Abstract
GABAergic amacrine cell feedback to bipolar cells in retina has been described, activating both GABAA and GABAC receptors. We explored whether metabotropic GABAB receptors also participate in this feedback pathway. CGP55845, a potent GABAB receptor antagonist, was employed to determine the endogenous role of these receptors. Ganglion cell EPSCs and IPSCs were monitored to measure the output of bipolar and amacrine cells. Using the tiger salamander slice preparation, we found that GABAB receptor pathways regulate bipolar cell release directly and indirectly. In the direct pathway, the GABAB receptor antagonist reduces EPSC amplitude, indicating that GABAB receptors cause enhanced glutamate release from bipolar cells to one set of ganglion cells. In the indirect pathway, the GABAB receptor antagonist reduces EPSC amplitude in another set of ganglion cells. The indirect pathway is only evident when GABAA receptors are inhibited, and is blocked by a glycine receptor antagonist. Thus, this second feedback pathway involves direct glycine feedback to the bipolar cell and this glycinergic amacrine cell is suppressed by GABAergic amacrine cells, through both GABAA and GABAB but not GABAC receptors. Overall, GABAB receptors do contribute to feedback regulation of bipolar cell transmitter release. However, unlike the ionotropic GABA receptor pathways, the metabotropic GABA receptor pathways act to enhance bipolar cell transmitter release. Furthermore, there are three discrete subsets of bipolar cell output regulated by GABAB receptor feedback (direct, indirect and null), implying three distinct, non-overlapping bipolar cell to ganglion cell circuits.
Introduction
In retina, the bipolar cells relay information from photoreceptors to ganglion cells. Horizontal cells modulate the input to bipolar cells, controlling the spatial and temporal balance of centre and surround signals driving the bipolar cells. However, the control of the bipolar cell output synapse, mediated by amacrine cells, provides a more varied control of the signals that eventually reach the ganglion cells. Much of this control is mediated by GABAergic and glycinergic amacrine cells through feedback and feedforward synapses (Masland, 2001; Sanes & Zipursky, 2010). One approach to exploring the complexity of these synapses is to examine the receptor diversity and how each receptor subtype uniquely influences inhibition in the inner plexiform layer. Attention has focused on regulation by inhibitory ionotropic receptors, notably the GABAA and GABAC receptors and the four subtypes of glycine receptor (Grünert, 2000; Shields et al. 2000; Cui et al. 2003; Wassle et al. 2010).
The clearest distinction currently is between GABAA and GABAC receptors, based on localization, ligand sensitivity and kinetics. GABAC receptors are concentrated at bipolar cell synaptic terminals (Lukasiewicz & Werblin, 1994), although they can be found in horizontal, amacrine and ganglion cells (Feigenspan et al. 1993; Qian & Dowling, 1993; Albrecht & Darlison, 1995; Koulen et al. 1997). Their activation kinetics are relatively slow, as is their desensitization (Lukasiewicz et al. 2004). Thus, GABAC receptors are well suited for delayed, sustained inhibition of bipolar cell synaptic terminals and can serve as high pass filters of ganglion cell excitation. In contrast, GABAA receptors are fast activating and rapidly desensitizing, serving as low pass filters. The sustained inhibition produced by the GABAC receptor has also been postulated to extend the dynamic range of the bipolar cell synapse (Lukasiewicz & Shields, 1998; Du & Yang, 2000; Shields et al. 2000).
Immunocytochemical studies show that glycine receptors in the mammalian retina are located on bipolar, amacrine and ganglion cells (Greferath et al. 1994; Sassoepognetto et al. 1994; Grunert & Wassle, 1996; Haverkamp et al. 2004; Jusuf et al. 2005; Ivanova et al. 2006; Heinze et al. 2007). However, electrophysiological evidence of glycinergic regulation at bipolar cell axon terminals indicates it is more limited than GABAergic feedback (Maple & Wu, 1998).
The metabotropic GABAB receptors also regulate synaptic communication in the proximal retina. GABABRs can activate inward rectifying potassium channels on amacrine and ganglion cells (Slaughter & Bai, 1989; Kaupmann et al. 1998), and suppress voltage-activated calcium channels in goldfish retinal bipolar and ganglion cells (Bindokas & Ishida, 1991; Matthews et al. 1994). Metabotropic GABA receptor activation facilitates L-type and inhibits N-type calcium channels on retinal ganglion cells in salamander (Zhang et al. 1997a; Shen & Slaughter, 1999). Immunolabelling of GABAB receptors indicates that they were expressed presynaptically in amacrine and horizontal cells and postsynaptically between amacrine cells or between amacrine and ganglion cells (Koulen et al. 1998). However, this approach has not revealed GABAB receptors in bipolar cells, at least in the rodent retina (Koulen et al. 1998; Zhang et al. 1998).
In the current experiments, we examined the influence of endogenous GABAB receptor activation on bipolar cell glutamatergic output to ganglion cells. Interesting, we found two discrete pathways that both lead to an enhancement of glutamate release, perhaps countering the action of ionotropic GABA receptors at bipolar cell terminals.
Methods
Slice preparation
Larval tiger salamanders (Ambystoma tigrinum) were obtained from Kons Scientific (Germantown, WI, USA) or Charles Sullivan (Nashville, TN, USA) and were maintained in tanks at 4°C on a 12:12 h light–dark cycle. Experiments were performed on retina slices according to the methods described previously (Wu, 1987; Awatramani & Slaughter, 2001). All procedures were performed in accordance with The Journal of Physiology standards and advice (Drummond, 2009), the US Animal Welfare Act and the National Institutes of Health's Guide for the Care and Use of Laboratory Animals, and were approved by the University Animal Care Committee. Salamanders were dark adapted overnight before the day of the experiment. The enucleation and preparation of the retinal slice were carried out under dim red light. Briefly, salamanders were stunned, decapitated and pithed. The retina was removed from the eyecup, placed ganglion cell side up on 0.22μm filter paper (Millipore, Bedford, MA, USA), and subsequently sliced at ∼250 μm intervals using a tissue slicer (Stoelting, Wood Dale, IL, USA). The retinal slice was mounted in a Warner RC-26 chamber on a PM-1 magnetic platform (Warner Instruments, Hamden, CT, USA). The retinal slices were continually bathed with control Ringer solution containing (in mm): 111 NaCl, 2.5 KCl, 1.8 CaCl2, 1 MgCl2, 10 dextrose, and 5 Hepes buffered to pH 7.8. All electrophysiological experiments were performed under infrared light (850 nm filter).
Whole cell patch-clamp
Recordings were obtained using 5–7 MΩ patch pipettes pulled from borosilicate glass capillaries (World Precision Instruments, Sarasota, FL, USA) using a Sutter P-97 puller (Sutter Instrument Co., Novato, CA, USA). The recording pipettes were filled with internal solution containing (mm): 100 potassium gluconate, 5 NaCl, 2 MgCl2, 5 EGTA and 5 Hepes, and buffered to pH 7.4 with KOH. Ganglion cells were identified based on their presence in the ganglion cell layer and their large sodium currents, exceeding 1 nA.
