Abstract
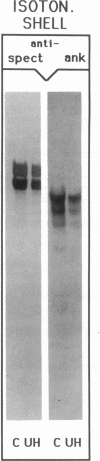
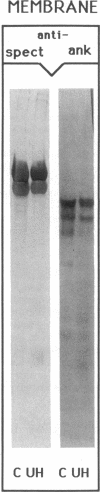
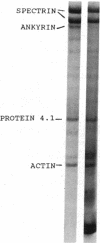
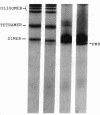
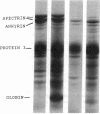
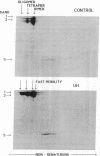
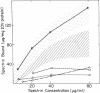
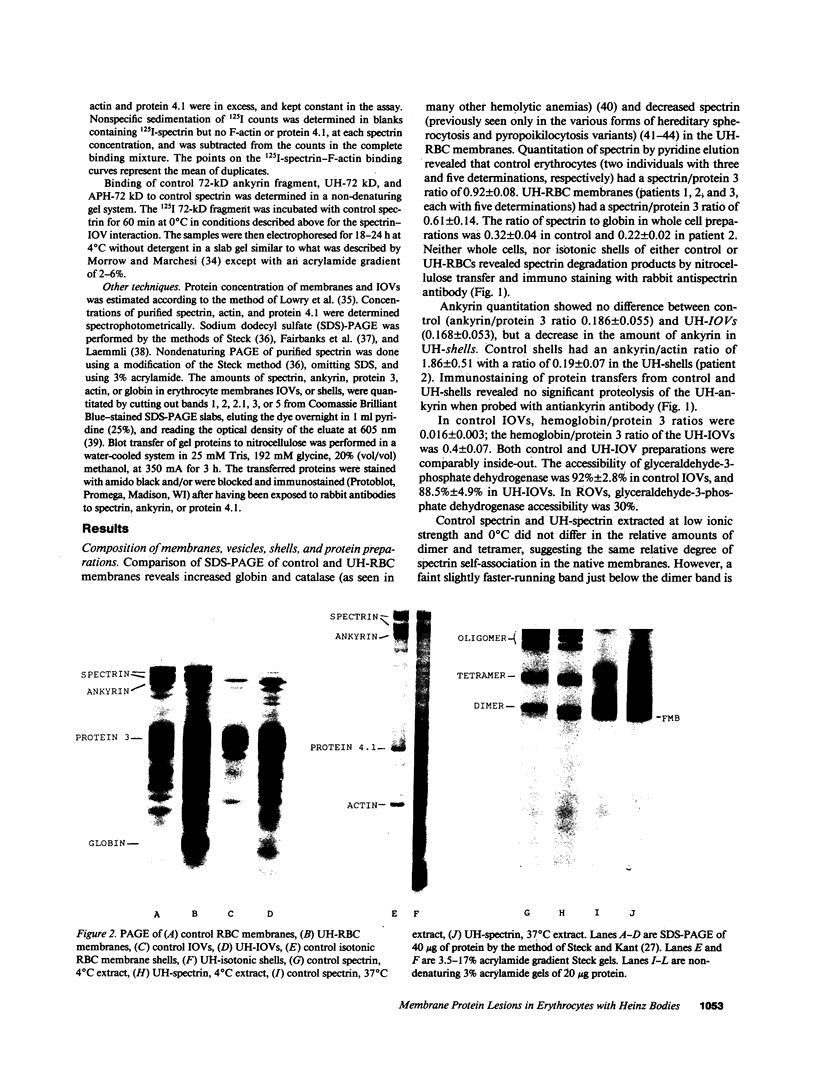
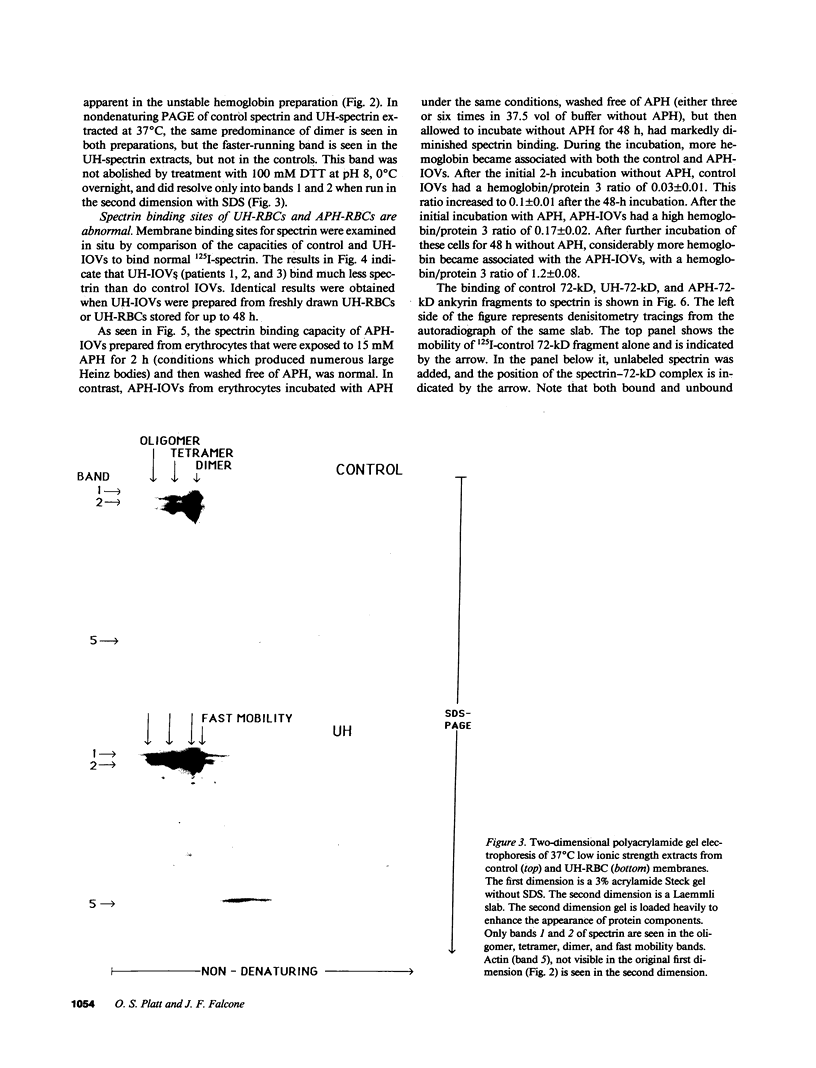
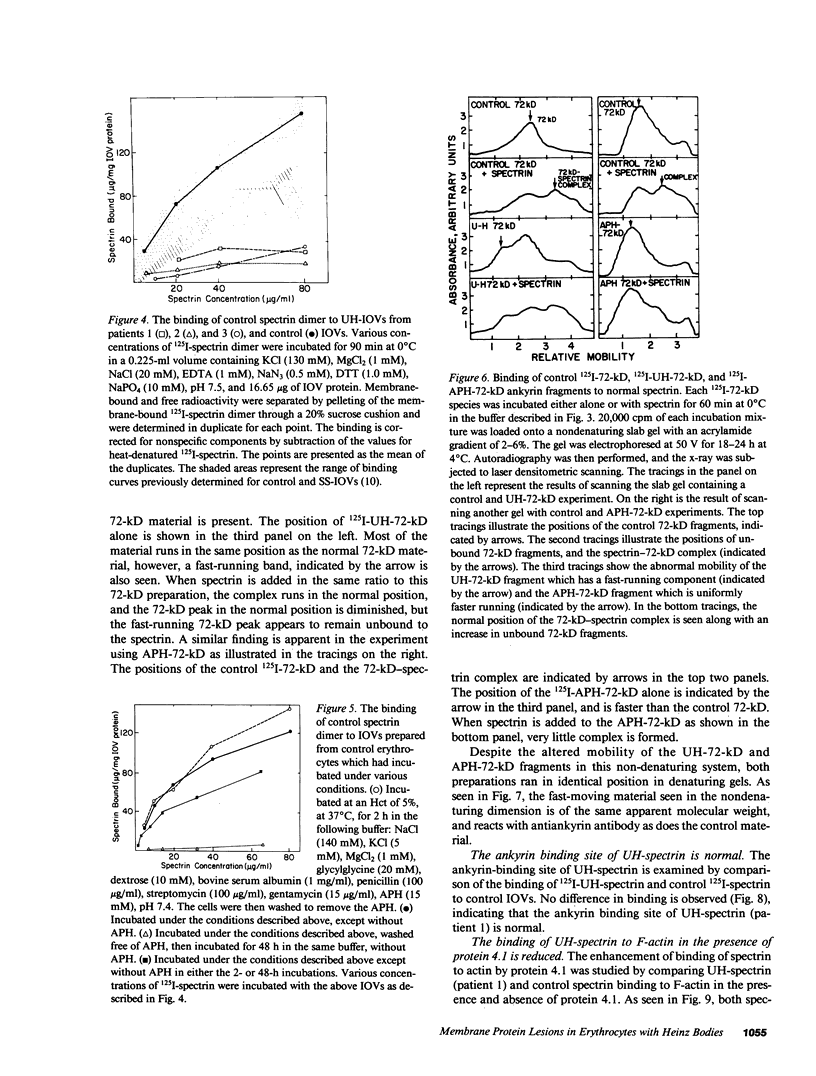
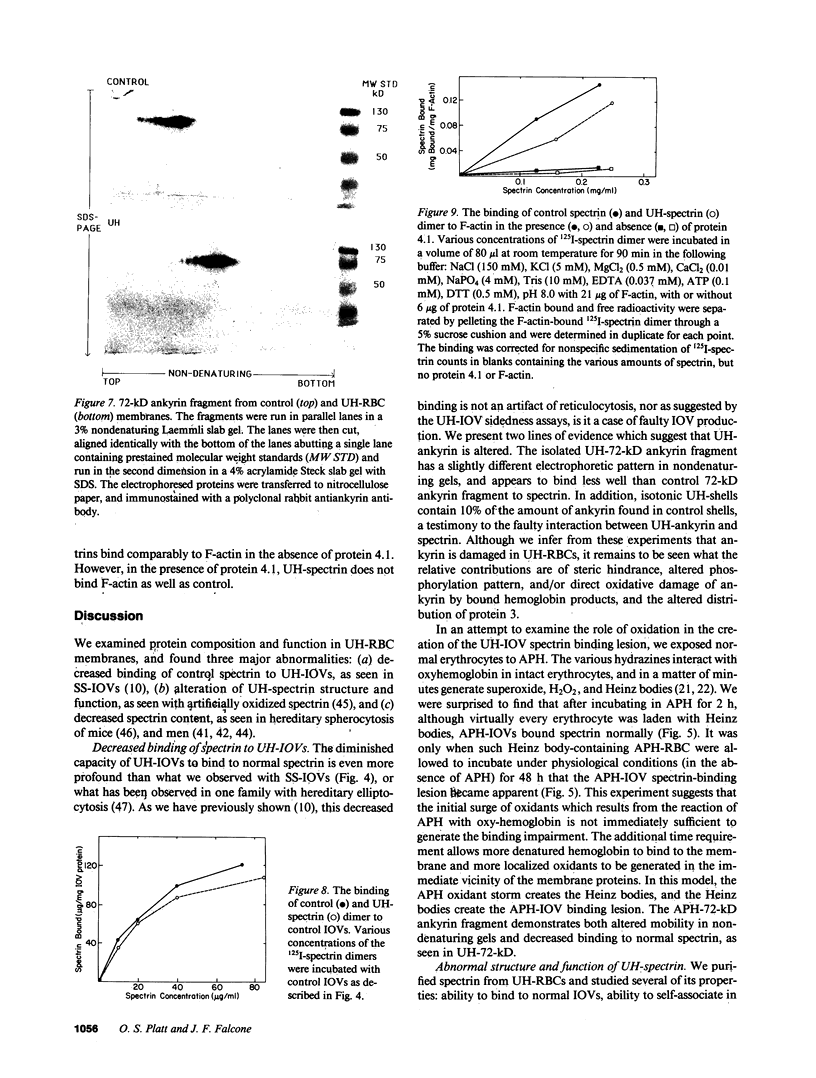
We studied Heinz body-containing erythrocytes with three different unstable hemoglobins: Nottingham, Brockton, and unclassified. We demonstrated two classes of membrane protein defects in unstable hemoglobin-containing cells (UH-RBCs), a defect of the spectrin-depleted inside-out vesicle (UH-IOV), and a defect of spectrin (UH-spectrin) itself. The composition of UH-IOVs is the same as control with respect to quantity of ankyrin and proportion inside-out. However, UH-IOVs bind even less spectrin than IOVs derived from sickle erythrocytes (SS-IOVs), suggesting a severe functional defect in the ankyrin of UH-RBCs (UH-ankyrin). Further evidence that UH-ankyrin is abnormal is demonstrated by the virtual absence of ankyrin in isotonic membrane shells of UH-RBCs (UH-shells), and abnormal mobility and decreased binding of the 72-kD (spectrin-binding) alpha-chymotryptic fragment of UH-ankyrin (UH-72-kD) to control spectrin. All UH-RBC membranes were spectrin-deficient (60% of control). In addition, spectrin isolated from UH-RBCs (UH-spectrin) was abnormal in two respects: (a) presence of a fast-moving band on nondenaturing polyacrylamide gels of both 0 degree C and 37 degrees C extracts, and (b) decreased binding to actin in the presence of protein 4.1. UH-spectrin did exhibit normal self-association, binding to IOVs and binding to actin in the absence of protein 4.1. This pattern of normal and abnormal spectrin functions has been described for spectrin