Abstract
Since accumulation of both H+ and extracellular K+ have been implicated in the reduction in dynamic contractile function during intense exercise, we investigated the effects of acidification and high K+ on muscle power and the force–velocity relation in non-fatigued rat soleus muscles. Contractions were elicited by supramaximal electrical stimulation at 60 Hz. Force–velocity (FV) curves were obtained by fitting data on force and shortening velocity at different loads to the Hill equation. Acidification of the muscles by incubation with up to 24 mm lactic acid produced no significant changes in maximal power (Pmax) at 30°C. More pronounced acidification, obtained by increasing CO2 levels in the equilibration gas from 5% to 53%, markedly decreased Pmax and maximal isometric force (Fmax), increased the curvature of the FV relation, but left maximal shortening velocity (Vmax) unchanged. Increase of extracellular K+ from 4 to 10 mm caused a depression of 58% in Pmax and 52% in Fmax, but had no significant effect on Vmax or curvature of the FV curve. When muscles at 10 mm K+ were acidified by 20 mm lactic acid, Pmax and Fmax recovered completely to the initial control level at 4 mm K+. CO2 acidification also induced significant recovery of dynamic contractions, but not entirely to control levels. These results demonstrate that in non-fatigued muscles severe acidification can be detrimental to dynamic contractile function, but in muscles depolarised by exposure to high extracellular [K+], approaching the [K+] level seen during intense fatiguing exercise, acidification can have positive protective effects on dynamic muscle function.
Introduction
Intense muscle activity leads to increased production of lactic acid and loss of potassium from the muscle causing lactic acid to accumulate in both the intra- and extracellular compartment of the muscles and potassium to increase in the extracellular compartment. Both changes have been considered as potentially important causative factors in peripheral muscle fatigue (Fitts, 1994; Sejersted & Sjøgaard, 2000), whereas a more recent review of the mechanisms of muscle fatigue downplayed the role of K+ and lactic acid (Allen et al. 2008). The effects of lactic acid on muscle contractile function are thought to be associated with intracellular acidification rather than the lactate ion per se. Evidence showing that acidification reduces the contractility of muscles comes mainly from experiments using single muscle fibres that are chemically or mechanically skinned of their sarcolemmal membrane in order to access the intracellular environment. In these preparations it has been found that lowering pH decreases maximal force production (Fmax) and maximal velocity of shortening (Vmax) (Chase & Kushmerick, 1988). Many early experiments were, however, performed at temperatures below the physiologically relevant range for mammals and more recently it was found that in rodents the detrimental effects of lowering pH on force were reduced considerably by raising the muscle temperature to 25–37°C (Pate et al. 1995; Westerblad et al. 1997). Moreover, we have shown that when muscles are exposed to elevated extracellular K+, corresponding to the values for extracellular K+ observed during intense exercise, reduction of intracellular pH significantly improves excitability of muscles leading to increased maximal isometric tetanic force (Nielsen et al. 2001). Based on these findings it has been argued that in mammals the detrimental effect of lactic acid on force production during intense exercise could be outweighed by protective effects on the excitability of the muscles (Lamb & Stephenson, 2006). If so, this suggests, that the reduction in pH during intense exercise delays the fatigue development rather than being the cause of it.
Recently, however, Knuth et al. (2006) showed that in dynamically contracting single rat fibres the effect of lowering pH on muscle power was considerable even at 30°C. Since power is, perhaps, the most important measure of muscle function in dynamic contractions, this could indicate that even at 30°C the balance between the detrimental effects of lactic acid on contractile function and its protective effect on excitability is shifted toward the detrimental effects when dynamic contractile function is considered. To address this question, we here examine the effects of acidification and elevated extracellular K+ on power production in isolated rat muscle maintained in a bicarbonate buffer at 30°C. To achieve reductions in both intra- and extracellular pH we used either addition of lactic acid or increased CO2 concentration in a bicarbonate buffer.
Methods
Ethical approval
No experiments were performed on live animals. All handling and use of animals complied with Danish animal welfare legislation, including the animal housing and termination that in addition was approved by the University Animal Welfare Officer. Our experiments comply with the policies and regulations of The Journal of Physiology (Drummond, 2009) and UK regulations on animal experimentation.