Data were acquired using an EPC-9 amplifier and HEKA Patchmaster software (HEKA Instruments Inc., Bellmore, NY, USA). Drug solutions were delivered through a gravity perfusion system and the flow speed was tuned by a FR-50 flow control valve (Warner Instruments). Picrotoxin, strychnine and salts were purchased from Sigma-Aldrich Corp. (St Louis, MO, USA). All other chemicals were obtained from Tocris Bioscience (Ellisville, MO, USA).
For light response experiments, retinal neurons were stimulated by a full-field red light-emitting diode (LED, λmax= 660 nm) (Nygaard & Frumkes, 1982). The irradiance of this red LED was ∼0.7 μW cm−2 at 660 nm, measured by a RPS900-R wideband spectroradiometer (International Light, Peabody, MA, USA). The total irradiance from 630 nm to 690 nm was ∼1.7 μW cm−2, equivalent to ∼6 × 104 photons μm−2 s−1. This light stimulus preferentially stimulated cones (Yang & Wu, 1997). A 2 s light stimulus was presented every 33 s.
Data analysis
Figures were processed in Patchmaster (HEKA) and exported to Origin 8 software (OriginLab Corp., Northampton, MA, USA). Pooled data are expressed as means ± standard error of the mean. Student's paired t test was used to compare values before and after drug applications for a cell dataset. Differences were considered significant when P≤ 0.05.
Results
Category 1: endogenous GABABRs enhance bipolar cell output
Previous studies showed that exogenous application of baclofen, a selective GABABR agonist, enhanced the light response in amacrine and ganglion cells but not in bipolar or horizontal cells (Slaughter & Bai, 1989). To identify the effects of endogenous GABABR activation, a selective antagonist of GABAB receptors, 5 μm CGP55845 (CGP), was tested on light evoked responses in ganglion cells. The ganglions cells were held at −70 mV to isolate the ON and OFF EPSCs evoked by 2 s red light stimulation. The responses of ganglion cells were found to fall into three categories: (1) neurons in which CGP55845 suppressed light responses, (2) neurons in which CGP55845 did not have an effect, and (3) neurons in which CGP55845 only suppressed light responses if GABAA receptors were blocked. An example of the first category is shown in Fig. 1A, illustrating that 5 μm CGP55845 suppressed both ON and OFF EPSCs and this effect was also observed when ionotropic GABA and glycine receptors were blocked with a combination of 100 μm picrotoxin (PTX) and 10 μm strychnine (Stry) (Fig. 1B). In the presence of picrotoxin and strychnine, a light-evoked IPSC was not observed or was very small when holding ganglion cells at 0 mV. Furthermore, CGP55845 had little or no effect on this IPSC, indicating that the GABAB receptor antagonist directly affected the light-evoked EPSC. This effect of CGP55845 was observed in 8 out of 38 cells. In these neurons, CGP55845 produced a mean suppression of 42 ± 8% of the ON response and 37 ± 16% of the OFF response (Fig. 1C). When inhibitory ionotropic receptors were blocked, then CGP55845 produced an average suppression of 40 ± 18% in the ON and 34 ± 19% in the OFF responses (n= 5) (Fig. 1D). Under either condition there are no statistically significant differences between ON and OFF responses (P > 0.05). These results are consistent with other reports that the endogenous GABABR acts to enhance bipolar cell output.
Figure 1. In ganglion cells classified as category 1, a GABAB receptor antagonist reduced the light response.
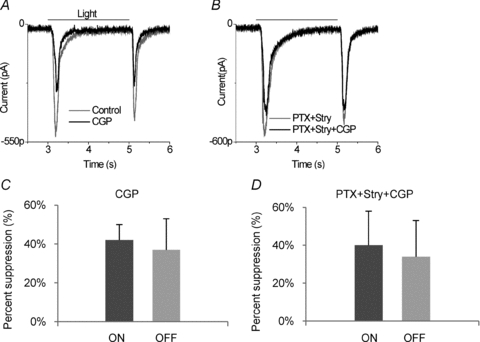
A, compared to control (grey trace), 5 μm CGP55845 (CGP) (black trace) suppressed both ON and OFF responses in a ganglion cell (n= 8). B, in the same cell, 100 μm picrotoxin (PTX) and 10 μm strychnine (Stry) prolonged and slightly enhanced the light responses (grey trace), and addition of 5 μm CGP55845 still suppressed both ON and OFF EPSCs (n= 5). Light-evoked EPSCs were recorded in a ganglion cell clamped at −70 mV and stimulated by 2 s red full field light. C, in the category 1 neurons, CGP55845 reduced ON and OFF EPSCs. There are no significant differences between suppression of ON and OFF responses. D, in the presence of PTX+Stry, CGP55845 still reduced both ON and OFF EPSCs.
Category 2: endogenous GABABRs do not regulate bipolar cell output
In the second category of cells, CGP55845 did not influence the light response of the neurons. As shown in Fig. 2Aa, 5 μm CGP55845 alone had little effect on the ON or OFF EPSCs. Picrotoxin and strychnine increased the amplitude of both the ON and OFF EPSCs, but CGP55845 had no apparent effect on the light-evoked EPSCs even when inhibitory ionotropic receptors were blocked (Fig. 2Ab and c). As will become relevant when comparing these neurons to the category 3 neurons, CGP55845 had negligible effects in the presence of picrotoxin. This ‘non-effect’ was observed in 18 of the 38 cells examined, where in the presence of 5 μm CGP55845 the mean ON response was 92 ± 8% of control amplitude and the OFF response was 94 ± 5% of control. We also examined the light-evoked IPSCs in this group of cells by clamping the neurons at 0 mV, close to the reversal potential of the glutamatergic EPSCs. The protocol was to first establish that the recorded neuron fitted into category 2 (Fig. 2Ba and b); then the holding voltage was switched from −70 mV to 0 mV, revealing ON and OFF IPSCs (Fig. 2Bc, black trace). Application of 5 μm CGP55845 did not alter the IPSCs. Then 100 μm picrotoxin was applied, suppressing the IPSCs (light grey trace) and leaving only a glycinergic IPSC (dark grey trace) that was unaltered by the addition of CGP55845 (black trace, Fig. 2Bd). The outward currents were eliminated by 10 μm strychnine (data not shown), demonstrating that these IPSCs were due to GABA and glycine ionotropic receptors.
Figure 2. In ganglion cells classified as category 2, a GABAB receptor antagonist does not affect bipolar cell output to ganglion cells.