subjected to mild diamide oxidation, suggesting the role of oxidation is the pathogenesis of membrane defect(s) of erythrocytes with abnormal hemoglobins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Asimos A., Casella J. F., McMillan C. Inheritance pattern and clinical response to splenectomy as a reflection of erythrocyte spectrin deficiency in hereditary spherocytosis. N Engl J Med. 1986 Dec 18;315(25):1579–1583. doi: 10.1056/NEJM198612183152504. [DOI] [PubMed] [Google Scholar]
- Agre P., Casella J. F., Zinkham W. H., McMillan C., Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. 1985 Mar 28-Apr 3Nature. 314(6009):380–383. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- Agre P., Orringer E. P., Bennett V. Deficient red-cell spectrin in severe, recessively inherited spherocytosis. N Engl J Med. 1982 May 13;306(19):1155–1161. doi: 10.1056/NEJM198205133061906. [DOI] [PubMed] [Google Scholar]
- Allen D. W., Cadman S., McCann S. R., Finkel B. Increased membrane binding of erythrocyte catalase in hereditary spherocytosis and in metabolically stressed normal cells. Blood. 1977 Jan;49(1):113–123. [PubMed] [Google Scholar]
- Aragoncillo C., Rodriguez-Loperena M. A., Carbonero P., Garcia-Olmeda F. Nigrosine staining of wheat endosperm proteolipid patterns on starch gels. Anal Biochem. 1975 Feb;63(2):603–606. doi: 10.1016/0003-2697(75)90387-5. [DOI] [PubMed] [Google Scholar]
- Becker P. S., Cohen C. M., Lux S. E. The effect of mild diamide oxidation on the structure and function of human erythrocyte spectrin. J Biol Chem. 1986 Apr 5;261(10):4620–4628. [PubMed] [Google Scholar]
- Becker P. S., Spiegel J. E., Wolfe L. C., Lux S. E. High yield purification of protein 4.1 from human erythrocyte membranes. Anal Biochem. 1983 Jul 1;132(1):195–201. doi: 10.1016/0003-2697(83)90447-5. [DOI] [PubMed] [Google Scholar]
- Bennett V. Purification of an active proteolytic fragment of the membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1978 Apr 10;253(7):2292–2299. [PubMed] [Google Scholar]