Muscle preparations and buffers
Experiments were carried out using soleus muscles or extensor digitorum longus (EDL) muscles from 4-week-old Wistar rats weighing 60–75 g (own breed). The rats were fed ad libitum and were kept at a constant temperature (21°C) and day length (12 h). The animals were killed by cervical dislocation followed by decapitation, and soleus or, in some instances, EDL muscles were isolated with the proximal end attached to the bone and the distal end with an intact tendon. The standard incubation medium was Krebs–Ringer bicarbonate (KRB) buffer containing (mm): 122 NaCl, 25 NaHCO3, 2.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.3 CaCl2 and 5.0 d-glucose. If not otherwise noted, buffers were maintained at 30°C and equilibrated with a mixture of 95% O2 and 5% CO2 throughout the experiment (pH 7.45 at 30°C). In K+-enriched buffers an equivalent amount of Na+ was omitted to maintain isoosmolarity. To avoid the exposure of the muscles to large and potentially damaging excursion in buffer pH, buffers in which l-lactic acid (Sigma-Aldrich Co.) was added were equilibrated for at least 30 min with 95% O2 and 5% CO2 before use. Other mixtures of O2 and CO2 were made using a gas mixing pump (type M201, Wösthoff oHG, Bochum, Germany). Addition of lactic acid and increased CO2 both led to reductions in intra- and extracellular pH as shown in Table 1. In all experiments, the muscles were initially mounted at optimal length for isometric twitch force generation in the standard incubation medium and equilibrated for at least 30 min before starting the experiments.
Table 1.
Changes in extracellular pH and expected changes in intracellular pH using different acidification procedures
| Acidification procedure | ΔpHe | ΔpHi |
|---|---|---|
| 20 mm lactic acid | −0.6 | −0.4 |
| 24 mm lactic acid | −0.7 | ∼−0.5 |
| 24% CO2 | −0.6 | −0.4 |
| 53% CO2 | −1.0 | ∼−0.7 |
ΔpHe are from measurements of buffer pH. ΔpHi are based on or extrapolated from measurements performed under similar conditions (Nielsen et al. 2001).
Dynamic contractions
Muscles were mounted on a length/force controlled lever system (model 305, Aurora scientific, Aurora, Ontario, Canada) at optimal muscle length for isometric force production (Lo). A personal computer generated the signal for electrical stimulation and force/length control of the levers during the contractions through commercial software (DMC v. 4.1.6, Aurora scientific), which also allowed for simultaneous sampling of force and length at 1000 Hz. Muscle contractions were evoked via field stimulation using supramaximal constant current pulses applied through two platinum plate electrodes. Pulses of 0.1 ms duration were used in brief trains of either 1.2 s at 60 Hz for soleus muscles or 0.8 s at 100 Hz for EDL muscles.
To determine length and force data for force–velocity (FV) curves under different conditions, series of brief tetanic contraction protocols were used. In each protocol, an isometric contraction was first elicited with the lever arm at a fixed position corresponding to Lo. When tetanic force was fully developed (about 0.6 s for soleus, 0.3 s for EDL) the holding force of the lever arm was reduced quickly to a level below the force generating capacity of the muscle. This caused the muscle to shorten and after a stable level of resisting force was reached (within 25 ms), the shortening velocity was measured over a period of 20–50 ms (see inset in Fig. 1). After each contraction the relaxed muscle was re-extended to its initial length (Lo).
Figure 1. Contraction protocol for determination of isometric force and FV curves.
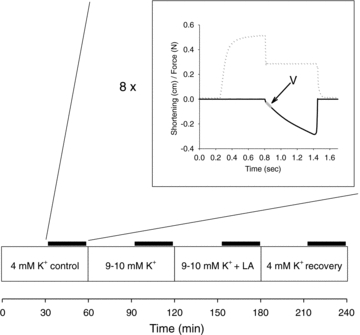
The lower part shows the time course of the whole experiment. Muscles were initially incubated for at 60–70 min in standard buffer (4 mm K+). During the last 30–40 min (indicated by thick line) 8 brief contraction protocols using different holding forces were elicited to record data for the force–velocity relationship. Thereafter the incubation buffer was changed as indicated. The inset shows a sample force (dotted line) and length trace from one contraction protocol where the holding force was reduced to a level below initial isometric contraction. The part of the length curve that is used for determination of velocity is indicated (V).