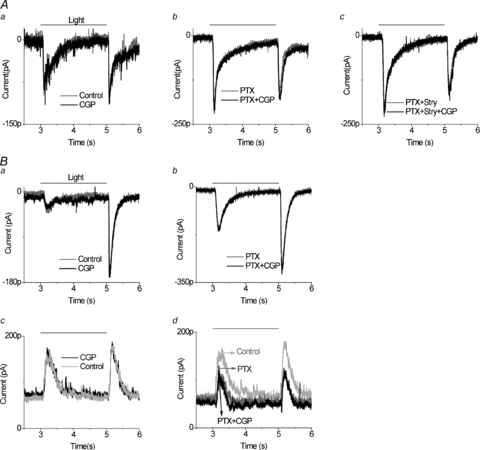
Aa, 5 μm CGP55845 alone had no effect on the ON or OFF EPSCs. Ab, 5 μm CGP55845 did not alter ganglion cell EPSCs in the presence of 100 μm PTX (n= 18). Ac, 5 μm CGP55845 changed neither ON nor OFF EPSCs after ionotropic GABA and glycine receptors were blocked by 100 μm PTX and 10 μm Stry, respectively. All recordings were from the same ganglion cell. Ba, in another ganglion cell, 5 μm CGP55845 did not alter the EPSCs compared to control. Bb, in the presence of 100 μm PTX, 5 μm CGP55845 still did not alter the light response. Bc, when IPSCs were measured in the same cell, 5 μm CGP55845 did not change light evoked IPSCs compared to the control. Bd, 100 μm PTX alone (dark grey trace) decreased IPSCs compared to the control (light grey trace), and this effect was not altered by the addition of 5 μm CGP55845. Light-evoked IPSCs were recorded in a ganglion cell clamped at 0 mV and stimulated by 2 s red LED light.
Category 3: endogenous GABABRs indirectly inhibit bipolar cell output
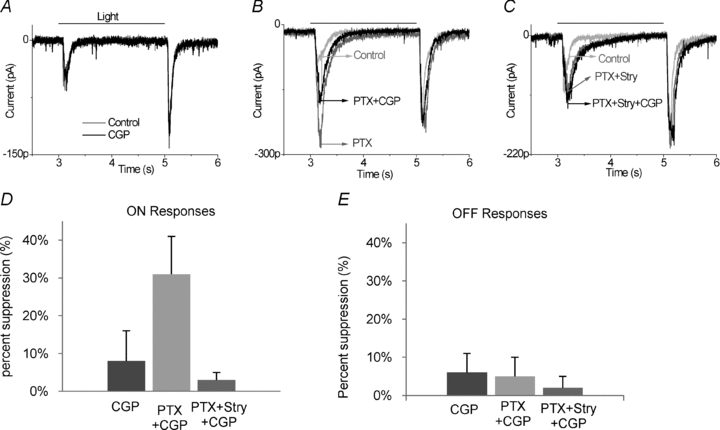
In a third category of ganglion cell light responses, CGP55845 alone had little effect on the ON or OFF EPSCs. Nor did CGP55845 have a significant effect on the light-evoked excitatory currents in the presence of picrotoxin and strychnine. Interestingly, in contrast to category 2 neurons, CGP55845 did significantly reduce EPSCs when ionotropic GABA receptors were blocked by 100 μm PTX (31 ± 10% mean suppression of ON EPSCs; 5 ± 5% mean suppression of OFF EPSCs, in 12 of 38 cells). Figure 3A illustrates the lack of effect of CGP55845 under control conditions. Picrotoxin produces a large enhancement of both ON and OFF responses (dark grey trace in Fig. 3B, note change in scale bar). In the continued presence of picrotoxin, CGP55845 induced a suppression of the ON EPSC (black trace in Fig. 3B). In this category of ganglion cell responses, the effect of CGP55845 was much more pronounced in ON responses. This CGP55845-induced suppression was reversible. The recovery confirmed that the suppressive effects were not due to long term application of picrotoxin, a phenomenon previously observed that probably required longer periods of drug application than we utilized (Cook et al. 2000). To determine whether CGP55845 was influencing the inhibitory inputs from amacrine cell terminals to these category 3 neurons, the cell was then clamped to 0 mV and IPSCs were observed, as shown in Fig. 3C. Picrotoxin reduced the IPSC, as expected since ganglion cells receive inhibitory GABAergic inhibition from amacrine cells. In fact, this suppression is the summation of three opposing effects of picrotoxin application. In addition to the block of GABAergic IPSCs, picrotoxin increases bipolar cell output, which enhances glycinergic amacrine cell signals in the ganglion cell. There is a second enhancement of glycinergic inhibition, which is the result of disinhibition of glycinergic amacrine cells (loss of GABAergic inhibition of glycinergic amacrine cells). In the presence of picrotoxin, CGP55845 increased the light-evoked IPSCs, particularly the ON inhibitory response. On average, in recordings from nine of the category 3 ganglion cells, CGP55845 in the presence of picrotoxin enhanced ON IPSCs by 33 ± 10% and OFF IPSCs by 13 ± 9%. This enhancement induced by CGP 55845 could also be reversed if CGP 55845 was removed. This indicates that the output of glycinergic amacrine cells is suppressed by endogenous GABABRs. It suggests that inhibitory glycinergic feedback to bipolar cell terminals is enhanced when GABABR receptors are inhibited.
Figure 3. In ganglion cells classified as category 3, a GABAB receptor antagonist did not affect bipolar cell output unless ionotropic GABA receptors were blocked.
A, application of 5 μm CGP55845 (black trace) had little effect on EPSCs compared to control. B, after application of 100 μm PTX, 5 μm CGP55845 reduced mainly the ON EPSC (black trace, n= 12) and this effect was reversible. C, when the IPSC was monitored in the same cell, 5 μm CGP55845 reversibly enhanced the IPSC in the presence of 100 μm PTX (n= 12) and this effect was reversible.
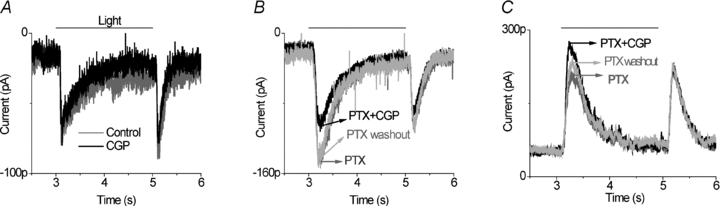
To test this hypothesis, EPSCs were recorded, in the presence of strychnine, in ganglion cells with identified category 3 response properties. Category 3 neurons were identified by determining that CPG55845 did not change EPSCs under control conditions (Fig. 4A) but did decrease light-evoked EPSCs in the presence of picrotoxin (Fig. 4B). We then tested the action of CGP55845 in the presence of both strychnine and picrotoxin. Strychnine blocked the CGP55845 effect (Fig. 4C). An example of this series of experiments is shown in Fig. 4. In a total of seven cells in which this protocol was performed, application of picrotoxin and strychnine, prior to application of CGP55845, increased the amplitude and prolonged the duration of EPSC currents. But CGP55845 in the presence of picrotoxin and strychnine had a negligible effect on the ON (97 ± 2% response remained) or the OFF (98 ± 3% remained) light response. This also contrasts with Fig. 4B, which showed that, when only ionotropic GABA receptors were blocked, the EPSC was suppressed by CGP55845. An interpretation of the data is that GABABR circuits suppress feed-forward and feed-back glycinergic inhibition by reducing transmitter release from glycinergic amacrine cells. A summary of CGP55845 effects on ON and OFF EPSCs in category 3 ganglion cells is presented in Fig. 4D and E. CGP55845 had negligible effects on the OFF responses. CGP55845 alone did not significantly affect EPSCs, but in the presence of picrotoxin it did suppress ON responses. This effect was blocked by strychnine.