- Cassoly R. Quantitative analysis of the association of human hemoglobin with the cytoplasmic fragment of band 3 protein. J Biol Chem. 1983 Mar 25;258(6):3859–3864. [PubMed] [Google Scholar]
- Coetzer T. L., Palek J. Partial spectrin deficiency in hereditary pyropoikilocytosis. Blood. 1986 Apr;67(4):919–924. [PubMed] [Google Scholar]
- Coetzer T., Zail S. S. Tryptic digestion of spectrin in variants of hereditary elliptocytosis. J Clin Invest. 1981 May;67(5):1241–1248. doi: 10.1172/JCI110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger J., Flores J., Salhany J. M. Association of cytosol hemoglobin with the membrane in intact erythrocytes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):408–412. doi: 10.1073/pnas.79.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Flynn T. P., Allen D. W., Johnson G. J., White J. G. Oxidant damage of the lipids and proteins of the erythrocyte membranes in unstable hemoglobin disease. Evidence for the role of lipid peroxidation. J Clin Invest. 1983 May;71(5):1215–1223. doi: 10.1172/JCI110870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. R., Shiffer K. A., Casoria L. A., Eyster M. E. Identification of the molecular defect in the erythrocyte membrane skeleton of some kindreds with hereditary spherocytosis. Blood. 1982 Sep;60(3):772–784. [PubMed] [Google Scholar]
- Goodman S. R., Weidner S. A. Binding of spectrin alpha 2-beta 2 tetramers to human erythrocyte membranes. J Biol Chem. 1980 Sep 10;255(17):8082–8086. [PubMed] [Google Scholar]
- Greenquist A. C., Shohet S. B., Bernstein S. E. Marked reduction of spectrinin hereditary spherocytosis in the common house mouse. Blood. 1978 Jun;51(6):1149–1155. [PubMed] [Google Scholar]