The dynamic contraction protocol was repeated 8 times separated by 3–5 min pauses using different levels of holding force of the lever arm: 35 g, 30 g, 25 g 20 g, 15 g, 10 g, 5 g and 3 g (2 g in some experiments). These force levels represents a range of values typically within 5–90% of isometric force. When muscles where incubated at high K+, the holding force levels were reduced to 15 g, 12 g, 10 g, 7 g, 5 g, 3.5 g, 2 g and 1 g to account for the lowering of force generating capacity by high K+.
Isometric force was recorded as the maximal force achieved immediately before the holding force step. For each experimental condition, the isometric force was calculated as the mean of this value for all eight contractions.
Experimental approach
The initial incubation was always standard buffer containing 4 mm K+ to establish a baseline. After 30 min equilibration eight dynamic contraction protocols were performed as described above. Here after muscles were incubated for 30 min in experimental buffers containing high K+ with or without lactic acid and again FV curves were constructed. Previous studies using similar muscles under similar conditions demonstrate that 30 min incubation is sufficient to obtain an almost full effect on muscle force of 10 mm K+ added to the incubation solution (Clausen & Nielsen, 2007). Finally muscles were transferred back to the standard buffer and a FV curve was again established (Fig. 1). This final incubation was used to check that muscle contractility was preserved throughout the experiment and that the effects of lactic acid, CO2 and high K+ could be reversed.
Curve fitting and statistics
The recorded force and length data were checked and relevant selections extracted using custom made software (SVX, Dept. Sport Science, Aarhus University, Denmark). Corresponding data points for velocity (v) and force (F) obtained from the dynamic contractions were fitted to the Hill equation:
For each muscle a FV relation was calculated for each experimental condition and the parameters a, b and Fo were obtained. From these parameters, Vmax, Pmax and curvature (a/Fo) were calculated. The curve fitting was performed using the curve fitting feature of the SigmaPlot 11.0 software (Systat Software Inc., San Jose, CA, USA).
All data are expressed as means ±s.e.m. The statistical significance of differences between treatments was ascertained using a one way ANOVA for repeated measures followed by a post hoc multiple comparisons analysis of pairwise differences between treatments using the Holm–Sidak method as provided by the statistical package in SigmaPlot 11.0. When comparing values between datasets obtained from different groups of muscles, Student's two-tailed t test for unpaired observations was used. Statistical significance was accepted at P < 0.05.
Results
Since muscles were used as their own control in most experiments, no systematic attempt was made to normalize the values of force, velocity and power to the size of the muscles. Due to the relatively narrow range of sizes in the rats used, the muscles (both soleus and EDL) were generally close to 25 mg. Thus in a subsample of experiments, soleus muscle weight and length were determined to be 25.3 ± 0.9 mg and 16.7 ± 0.2 mm, respectively (n= 7). This corresponds to a muscle volume of 23.9 ± 0.8 μl, when assuming a density of 1.06 g ml−1. Under control conditions (30°C at 4 mm K+) the Pmax of these muscles was 162 ± 6 g mm s−1 corresponding to 1594 ± 59 μW. This yielded a specific power of 67 ± 3 W (l muscle)−1.
Effects of acidification using lactic acid
In soleus muscles pre-incubated at 30°C at 4 mm K+, the addition of 20 mm lactic acid led to a slight increase in isometric force (8%, P < 0.05). However, Vmax was not significantly affected (Table 2). Furthermore, maximal power was unaffected by 20 mm lactic acid demonstrating that at the concentrations used here, no negative effects on dynamic muscle function could be attributed to lactic acid. Experiments using a higher concentration of lactic acid (24 mm) did give rise to a small but significant decrease in Vmax (9%, P < 0.05), and again a small but significant increase in isometric force (Table 2). However, Pmax was not significantly affected by incubation at 24 mm despite a further lowering of buffer pH and pHi (Table 1) and as shown in Fig. 2, the changes in the force–velocity relationship were only marginal. The curvature of the FV relation (a/Fo) was not significantly affected by incubation with lactic acid in any of the abovementioned experiments (Table 2).
Table 2.