Figure 4. Strychnine blocked the GABAB receptor effects in category 3 ganglion cells.
A, application of 5 μm CGP55845 alone did not alter EPSCs compared to control. B, 100 μm PTX enhanced and prolonged the light response, particularly at light onset. Then 5 μm CGP in the presence of PTX reduced ON EPSCs (n= 7), indicative of a category 3 neuron. C, in the presence of 100 μm PTX and 10 μm strychnine, 5 μm CGP55845 had a negligible effect on the ON or the OFF light responses (n= 7). D and E, in the category 3 ganglion cells, CGP55845 reduced ON responses significantly, but had little effect on OFF responses. The effect on ON responses required picrotoxin and was blocked by strychnine.
GABAARs occlude GABABR effects on Category 3 ganglion cells
Why is the CGP55845 effect on category 3 ganglion cells only evident when ionotropic GABA receptors are blocked? And does the action of picrotoxin mean that both GABAA and GABAC receptors have to be blocked? The answer to the first question is that glycine output is likely to be inhibited by both ionotropic and metabotropic GABA receptors, and perhaps under the conditions of our experiments the two forms of inhibition are redundant. To answer the second question, EPSCs were recorded in ganglion cells and the actions of specific GABAA or GABAC receptor antagonists were tested.
CGP55845 reduced light-evoked EPSCs after GABAA receptors were blocked with SR95531 (SR). On average in eight category 3 neurons, CGP55845 in the presence of SR95531 suppressed 50.6 ± 14% of ON responses and 15.6 ± 8% of OFF responses (example shown in Fig. 5C). This is similar to the response shown in Fig. 3B after both GABAA and GABAC receptors were blocked. The experimental protocol in these eight cells is shown in Fig. 5. First it was established that the recording was from a category 3 ganglion cell, in which CGP55845 alone did not affect the light-evoked EPSCs (Fig. 5A), but did reduce responses in the presence of picrotoxin (Fig. 5B). Then picrotoxin was removed and, after recovery, SR95531 was added. CGP55845 again reduced the EPSC in the presence of the GABAA receptor antagonist (Fig. 5C). However, when strychnine was added to SR95531, CGP55845 had no effect on the light-evoked EPSCs (Fig. 5D). These results indicate that retinal circuits that activate GABAA receptors can occlude the effect of GABAB receptor circuits.
Figure 5. GABAA receptors occlude GABABR effects in category 3 neurons.
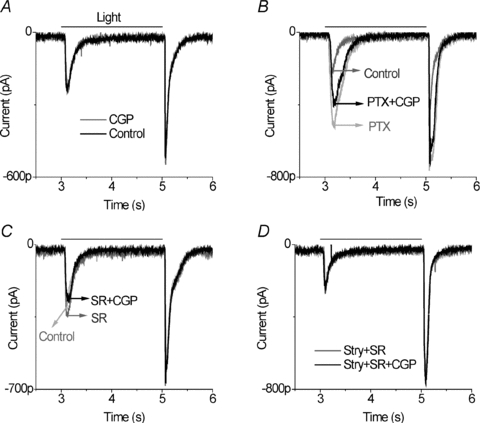
A, application of 5 μm CGP55845 did not alter EPSCs compared to control. B, in the same cell, after 100 μm PTX application, 5 μm CGP reduced ON EPSCs, indicative of a category 3 neuron. C, in the presence of 10 μm SR95531, 5 μm CGP55845 suppressed ON responses (n= 8). D, in the presence of 10 μm strychnine and 10 μm SR95531, 5 μm CGP55845 did not affect the light response. All of the indicated currents were recorded from the same ganglion cell.
However, after GABAC receptors were blocked by 100 μm TPMPA (1,2,5,6-Tetrahydropyridin-4-yl) methylphosphinic acid, CGP55845 had only a very small effect on light-evoked EPSCs (ON responses reduced on average by 6.3 ± 4%, OFF responses reduced by 7.3 ± 3%, n= 5). An example of this experiment is shown in Fig. 6. Application of CGP55845 alone did not alter the light evoked EPSCs (Fig. 6A), but it did suppress the ON and OFF EPSCs in the presence of picrotoxin (Fig. 6B). This established that this was a category 3 neuron. CGP55845 also reduced the ON and OFF EPSCs in the presence of the GABAA receptor blocker (Fig. 6C), but did not alter the EPSCs in the presence of the GABAC receptor blocker, TPMPA (Fig. 6D). Note that picrotoxin and TPMPA both prolonged the light-evoked EPSCs, while picrotoxin also increased the peak amplitude. In contrast, block of GABAA receptors increased the amplitude but did not prolong the time course of the light responses. The results of experiments illustrated in Figs 5 and 6 indicate that GABAA receptors occlude the GABAB receptor effects, while GABAC receptors have little if any effect on the GABAB receptor circuit. This might be expected if the site of GABA action was on glycinergic amacrine cells and not bipolar cell terminals because GABAC receptors are predominantly localized to bipolar cell terminals.
Figure 6. GABAc receptors do not occlude GABAB receptor effects in category 3 neurons.
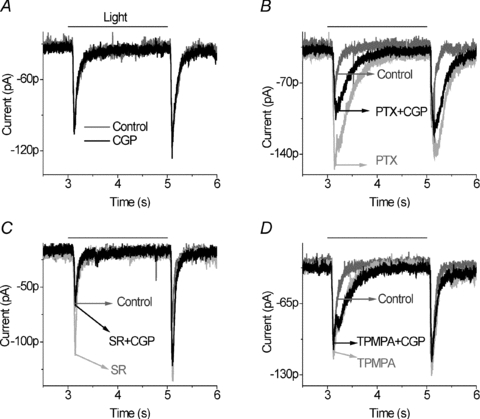
A, application of 5 μm CGP55845 alone did not alter EPSCs compared to control. B, however, 100 μm PTX enhanced the light responses (light grey trace) and 5 μm CGP55845 in the presence of PTX reduced ON and OFF EPSCs (black trace). C, 5 μm CGP55845 in the presence of SR95531 produced a large suppression of the ON response and a small reduction in the OFF response. D, 100 μm TPMPA prolonged the light responses, particularly at light onset (light grey trace). But 5 μm CGP55845 in the presence of TPMPA (black trace) had a negligible effect on light-evoked EPSCs (n= 5). All of the indicated currents were recorded from a same ganglion cell.