- Hebbel R. P., Eaton J. W., Balasingam M., Steinberg M. H. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest. 1982 Dec;70(6):1253–1259. doi: 10.1172/JCI110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. K., Hochstein P. Polymerization of membrane components in aging red blood cells. Biochem Biophys Res Commun. 1980 Jan 15;92(1):247–254. doi: 10.1016/0006-291x(80)91545-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liljas L., Lundahl P., Hjertén S. Selective solubilization with Tween 20 of proteins from water-extracted human erythrocyte membranes. Analysis by gel electrophoresis in dodecylsulfate and in Tween 20. Biochim Biophys Acta. 1974 Jun 29;352(3):327–337. doi: 10.1016/0005-2736(74)90224-7. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J. Hemoglobin enhances the self-association of spectrin heterodimers in human erythrocytes. J Biol Chem. 1984 Sep 25;259(18):11556–11562. [PubMed] [Google Scholar]
- Liu S. C., Palek J., Prchal J., Castleberry R. P. Altered spectrin dimer-dimer association and instability of erythrocyte membrane skeletons in hereditary pyropoikilocytosis. J Clin Invest. 1981 Sep;68(3):597–605. doi: 10.1172/JCI110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. S., Waugh S. M., Zinke K., Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985 Feb 1;227(4686):531–533. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Ukena T. E. Diminished spectrin extraction from ATP-depleted human erythrocytes. Evidence relating spectrin to changes in erythrocyte shape and deformability. J Clin Invest. 1978 Mar;61(3):815–827. doi: 10.1172/JCI108996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian V., Gratzer W. Preparation of red-cell-membrane cytoskeletal constituents and characterisation of protein 4.1. Eur J Biochem. 1984 Oct 15;144(2):375–379. doi: 10.1111/j.1432-1033.1984.tb08474.x. [DOI] [PubMed] [Google Scholar]