Maximal force, velocity and power and curvature of the force–velocity relation in rat soleus muscles incubated in standard KRB buffer without or with lactic acid at 30°C
| Fmax (g) | Vmax (mm s−1) | Pmax (g mm s−1) | Curvature | |
|---|---|---|---|---|
| Control (n= 7) | 40.6 ± 1.9 | 34.7 ± 1.6 | 164 ± 11 | 0.36 ± 0.04 |
| + 20 mm LA (n= 7) | 42.6 ± 1.7*** | 34.0 ± 1.2 | 161 ± 7 | 0.33 ± 0.01 |
| Control (n= 6) | 41.5 ± 1.6 | 38.6 ± 2.2 | 177 ± 11 | 0.35 ± 0.02 |
| + 24 mm LA (n= 6) | 43.6 ± 0.7* | 35.6 ± 2.5*** | 172 ± 9 | 0.37 ± 0.03 |
LA: Lactic acid. Means ±s.e.m. Lactic acid induced difference from control conditions, *P < 0.05, ***P < 0.001
Figure 2. Effect of 24 mm lactic acid on the force–velocity curve of rat soleus at 30°C.
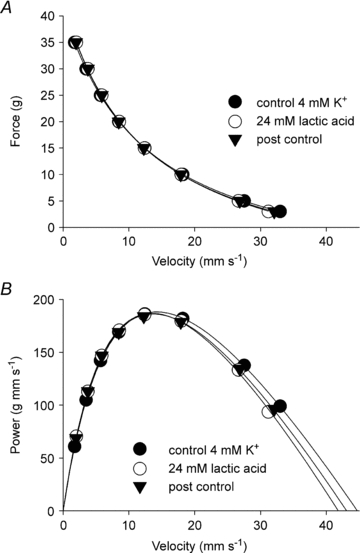
A, force–velocity curves fitted to data points from a representative muscle incubated at 4 mm K+ without lactic acid (pH 7.45, control), 4 mm K+ with 24 mm lactic acid (pH 6.8) and finally at 4 mm K+ again (pH 7.45, post control). B, same data as above but expressed as power versus velocity and fitted to power–velocity curves using the parameters obtained from the Hill fits in the top panel.
Many earlier observations have pointed toward a temperature sensitivity of the pH-induced effects on muscle contractility. We therefore repeated the experiments at a lower temperature (16°C, n= 11). As expected, in these experiments the baseline values of Fmax, Vmax and Pmax were considerably reduced compared to muscles incubated at 30°C. Furthermore it was found that incubation with 24 mm lactic acid significantly lowered Fmax, by 10% (33.0 ± 1.1g vs. 29.6 ± 0.9 g, P < 0.001), Vmax by 11% (8.0 ± 0.5 mm s−1vs. 7.1 ± 0.5 mm s−1, P < 0.01) and Pmax by 19% (25.1 ± 1.2 g mm s−1vs. 20.4 ± 0.8 g mm s−1, P < 0.001) compared to control conditions.
To determine if the effects of lactic acidosis were reversible, muscles were at the end of the experiment re-incubated in standard buffer with no lactic acid added and the determination of the FV relation was repeated. All measured variables returned to baseline levels in these experiments (data not shown).
In order to determine if the effects of lactic acid were related to fibre type, experiments were performed also on EDL muscles. EDL muscles of rats contain predominantly type IIb fibres, whereas solues muscles in the Wistar strain of rats contain predominantly type I fibres (Bar & Pette, 1988; Smith et al. 1988; Desplanches et al. 1990).
Not surprisingly, in the faster EDL muscles dynamic contractile function was considerably enhanced compared to soleus muscles. However, with respect to the effect of lactic acid the results were essentially similar to those obtained in soleus. Thus, incubation with 24 mm lactic acid increased isometric force from 43.0 ± 1.0 g to 47.2 ± 0.9 g (P < 0.01, n= 9) and decreased Vmax from 73.2 ± 3.4 mm s−1 to 62.2 ± 3.4 (P < 0.01, n= 9), whereas Pmax was not significantly changed (433 ± 37 g mm s−1vs. 475 ± 35 g mm s−1, P > 0.05, n= 9). In contrast to the experiments performed on soleus muscles, Fmax remained elevated and Vmax remained depressed when muscles were re-incubated for 30 min in standard buffer without lactic acid, indicating that these changes were not entirely reversible in EDL muscles (data not shown).