GABABRs do not influence GABAergic feedback to bipolar cells
The results indicate that glycinergic amacrine cell output is suppressed by GABAergic inhibition, and this is mediated by GABAA and GABABR receptors. GABAergic neurons often receive metabotropic autoreceptor feedback. Therefore, we examined whether a GABAB receptor mediated pathway also suppressed GABA feedback to bipolar cells. Once again we focused on ganglion cells in which CGP55845 had no effect under control condition (Fig. 7A). In the presence of strychnine, CGP55845 did not significantly reduce the EPSC (Fig. 7B). In the presence of strychnine in a total of eight cells, CGP55845 produced a mean reduction of 4.8 ± 3% in ON EPSCs and of 8.7 ± 6% in OFF EPSCs.
Figure 7. GABAB receptors do not influence ionotropic GABAergic action on bipolar cell output.
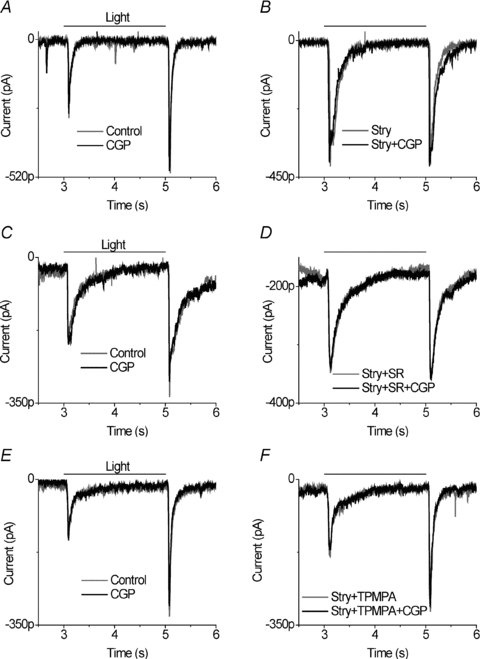
A, application of 5 μm CGP55845 did not alter EPSCs compared to control. B, in the same cell, in the presence of strychnine, 5 μm CGP55845 did not reduce the EPSCs. GABAB receptors do not alter GABAAR or GABAcR influence on bipolar cell output. C, application of 5 μm CGP55845 did not alter EPSCs compared to control. D, in the presence of 10 μm strychnine and 10 μm SR95531, 5 μm CGP55845 did not reduce the EPSCs. Currents were recorded from the same neuron. E, in another ganglion cell, application of 5 μm CGP55845 alone did not alter EPSCs compared to control. F, in the same cell, 5 μm CGP55845 had no effect on EPSCs in the presence of 10 μm strychnine and 100 μm TPMPA.
Since much of the GABAergic feedback to bipolar cells acts through GABAC receptors, yet most of the cross-inhibition between GABAergic amacrine cells is through GABAA receptors, we also tested the GABAergic feedback to bipolar cells when only GABAA and glycine receptors were blocked, using SR95531 and strychnine, respectively. Alternatively, we tested whether CGP55845 affected GABAA receptor feedback to bipolar cells when GABAC and glycine receptors were blocked with TPMPA and strychnine, respectively. We found that CGP55845 in the presence of strychnine and SR95531 produced a small, statistically insignificant suppression of light-evoked EPSCs (Fig. 7C and D). In 11 cells tested, the ON responses were suppressed by 7 ± 5% and the OFF responses were reduced by 9 ± 4%. Similarly, CGP55845 in the presence of strychnine and TPMPA produced a small and statistically insignificant reduction in light stimulated EPSCs (Fig. 7E and F). In five cells, the mean ON and OFF responses were both reduced by 7 ± 3%. Thus, we did not find evidence that GABAB receptors modulated the GABAA or GABAC feedback to bipolar cells.
Discussion
Here we probed the regulation of bipolar cell output mediated by synaptically activated GABAB receptors. Our data indicate that this regulation has multiple mechanisms, involving both direct and indirect pathways. First, we found evidence of direct GABABR feedback to bipolar cell terminals. The potent GABAB receptor antagonist, CGP55845, suppressed light evoked EPSCs in ganglion cells. One mechanism that increases bipolar cell output is the block of ionotropic GABA or glycine feedback to bipolar cells, especially the former. However, even when this feedback was blocked, inhibition of GABABRs suppressed bipolar cell output. This implies that endogenous GABABR activation directly enhances bipolar cell transmitter release (Fig. 8A). This is the opposite of the effect of GABA acting on bipolar cell GABAA or GABAC receptors (Tachibana & Kaneko, 1988; Lukasiewicz & Werblin, 1994; Pan & Lipton, 1995; Lukasiewicz & Shields, 1998; Shen & Slaughter, 2001).
Figure 8. A model of GABAB receptor mediated regulation of bipolar cell output.
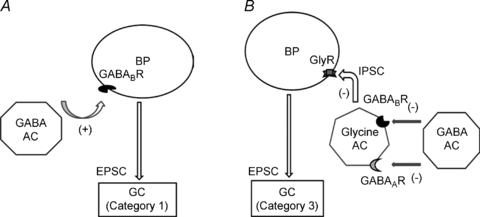
These two forms of regulation, direct (A) and indirect (B), both enhance bipolar cell transmitter release. A, in the direct pathway, GABAergic amacrine cells activate GABAB receptors on some bipolar cell terminals, leading to enhanced glutamate release. B, in the indirect pathway, glycinergic amacrine cells can inhibit glutamate release at a different set of bipolar cell terminals. But this glycinergic feedback is normally suppressed by GABAergic amacrine cells, which inhibit the glycinergic amacrine cell through activation of inhibitory GABAA and GABAB receptors. Abbreviations: BP, bipolar cell; AC, amacrine cell; GC, ganglion cell; (−), inhibition; (+), enhancement.
Second, we found that an indirect pathway also plays a role in enhancement of bipolar cell output. This indirect effect is mediated by GABAB receptors that inhibit glycinergic amacrine cells, which in turn disinhibits bipolar cell transmitter output (Fig. 8B). This circuit may be redundant with a GABAAR pathway, although that was not determined. Interestingly and counter-intuitively, both the direct and indirect GABABR circuits increase bipolar cell signalling to ganglion cells. Thus, ionotropic GABA feedback is inhibitory but metabotropic GABA feedback is facilitatory. In addition, there is a third group of bipolar cell inputs to ganglion cells for which we found no GABABR regulation, even in the presence of picrotoxin or strychnine or both.
With respect to GABAB receptor modulation of bipolar cell signals, there are three distinct, non-overlapping groups of ganglion cells. In one subset of ganglion cells, observed in 21% of our recordings, GABAB receptors directly enhance excitation, but there does not appear to be a secondary, indirect GABAB receptor regulation of the ganglion cell excitation that is revealed by strychnine or picrotoxin. This feedback affects ON and OFF bipolar cells similarly. In another group of ganglion cells, representing 32% of our recordings, a direct effect is absent and an indirect glycinergic modulation of excitation is dominant. This pathway affects primarily the ON pathway. In the third and largest group of ganglion cells (47%) we found no evidence that GABAB receptors modulate excitatory synaptic input, although perhaps this could have been observed with a different light stimulation.