- Platt O. S., Falcone J. F., Lux S. E. Molecular defect in the sickle erythrocyte skeleton. Abnormal spectrin binding to sickle inside-our vesicles. J Clin Invest. 1985 Jan;75(1):266–271. doi: 10.1172/JCI111684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premachandra B. R. Interaction of hemoglobin and its component alpha and beta chains with band 3 protein. Biochemistry. 1986 Jun 3;25(11):3455–3462. doi: 10.1021/bi00359a054. [DOI] [PubMed] [Google Scholar]
- Rank B. H., Carlsson J., Hebbel R. P. Abnormal redox status of membrane-protein thiols in sickle erythrocytes. J Clin Invest. 1985 May;75(5):1531–1537. doi: 10.1172/JCI111857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice-Evans C., Hochstein P. Alterations in erythrocyte membrane fluidity by phenylhydrazine-induced peroxidation of lipids. Biochem Biophys Res Commun. 1981 Jun;100(4):1537–1542. doi: 10.1016/0006-291x(81)90693-8. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Cordes K. A., Gaines E. D. Light-scattering measurements of hemoglobin binding to the erythrocyte membrane. Evidence for transmembrane effects related to a disulfonic stilbene binding to band 3. Biochemistry. 1980 Apr 1;19(7):1447–1454. doi: 10.1021/bi00548a028. [DOI] [PubMed] [Google Scholar]
- Sayare M., Fikiet M. Cross-linking of hemoglobin to the cytoplasmic surface of human erythrocyte membranes. Identification of band 3 as a site for hemoglobin binding in Cu2+-o-phenanthroline catalyzed cross-linking. J Biol Chem. 1981 Dec 25;256(24):13152–13158. [PubMed] [Google Scholar]
- Schlüter K., Drenckhahn D. Co-clustering of denatured hemoglobin with band 3: its role in binding of autoantibodies against band 3 to abnormal and aged erythrocytes. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6137–6141. doi: 10.1073/pnas.83.16.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaklai N., Sharma V. S., Ranney H. M. Interaction of sickle cell hemoglobin with erythrocyte membranes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):65–68. doi: 10.1073/pnas.78.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaklai N., Yguerabide J., Ranney H. M. Classification and localization of hemoglobin binding sites on the red blood cell membrane. Biochemistry. 1977 Dec 13;16(25):5593–5597. doi: 10.1021/bi00644a032. [DOI] [PubMed] [Google Scholar]
