Cycle_Evaluation.pdf
Appendix D
Astudy of the in vivo evaluation of alterations in the vaginal microflora during use of Instead® for 8, 12, or 24 hours. Available at http://softcup.com/download_public/1993_market_study/Appendix_D-Extended_Use_Study_Softcup.pdf
Acknowledgments
We thank Richard Soderstrom, M.D., Seattle Washington; Nancy Tipton, M.D., Seattle Planned Parenthood; Sharon Hillier, Ph.D., University of Washington; Steven D. Smith, M.D., Missoula, MT.; Ana Rosa Marcial, M.D., University of Central Caribe, Puerto Rico; W. White, M.D., Riverside and San Diego California Planned Parenthood; and Pamela L. Ciesielski, M.D., University of Oregon Health Service, for their participation in this study. This study was funded by Ultrafem, Inc.
Disclosure Statement
B.B.N. was Medical Director for Ultrafem, Inc., the developer of the Softcup and Principal Investigator of the reported clinical trials. She is Medical Director of the successor firms Instead, LLC, and Evofem, Inc. M.J.O. was Vice President of Regulatory and Technical Affairs for Ultrafem and has been a consultant to the successor firms Instead, LLC, and Evofem, Inc.
References
- 1.Hockert S. Catamenial Sack. U.S. Patent and Trademark Office, 1867. Patient No. 70843.
- 2.Ten VTA. Menstrual hygiene: A neglected condition for the achievement of several millennium development goals. Europe External Policy Advisors. Oct, 2007.
- 3.Bharadwaj S. Patkar A. Menstrual hygiene and management in developing countries: Taking stock. Junction Social; India: 2004. [Google Scholar]
- 4.Delaney J. Lupton M. Toth E. The curse—A cultural history of menstruation. Urbana: University of Illinois Press; 1988. [Google Scholar]
- 5.Sommer M. Where the education system and women's bodies collide: The social and health impact of girls' experiences of menstruation and schooling in Tanzania. J Adolesc. 2010;33:521–529. doi: 10.1016/j.adolescence.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Houppert K. The curse—Confronting the last unmentionable taboo: Menstruation. New York: Farrar Straus and Giraux; 1999. [Google Scholar]
- 7.Shehin S. Jones MB. Hochwalt AE. Sarbaugh FC. Nunn S. Clinical safety-in-use study of a new tampon design. Infect Dis Obstet Gynecol. 2003;11:89–99. doi: 10.1080/10647440300025504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalmers L. Catamenial Appliance. U.S. Patent and Trademark Office, 1937. Patent no. 2089113.
- 9.Liswood R. Internal menstrual protection. Use of a safe and sanitary menstrual cup. Obstet Gynecol. 1959;13:539–543. [PubMed] [Google Scholar]
- 10.Gleeson N. Devitt M. Buggy F. Bonnar J. Menstrual blood loss measurement with Gynaeseal. Aus NZ J Obstet Gynaecol. 1993;33:79–80. doi: 10.1111/j.1479-828x.1993.tb02061.x. [DOI] [PubMed] [Google Scholar]
- 11.Wysocki S. New options in menstrual protection. A guide for nurse practitioners. Adv Nurse Pract. 1997;5:51–54. [PubMed] [Google Scholar]
- 12.Stewart K. Powell M. Greer R. An alternative to conventional sanitary protection: Would women use a menstrual cup? J Obstet Gynecol. 2009;29:49–52. doi: 10.1080/01443610802628841. [DOI] [PubMed] [Google Scholar]
- 13.Pena E. Menstrual protection. Advantages of the menstrual cup. Obstet Gynecol. 1962;19:684–714. [PubMed] [Google Scholar]
- 14.Karnaky K. Internal menstrual protection with the rubber menstrual cup. Obstet Gynecol. 1962;19:688–691. [PubMed] [Google Scholar]
- 15.U.S. FDA. Tripartite biocompatibility guidance for medical devices. Sep, 1986.
- 16.United States pharmacopeia. Classification of plastics (Class VI) testing, USP XXII. 1990:1497–1500. [Google Scholar]
- 17.Cheng M. Kung R. Hannah M. Wilansky D. Shime J. Menses cup evaluation study. Fertil Steril. 1995;64:661–663. [PubMed] [Google Scholar]
- 18.Bright R. Dwyer D. Retained menstrual tampons: Hazards and epidemiology. International Society of Technology Assessment in Health Care Meeting; Canmore, Alberta, Canada. 2003. [Google Scholar]
- 19.Hardy E. de Padua KS. Hebling E. Osis MJ. Zaneveld LJ. Women's preferences for vaginal antimicrobial contraceptives. V: Attitudes of Brazilian women to the insertion of vaginal products. Contraception. 2003;67:391–395. doi: 10.1016/s0010-7824(03)00026-x. [DOI] [PubMed] [Google Scholar]
- 20.Schooler D. Ward L. Merriwether A. Caruthers A. Cycles of shame: Menstrual shame, body shame, and sexual decision-making. J Sex Res. 2005;42:324–334. doi: 10.1080/00224490509552288. [DOI] [PubMed] [Google Scholar]
- 21.Oster EFT. Thornton RE. Menstruation and education in Nepal. National Bureau of Economic Research Working Paper Series. 2009:Vol.w14853. [Google Scholar]
- 22.van der Straten A. Sahin-Hodoglugil N. Mtetwa S, et al. Feasibility and acceptability of cervical barriers among vulnerable youth: A pilot study in Zimbabwe. 4th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; Sydney, Australia. 2007. [Google Scholar]
- 23.Averbach S. Sahin-Hodoglugil N. Musara P. Chipato T. van der Straten A. Duet® for menstrual protection: A feasibility study in Zimbabwe. Contraception. 2009;79:463–468. doi: 10.1016/j.contraception.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Padian N. van der Straten A. Ramjee G, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: A randomised controlled trial. Lancet. 2007;370:251–261. doi: 10.1016/S0140-6736(07)60950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane M. Arceo R. Sobrero AJ. Successful use of the diaphragm and jelly by a young population: Report of a clinical study. Fam Plan Perspect. 1976;8:81–86. [PubMed] [Google Scholar]
- 26.Maher J. Harvey SM. Bird ST. Stevens VJ. Beckman LJ. Acceptability of the vaginal diaphragm among current users. Perspect Sex Reprod Health. 2004;36:64–71. doi: 10.1363/psrh.36.64.04. [DOI] [PubMed] [Google Scholar]
- 27.Sharma A. Bukusi E. Posner S. Feldman D. Ngugi E. Cohen CR. Sex preparation and diaphragm acceptability in sex workers in Nairobi, Kenya. Sex Health. 2006;3:261–268. doi: 10.1071/sh06021. [DOI] [PubMed] [Google Scholar]