Direct regulation of bipolar cell output by GABABR
CGP55845, a potent GABABR antagonist, was used to demonstrate the role that endogenous GABABRs play in regulation of bipolar cell output. Since a reduction in the postsynaptic EPSC is generally interpreted to signify a reduction of presynaptic release, we postulate that GABABRs at the bipolar cell terminal enhance release onto category 1 ganglion cells. However, CGP55845 might act postsynaptically to suppress the EPSC by either enhancing the IPSC or by suppressing the glutamate receptor on ganglion cells. Three factors argue against the first possibility: (1) we were presumably negating the IPSC by holding at the chloride reversal potential, (2) the effect of CGP55845 was observed when both GABA and glycine ionotropic receptors were blocked, and (3) if CGP55845 blocked a postsynaptic GABAB receptor mediated IPSC (Slaughter & Bai, 1989; Kaupmann et al. 1998), that would enhance, not suppress, the light-evoked EPSC. With respect to the second possibility, CGP55845 did not alter EPSCs in category 2 cells or in category 3 cells under control conditions, suggesting that postsynaptic glutamate receptors are not suppressed by CGP55845. Consequently, we conclude that direct synaptic feedback to the GABAB receptor on a set of ON and OFF bipolar cells enhances their transmitter release. This is consistent with several previous experiments showing that exogenous activation of GABABRs, using baclofen, enhanced light evoked excitation in transient ganglion cells (Bai & Slaughter, 1989; Hisako et al. 1990; Muller et al. 1992; Lukasiewicz & Werblin, 1994).
The current experiments show that GABAB receptor mediated feedback to bipolar cells occurs endogenously but also indicates that this is found in only a subset of ganglion cells, while the effect of applied baclofen was more ubiquitous. The implication is that applied baclofen reached non-synaptic, as well as synaptic, receptors. A possible mechanism for the enhanced bipolar cell release is a report that baclofen enhances L-type voltage-gated calcium channels in ganglion cells (Shen & Slaughter, 1999). Since bipolar cells use L-type calcium channels at the synapse, a similar effect could enhance their glutamate release. But the only direct studies of bipolar cell calcium channels conclude that applied baclofen either suppressed L-type calcium channels (Maguire et al. 1989) or had no effect (Lukasiewicz & Werblin, 1994). However, those experiments utilized applied agonist, while the current experiments were presumably examining endogenous activation of synaptic GABAB receptors. In unpublished experiments we found that applied baclofen has two effects: one that enhances bipolar cell synaptic release and a second that suppresses release (GB. Awatramani, Y. Song and M. M. Slaughter, unpublished observations). Thus, baclofen may be activating synaptic and non-synaptic receptors, while the current study only explores the synaptic GABAB receptors.
Indirect regulation of bipolar cell output: serial inhibition
There are various subtypes of glycinergic and GABAergic amacrine cell in the retinal inner plexiform layer, leading to serial synapses and concatenated inhibition. Several instances have been described at GABAAR, GABACR, and glycine receptor synapses. For example, in amphibian retina suppression of GABA inhibition often results in enhanced glycine inhibition, and vice versa (Zhang et al. 1997b). In rabbit retina starburst amacrine cells inhibit each other using GABA (Fried et al. 2002, 2005; Lee & Zhou, 2006). In mouse retina, serial inhibition forms long range lateral interactions that influence bipolar cell output in response to large field light stimuli (Eggers & Lukasiewicz, 2010). The current report demonstrates that the metabotropic GABA system also contributes to serial inhibition between amacrine cells. In our experiments on category 3 neurons, blocking of GABAB receptors produced both an enhancement of glycinergic inhibition and a reduction of glutamatergic excitation in ganglion cells. Both effects were blocked by strychnine. The simplest interpretation is that GABAergic amacrine cells, acting at GABABRs, suppressed glycinergic feedback to bipolar cells and feedforward to ganglion cells. This may be similar to a pathway affecting cholinergic amacrine cells in rabbit retina, in which exogenous activation of GABABRs suppressed glycinergic inhibition of starburst cells (Neal & Cunningham, 1995) although (Zucker et al. 2005) argue that GABAB receptors are not present on these glycinergic amacrine cells. The experiments of Neal & Cunningham (1995) focused on the ON pathway and concluded that baclofen suppressed glycine release and augmented acetylcholine release. Although our results were similar, they differ in some respects. We were able to conclude that only the ON pathway was affected, that the glycinergic feedback suppressed bipolar cell output, and that the GABAB receptors were synaptically activated.
The action of GABAB receptors in this serial pathway was only evident when GABAA receptors were blocked. Thus, the effect of glycinergic amacrine cell feedback is suppressed by both ionotropic and metabotropic receptor synapses. Generally, it has been found that GABAergic feedback to bipolar cells is much more pronounced than glycinergic feedback (Euler & Wassle, 1998; Maple & Wu, 1998; Flores-Herr et al. 2001; Ivanova et al. 2006). Apparently this is not due to a lack of glycinergic synapses; instead it is because the glycinergic feedback is tightly regulated. The GABAAR pathway alone is strong enough to suppress glycine feedback to bipolar cells and thereby occlude the action of GABABRs. Blocking the GABACR does not influence the GABABR feedback to bipolar cells. Since GABACRs are believed to be located mainly at bipolar cell synapses, they presumably do not contribute significantly to serial inhibition between amacrine cells. While GABAB receptor circuits modulate glycinergic input to bipolar cells and ganglion cells, GABAB receptors do not appear to regulate GABAergic inhibition in our experiments, despite the presence of presynaptic GABAB receptors on GABAergic amacrine cells (Koulen et al. 1998). CGP55845, in the presence of strychnine, did not alter EPSCs in ganglion cells. Thus, we have no evidence that ionotropic GABA feedback to bipolar cells is regulated by the metabotropic GABA system. This is similar to the conclusion reached by Neal & Cunningham (1995) that baclofen did not alter GABA release evoked by high potassium.
Implications for retinal circuitry
Our results imply that there are distinct subsets of bipolar cell inputs that stimulate three categories of ganglion cell. In category 1 ganglion cells the excitatory inputs are suppressed by CGP55845 under control conditions. However, in the presence of picrotoxin there is no additional effect of CGP55845 in category 1 cells although there is in category 3 cells. This indicates that the bipolar cell synapses that receive glycine feedback in category 3 neurons are not driving the category 1 neurons. Similarly, the category 2 ganglion cells are unaffected by CGP55845 under any of our experimental conditions, indicating that the bipolar cell inputs that stimulate this group of ganglion cells are different from those driving either category 1 or category 3 ganglion cells. Different inputs may not be equivalent to different bipolar cells, since the various synapses from a bipolar cell may be differentially regulated. However, it does suggest the GABAB receptors identify separate information channels reaching the ganglion cells through discrete bipolar cell circuits.