Effects of acidification using increased CO2 levels in equilibration gas
The acidification in the experiments described above was performed by adding lactic acid to a buffer containing 25 mm HCO3−. Using this buffering system an increase in the concentration of lactic acid above 25 mm would exceed the buffering capacity and produce a very large and uncontrollable acidification. For this reason, it was not possible to reduce pH further by increasing the concentration of lactic acid. Instead, further investigations of the dose–response relationship of pH change in dynamic contractions were based on CO2-induced acidification. Incubation with 24% CO2 induced pH changes comparable to those seen with 20 and 24 mm lactic acid (Table 1). Contractile parameters were affected only moderately (Fig. 3 and Table 3). Fmax, Vmax and curvature were not significantly changed, whereas, Pmax was reduced by 14% during incubation, and did not recover entirely upon returning to 5% CO2.
Figure 3. Effect of 24 and 53% CO2 on the force–velocity curve of rat soleus at 30°C.
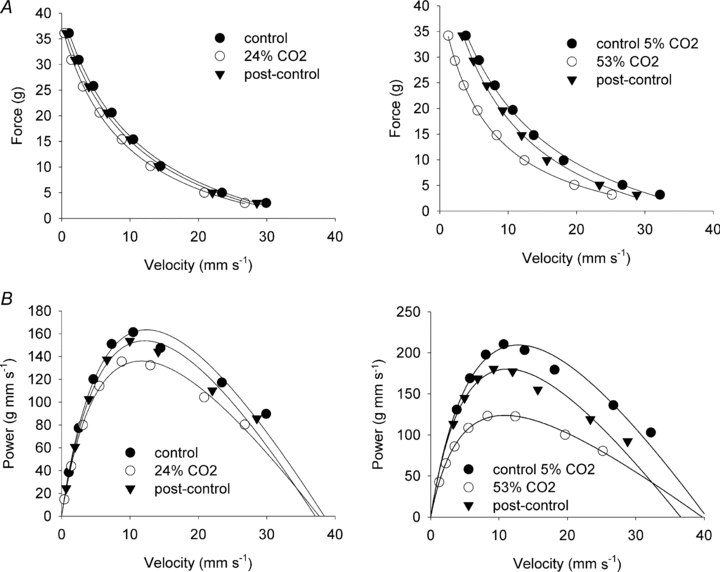
A, force–velocity curves fitted to data points from representative muscles incubated at 4 mm K+ using 5% CO2 in the equilibration gas (pH 7.45, control), followed by 24% CO2 (pH 6.9, left panel) or 53% (pH 6.5, right panel) in the equilibration gas and then again using 5% CO2 (pH 7.45, post control). B, same data as above but expressed as power versus velocity and fitted to power–velocity curves using the parameters obtained from the Hill fits in A.
Table 3.
Maximal force, velocity and power and curvature of the force–velocity relation in rat soleus muscles incubated in standard KRB buffer with 5% CO2, 24% CO2 or 53% CO2 at 30°C
| Fmax (g) | Vmax (mm s−1) | Pmax (g mm s−1) | Curvature | V3g | |
|---|---|---|---|---|---|
| 5% CO2 control (n= 5) | 41.2 ± 0.7 | 38.7 ± 1.7 | 165 ± 7 | 0.29 ± 0.02 | 29.6 ± 1.2 |
| 24% CO2 (n= 5) | 39.3 ± 0,3 | 37.3 ± 2.0 | 142 ± 6*** | 0.27 ± 0.02 | 26.9 ± 1.4*** |
| 5% CO2 recovery (n= 5) | 38.3 ± 1.7 | 37.7 ± 1.6 | 153 ± 11* | 0.30 ± 0.02 | 28.4 ± 1.4 ** |
| 5% CO2 control (n= 6) | 46.9 ± 1.2 | 43.6 ± 1.1 | 209 ± 6 | 0.28 ± 0.01 | 34.1 ± 0.6 |
| 53% CO2 (n= 6) | 39.9 ± 1.0*** | 43.9 ± 1,1 | 127 ± 5*** | 0.17 ± 0.01*** | 27.2 ± 0.6*** |
| 5% CO2 recovery (n= 6) | 42.9 ± 2.3 ** | 39.8 ± 1.3 * | 175 ± 8*** | 0.27 ± 0.01 | 30.8 ± 0.6 *** |
V3g: shortening velocity at 3 g holding force. Means ±s.e.m. Difference from control value, *P < 0.05, **P < 0.01, ***P < 0.001
Incubation with 53% CO2 reduced intra- and extracellular pH even further (Table 1) and gave a more pronounced negative effect on most aspects of dynamic contractile function in soleus muscles as shown in Fig. 3 and Table 3. Thus, Fmax was reduced by 16% and Pmax was reduced by 39%. Vmax was not changed compared to control conditions. A marked and consistent increase in curvature (a/Fo decreased significantly, Table 3) accounted for the large drop in Pmax despite maintenance of Vmax. Upon return to 5% CO2, force, power and curvature recovered towards initial control levels, although contractile function did remain somewhat depressed at the end of the experiment compared to initial control levels (Table 3).