- Shaklai N., Yguerabide J., Ranney H. M. Interaction of hemoglobin with red blood cell membranes as shown by a fluorescent chromophore. Biochemistry. 1977 Dec 13;16(25):5585–5592. doi: 10.1021/bi00644a031. [DOI] [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Tyler J. M., Reinhardt B. N., Branton D. Associations of erythrocyte membrane proteins. Binding of purified bands 2.1 and 4.1 to spectrin. J Biol Chem. 1980 Jul 25;255(14):7034–7039. [PubMed] [Google Scholar]
- Walder J. A., Chatterjee R., Steck T. L., Low P. S., Musso G. F., Kaiser E. T., Rogers P. H., Arnone A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J Biol Chem. 1984 Aug 25;259(16):10238–10246. [PubMed] [Google Scholar]
- Waugh S. M., Low P. S. Hemichrome binding to band 3: nucleation of Heinz bodies on the erythrocyte membrane. Biochemistry. 1985 Jan 1;24(1):34–39. doi: 10.1021/bi00322a006. [DOI] [PubMed] [Google Scholar]
- Waugh S. M., Walder J. A., Low P. S. Partial characterization of the copolymerization reaction of erythrocyte membrane band 3 with hemichromes. Biochemistry. 1987 Mar 24;26(6):1777–1783. doi: 10.1021/bi00380a041. [DOI] [PubMed] [Google Scholar]
- Waugh S. M., Willardson B. M., Kannan R., Labotka R. J., Low P. S. Heinz bodies induce clustering of band 3, glycophorin, and ankyrin in sickle cell erythrocytes. J Clin Invest. 1986 Nov;78(5):1155–1160. doi: 10.1172/JCI112696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe L. C., John K. M., Falcone J. C., Byrne A. M., Lux S. E. A genetic defect in the binding of protein 4.1 to spectrin in a kindred with hereditary spherocytosis. N Engl J Med. 1982 Nov 25;307(22):1367–1374. doi: 10.1056/NEJM198211253072203. [DOI] [PubMed] [Google Scholar]