The dendrites of glycinergic amacrine cells are found to be local and vertical (Pourcho & Goebel, 1985) while those of GABAergic amacrine cells are more diffuse (Lin & Masland, 2006). Thus, glycine may function predominantly between the synaptic layers within the inner plexiform layer. One ramification is that glycine mediates cross-inhibition between ON and OFF pathways in the inner plexiform layer. Cross inhibition can serve as a push–pull mechanism to enhance the differential ON–OFF signals in some bipolar, amacrine and ganglion cells (Molnar & Werblin, 2007; Hsueh et al. 2008; Manookin et al. 2008). Thus, GABAB receptor mediated inhibition of glycinergic amacrine cells may suppress cross-inhibition to the OFF pathway. However, since the GABA regulation of glycine feedback was only observed in 32% of the ganglion cells, this GABA inhibition may not affect cross-inhibition. Instead, it might act to enhance the ON–OFF differential by augmenting the ON response. In addition, the direct GABAB receptor pathway can also serve to enhance the ON–OFF differential.
Pang et al. (2007) describe three types of ON–OFF ganglion cells in the salamander retina. Type I received direct excitatory input from both ON and OFF bipolar cells while types II and III received direct input from only OFF or ON bipolar cells, respectively. In type II ganglion cells the ON excitatory responses arose from disinhibitory cross-talk through the OFF pathway; the opposite was observed in type III neurons. The disinhibition could be blocked by ionotropic GABA and glycine receptor blockers. We did not block ON or OFF excitation with these antagonists, indicating that both the direct and indirect GABAB receptor pathways act on type I ganglion cells.
Acknowledgments
This work was supported by a grant from National Eye Institute no. 05725.
Author contributions
Both authors have been involved in the conception and design of experiments, analysis and interpretation of data, and drafting the article. Y.S. collected the data.
References
- Albrecht BE, Darlison MG. Localization of the ρ1- and ρ2-subunit messenger RNAs in chick retina by in situ hybridization predicts the existence of γ-aminobutyric acid type C receptor subtypes. Neurosci Lett. 1995;189:155–158. doi: 10.1016/0304-3940(95)11479-g. [DOI] [PubMed] [Google Scholar]
- Awatramani GB, Slaughter MM. Intensity-dependent, rapid activation of presynaptic metabotropic glutamate receptors at a central synapse. J Neurosci. 2001;21:741–749. doi: 10.1523/JNEUROSCI.21-02-00741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai SH, Slaughter MM. Effects of baclofen on transient neurons in the mudpuppy retina: electrogenic and network actions. J Neurophysiol. 1989;61:382–390. doi: 10.1152/jn.1989.61.2.382. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Ishida AT. (–)-Baclofen and γ-aminobutyric-acid inhibit calcium currents in isolated retinal ganglion cells. Proc Natl Acad Sci U S A. 1991;88:10759–10763. doi: 10.1073/pnas.88.23.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PB, Lukasiewicz PD, McReynolds JS. GABAC receptors control adaptive changes in a glycinergic inhibitory pathway in salamander retina. J Neurosci. 2000;20:806–812. doi: 10.1523/JNEUROSCI.20-02-00806.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui JJ, Ma YP, Lipton SA, Pan ZH. Glycine receptors and glycinergic synaptic input at the axon terminals of mammalian retinal rod bipolar cells. J Physiol. 2003;553:895–909. doi: 10.1113/jphysiol.2003.052092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J-L, Yang X-L. Subcellular localization and complements of GABAA and GABAC receptors on bullfrog retinal bipolar cells. J Neurophysiol. 2000;84:666–676. doi: 10.1152/jn.2000.84.2.666. [DOI] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. J Neurophysiol. 2010;103:25–37. doi: 10.1152/jn.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Wassle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol. 1998;79:1384–1395. doi: 10.1152/jn.1998.79.3.1384. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Wassle H, Bormann J. Pharmacology of GABA receptor Cl− channels in rat retinal bipolar cells. Nature. 1993;361:159–162. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- Flores-Herr N, Protti DA, Wassle H. Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. J Neurosci. 2001;21:4852–4863. doi: 10.1523/JNEUROSCI.21-13-04852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Directional selectivity is formed at multiple levels by laterally offset inhibition in the rabbit retina. Neuron. 2005;46:117–127. doi: 10.1016/j.neuron.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Greferath U, Brandstatter JH, Wassle H, Kirsch J, Kuhse J, Grunert U. Differential expression of glycine receptor subunits in the retina of the rat: a study using immunohistochemistry and in-situ hybridization. Vis Neurosci. 1994;11:721–729. doi: 10.1017/s0952523800003023. [DOI] [PubMed] [Google Scholar]
- Grünert U. Distribution of GABA and glycine receptors on bipolar and ganglion cells in the mammalian retina. Microsc Res Tech. 2000;50:130–140. doi: 10.1002/1097-0029(20000715)50:2<130::AID-JEMT5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Grunert U, Wassle H. Glycine receptors in the rod pathway of the macaque monkey retina. Vis Neurosci. 1996;13:101–115. doi: 10.1017/s0952523800007161. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Müller U, Zeilhofer HU, Harvey RJ, Wässle H. Diversity of glycine receptors in the mouse retina: Localization of the α2 subunit. J Comp Neurol. 2004;477:399–411. doi: 10.1002/cne.20267. [DOI] [PubMed] [Google Scholar]
- Heinze L, Harvey RJ, Haverkamp S, Wässle H. Diversity of glycine receptors in the mouse retina: Localization of the α4 subunit. J Comp Neurol. 2007;500:693–707. doi: 10.1002/cne.21201. [DOI] [PubMed] [Google Scholar]
- Hisako I, Hankms MW, Kay CD. Actions of baelofen and phaclofen upon ON- and OFF-ganglion cells in the cat retina. Eur J Pharmacol. 1990;190:1–9. doi: 10.1016/0014-2999(90)94106-8. [DOI] [PubMed] [Google Scholar]
- Hsueh H-A, Molnar A, Werblin FS. Amacrine-to-amacrine cell inhibition in the rabbit retina. J Neurophysiol. 2008;100:2077–2088. doi: 10.1152/jn.90417.2008. [DOI] [PubMed] [Google Scholar]
- Ivanova E, Müller U, Wässle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci. 2006;23:350–364. doi: 10.1111/j.1460-9568.2005.04557.x. [DOI] [PubMed] [Google Scholar]