Despite the lack of effect on Vmax it was evident that increased levels of CO2 slowed the muscle at all the levels of holding force used in our experiments (Fig. 3). Thus, even at the lowest holding force (3 g) there was a significant reduction in the measured velocity when CO2 was raised compared to the control situation (Table 3).
When the percentage of CO2 in the equilibration gas was increased, the percentage of O2 was reduced by an equal amount. Therefore, to investigate whether reduction of O2 in the equilibration gas per se affected dynamic contractility, experiments were done where half of the O2 was replaced by N2, while maintaining CO2 at 5%. In these experiments buffer pH remained constant at 7.45 and no significant effects on muscle dynamic contractility were found (e.g. Pmax was 172.8 ± 9.9 at 95% O2vs. 169.0 ± 11.6 at 47.5% O2, P > 0.05, n= 5), indicating that the effects of increased CO2 could not be ascribed to reduced O2 content.
Effects of high [K+]o on the FV curve
As shown in Fig. 4, there was a marked shift in the FV curve toward lower force and velocity when the [K+] of the incubation medium was increased from 4 to 10 mm. This change was particularly notable in the region of low velocities and high forces, whereas the high K+ curve converged towards the control curve at higher velocities.
Figure 4. Effects of 10 mm K+ on FV curves and power–velocity curves in soleus muscles before and after addition of 20 mm lactic acid.
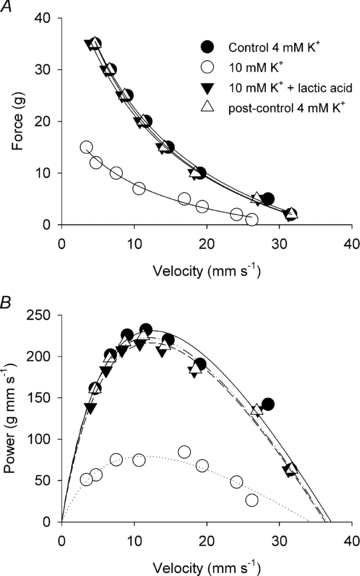
A, force–velocity curves fitted to data points from a representative muscle incubated at 4 mm K+ (control), 10 mm K+, 10 mm K+ with 20 mm lactic acid, and finally at 4 mm K+ again (post control). B, same data as above expressed as power versus velocity and fitted to power–velocity curves using the parameters obtained from the Hill fits in the top panel.
As shown in Figs 4, 5 and 6, increasing the [K+] of the incubation medium from 4 to 10 mm caused a significant decrease of 52 ± 5% in Fmax and a slightly larger and significant decrease in Pmax of 58 ± 5% (P < 0.001). However, Vmax and the curvature of the FV relation were not consistently changed by 10 mm[K+] (Fig. 5). A dose relation of the effects of high [K+] was observed in a series of experiments using 9 mm K+. Increasing the [K+]o from 4 to 9 mm caused a decrease in Fmax of 16 ± 2% accompanied by a decrease of 15 ± 2% in Pmax, whereas Vmax and curvature remained unchanged (n= 6).
Figure 5. Effects of 10 mm K+ on dynamic contractile variables in soleus muscles before and after addition of 20 mm lactic acid or 24% CO2.