- Jusuf PR, Haverkamp S, Grünert U. Localization of glycine receptor α subunits on bipolar and amacrine cells in primate retina. J Comp Neurol. 2005;488:113–128. doi: 10.1002/cne.20555. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Schuler V, Mosbacher J, Bischoff S, Bittiger H, Heid J, Froestl W, Leonhard S, Pfaff T, Karschin A, Bettler B. Human γ-aminobutyric acid type B receptors are differentially expressed and regulate inwardly rectifying K+ channels. Proc Natl Acad Sci U S A. 1998;95:14991–14996. doi: 10.1073/pnas.95.25.14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P, Brandstätter JH, Kröger S, Enz R, Bormann J, Wässle H. Immunocytochemical localization of the GABAC receptor rho subunits in the cat, goldfish, and chicken retina. J Comp Neurol. 1997;380:520–532. doi: 10.1002/(sici)1096-9861(19970421)380:4<520::aid-cne8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Koulen P, Malitschek B, Kuhn R, Bettler B, Wässle H, Brandstätter JH. Presynaptic and postsynaptic localization of GABAB receptors in neurons of the rat retina. Eur J Neurosci. 1998;10:1446–1456. doi: 10.1046/j.1460-9568.1998.00156.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Zhou ZJ. The synaptic mechanism of direction selectivity in distal processes of starburst amacrine cells. Neuron. 2006;51:787–799. doi: 10.1016/j.neuron.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Masland RH. Populations of wide-field amacrine cells in the mouse retina. J Comp Neurol. 2006;499:797–809. doi: 10.1002/cne.21126. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz P, Werblin F. A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. J Neurosci. 1994;14:1213–1223. doi: 10.1523/JNEUROSCI.14-03-01213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz PD, Eggers ED, Sagdullaev BT, McCall MA. GABAC receptor-mediated inhibition in the retina. Vision Res. 2004;44:3289–3296. doi: 10.1016/j.visres.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Shields CR. Different combinations of GABAA and GABAC receptors confer distinct temporal properties to retinal synaptic responses. J Neurophysiol. 1998;79:3157–3167. doi: 10.1152/jn.1998.79.6.3157. [DOI] [PubMed] [Google Scholar]
- Maguire G, Maple B, Lukasiewicz P, Werblin F. Gamma-aminobutyrate type-B receptor modulation of L-Type calcium-channel current at bipolar cell terminals in the retina of the tiger salamander. P Natl Acad Sci USA. 1989;86:10144–10147. doi: 10.1073/pnas.86.24.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple BR, Wu SM. Glycinergic synaptic inputs to bipolar cells in the salamander retina. J Physiol. 1998;506:731–744. doi: 10.1111/j.1469-7793.1998.731bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- Matthews G, Ayoub G, Heidelberger R. Presynaptic inhibition by GABA is mediated via two distinct GABA receptors with novel pharmacology. J Neurosci. 1994;14:1079–1090. doi: 10.1523/JNEUROSCI.14-03-01079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Werblin F. Inhibitory feedback shapes bipolar cell responses in the rabbit retina. J Neurophysiol. 2007;98:3423–3435. doi: 10.1152/jn.00838.2007. [DOI] [PubMed] [Google Scholar]
- Muller F, Boos R, Wassle H. Actions of GABA(ergic) ligands on brisk ganglion-cells in the cat retina. Vis Neurosci. 1992;9:415–425. doi: 10.1017/s0952523800010828. [DOI] [PubMed] [Google Scholar]
- Neal MJ, Cunningham JR. Baclofen enhancement of acetylcholine-release from amacrine cells in the rabbit retina by reduction of glycinergic inhibition. J Physiol. 1995;482:363–372. doi: 10.1113/jphysiol.1995.sp020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard RW, Frumkes TE. LEDs: Convenient, inexpensive sources for visual experimentation. Vision Res. 1982;22:435–440. doi: 10.1016/0042-6989(82)90190-0. [DOI] [PubMed] [Google Scholar]
- Pan Z, Lipton S. Multiple GABA receptor subtypes mediate inhibition of calcium influx at rat retinal bipolar cell terminals. J Neurosci. 1995;15:2668–2679. doi: 10.1523/JNEUROSCI.15-04-02668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J-J, Gao F, Wu SM. Cross-talk between ON and OFF channels in the salamander retina: Indirect bipolar cell inputs to ON-OFF ganglion cells. Vision Res. 2007;47:384–392. doi: 10.1016/j.visres.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. A combined golgi and autoradiographic study of (3H)glycine-accumulating amacrine cells in the cat retina. J Comp Neurol. 1985;233:473–480. doi: 10.1002/cne.902330406. [DOI] [PubMed] [Google Scholar]
- Qian H, Dowling JE. Novel GABA responses from rod-driven retinal horizontal cells. Nature. 1993;361:162–164. doi: 10.1038/361162a0. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoepognetto M, Wassle H, Grunert U. Glycinergic synapses in the rod pathway of the rat retina – cone bipolar cells express the α-1 subunit of the glycine receptor. J Neurosci. 1994;14:5131–5146. doi: 10.1523/JNEUROSCI.14-08-05131.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Slaughter MM. Metabotropic GABA receptors facilitate L-type and inhibit N-type calcium channels in single salamander retinal neurons. J Physiol. 1999;516:711–718. doi: 10.1111/j.1469-7793.1999.0711u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Slaughter MM. Multireceptor GABAergic regulation of synaptic communication in amphibian retina. J Physiol. 2001;530:55–67. doi: 10.1111/j.1469-7793.2001.0055m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CR, Tran MN, Wong ROL, Lukasiewicz PD. Distinct ionotropic GABA receptors mediate presynaptic and postsynaptic inhibition in retinal bipolar cells. J Neurosci. 2000;20:2673–2682. doi: 10.1523/JNEUROSCI.20-07-02673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter MM, Bai SH. Differential effects of baclofen on sustained and transient cells in the mudpuppy retina. J Neurophysiol. 1989;61:374–381. doi: 10.1152/jn.1989.61.2.374. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Kaneko A. Retinal bipolar cells receive negative feedback input from GABAergic amacrine cells. Vis Neurosci. 1988;1:297–305. doi: 10.1017/s0952523800001954. [DOI] [PubMed] [Google Scholar]
- Wassle H, Heinze L, Ivanova E, Majumdar S, Weiss J, Harvey RJ, Haverkamp S. Glycinergic transmission in the mammalian retina. Front Mol Neurosci. 2010;3:12. doi: 10.3389/neuro.02.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SMS. Synaptic connections between neurons in living slices of the larval tiger salamander retina. J Neurosci Methods. 1987;20:139–149. doi: 10.1016/0165-0270(87)90046-x. [DOI] [PubMed] [Google Scholar]
- Yang X-L, Wu SM. Response sensitivity and voltage gain of the rod- and cone-bipolar cell synapses in dark-adapted tiger salamander retina. J Neurophysiol. 1997;78:2662–2673. doi: 10.1152/jn.1997.78.5.2662. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bettler B, Duvoisin RM. Differential localization of GABAB receptors in the mouse retina. NeuroReport. 1998;9:3493–3497. doi: 10.1097/00001756-199810260-00029. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shen W, Slaughter MM. Two metabotropic γ-aminobutyric acid receptors differentially modulate calcium currents in retinal ganglion cells. J Gen Physiol. 1997a;110:45–58. doi: 10.1085/jgp.110.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JA, Jung CS, Slaughter MM. Serial inhibitory synapses in retina. Vis Neurosci. 1997b;14:553–563. doi: 10.1017/s0952523800012219. [DOI] [PubMed] [Google Scholar]
- Zucker CL, Nilson JE, Ehinger B, Grzywacz NM. Compartmental localization of gamma-aminobutyric acid type B receptors in the cholinergic circuitry of the rabbit retina. J Comp Neurol. 2005;493:448–459. doi: 10.1002/cne.20766. [DOI] [PMC free article] [PubMed] [Google Scholar]