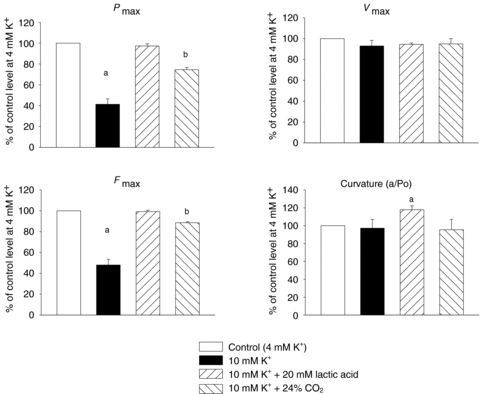
Pmax, Vmax and curvature were obtained from Hill fits of force–velocity data obtained during the indicated incubations. Fmax is average isometric force measured during the indicated incubations. All values are expressed as a percentage of the control level obtained at 4 mm K+. Bars represent means of 13 (10 mm K+), 8 (10 mm K++ 20 mm lactic acid) or 5 (10 mm K++ 24% CO2) muscles with error bars indicating standard error of means. aSignificant different from control conditions (P < 0.05); bsignificant different from control conditions and from 10 mm K+.
Figure 6. Relation between Pmax and Fmax in soleus muscles incubated at 9 or 10 mm K+.
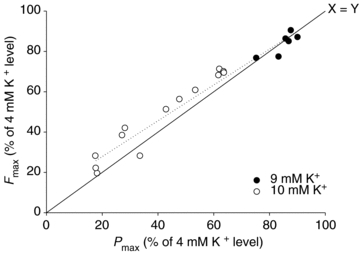
Data points from 18 individual muscles incubated at high K+ are plotted as a percentage of control levels obtained at 4 mm K+. Dotted line represents best linear fit of the data points (r= 0.98).
These results indicated that high [K+] predominantly affects the isometric force generating capacity of the muscles and that the change in Pmax could be seen as a direct consequence of this force loss. When plotting corresponding data sets of Fmax and Pmax from individual muscles, expressed relative to the control value, a tight linear correlation (r= 0.98) was obtained indicating that changes in isometric force may be considered a good indicator of maximal power in muscles exposed to high [K+], although the loss of power was slightly underestimated by the change in isometric force (Fig. 6).
Effects of acidification in muscles incubated at high [K+]o
When adding 20 mm lactic acid to muscles already incubated in 10 mm[K+]o, a near complete recovery of the FV curve was observed (Fig. 4). Thus, as summarized in Fig. 5, Fmax, Pmax, Vmax recovered completely to a level not significantly different from the control level obtained at 4 mm K+. The curvature was, however, significantly decreased as showed by the increase in a/Fo ratio (Fig. 5).
Following experiments with high K+ and lactic acid, muscles were re-incubated in standard buffer (4 mm K+) with no lactic acid and the dynamic contractility variables were measured again. These experiments confirmed that muscles could still perform at the initial baseline level and that effects of high [K+] and lactic acid were reversible (data not shown).
In muscles incubated at 10 mm K+ an increase of CO2 to 24% induced a significant recovery of Fmax and Pmax. However in contrast to the experiments with lactic acid, a full recovery was not obtained in Pmax or Fmax, which remained depressed by 24% and 12% compared to the initial control level, respectively (Fig. 5). Furthermore, in experiments where CO2 was increased to 53% in muscles incubated at 10 mm K+, significant but incomplete recovery was observed of Fmax and Pmax (from 34 to 70% and from 30 to 48% of initial control values, respectively, n= 3, P < 0.001).
Discussion
The present results show that in isolated whole muscle at near physiological temperatures the effects of moderate acidosis on dynamic muscle function were limited to modest changes in Fmax and Vmax that did not lead to significant impairment of Pmax. However, more severe levels of acidosis obtained with 53% CO2 significantly reduced Pmax in soleus muscles. Furthermore, it is shown that the elevation of [K+]o to 9–10 mm led to a pronounced depression of Pmax, which was of similar magnitude to the concomitant decrease in Fmax, while Vmax was unchanged. However, induction of moderate acidosis by addition of lactic acid elicited complete recovery of Pmax in muscles where dynamic function was depressed by high [K+]o, indicating that the previously described positive or protective effects of acidification dominated in this situation. Also acidification with CO2 produced positive effects on dynamic muscle function in muscles depressed by high [K+]o, although full recovery was not obtained using this procedure.
Effects of acidification with lactic acid and CO2
The present results show that in isolated rat soleus muscle at near physiological temperatures the effects on dynamic muscle function of acidosis induced by 20–24 mm lactic were limited to modest changes in Fmax and Vmax that did not lead to significant impairment of Pmax. Similar results were obtained with EDL muscles, which indicates that dynamic function is well preserved in both type I and type II fibres with this level of acidification. Previous studies have shown that incubating muscles with 20 mm lactic acid under similar conditions causes a shift of buffer pH from about 7.45 to 6.80 and of intracellular pH (pHi) from about 7.30 to 6.90 (Nielsen et al. 2001). With more severe acidification obtained with 53% CO2, which reduces buffer pH by about 1 pH unit to pH 6.5 and from extrapolation of previous data would be expected to reduce pHi by about 0.7 pH units (Nielsen et al. 2001), the present study did, however, observe pronounced negative effects on Fmax, Pmax and FV curvature. In contrast, we did not observe a decrease in Vmax even at these low pH levels. The Vmax is obtained by extrapolation from the Hill curve fitted to the force–velocity data points and may not reflect the true maximal unloaded shortening velocity. Looking at the actually measured data points it is clear that the 53% CO2 incubation slowed the muscle considerably at all levels of holding force (see example in Fig. 3), and even at the lowest force level measured (3 g) the shortening velocity was significantly slowed by 53% CO2 (Table 3). This indicated that slowing of the muscle is induced by acidification at all contractions where the load exceeds about 5% of Fmax. In dynamic actions in vivo the muscle will almost always be influenced by some external tension during activity due to its attachment to the skeleton. Thus, even shortening of the muscle without additional external loads will require at least lifting the load of the attached limb and, therefore, it can be argued that for practical considerations of muscle function it is less relevant to consider Vmax and more relevant to consider the changes to the FV curve at loads that are also found during movement under in vivo conditions. These results therefore indicate that in intact muscles, moderate acidification (pHi∼ 6.9) is without detrimental effects on dynamic contractile function, whereas severe acidification (pHi below 6.6) may reduce Fmax, Pmax and shortening velocity. This notion tallies with other studies on the effect of moderate reductions of pH in isolated muscle where the effects on Fmax and Vmax were determined from measurements of isometric force or muscle shortening at a single point on the FV curve (Adams et al. 1991; Nielsen et al. 2001; Nielsen & de Paoli, 2007). The notion also agree with studies on intact or skinned fibres showing that at near physiological temperatures, severe reductions in pH are necessary to significantly compromise dynamic muscle function (Pate et al. 1995; Westerblad et al. 1997; Knuth et al. 2006). Notably, however, Adams et al. (1991) found that even at a pHi as low as 6.4–6.5, increased CO2 had no effect on isometric force in perfused cat muscles. The reason for this high tolerance of these muscles to lowered pHi is not clear but may relate to the high experimental temperature (37°C).
In contrast to acidification with 20 or 24 mm lactic acid, acidification with 24% CO2 led to a small but significant reduction in Pmax despite the fact that the reductions in buffer and intracellular pH are almost equal under the two conditions (Nielsen et al. 2001; Hansen et al. 2005). The reason for this difference was probably that the tendency of a lower Vmax after addition of lactic acid was balanced by a small increase in Fmax that was not seen with CO2. A similar difference in the effect of acidification with lactic acid and CO2 has been observed previously for maximal tetanic force (Nielsen et al. 2001). The explanation for this effect of lactic acid on maximal force remains obscure but lately it was shown that a similar increase in maximal tetanic force can be induced by addition of 10 mm lactate at neutral pH despite this treatment having very little effect on pHi (de Paoli et al. 2010), indicating that it is related to an effect of the lactate molecules per se.
Effects of high K+
The force-depressing effect of extracellular potassium is well known, and is associated with the loss of excitability which occurs due to the depolarization of the membrane leading to inhibition of the generation and propagation of action potentials (for review see Nielsen & de Paoli, 2007). The dose-dependent decrease in isometric force at high K+ observed in this study was similar to earlier studies on isolated rat muscle (Cairns et al. 1995; de Paoli et al. 2007). However, we are not aware of any previously published data on the effects of high [K+]o on dynamic muscle function.
The effects of K+ on contractile function are mediated via a decrease in excitability of the muscle fibre membrane. Since this causes a block of the activation of the muscle fibres at a step that occurs prior to and is independent of the excitation–contraction coupling and cross-bridge cycling, elevated K+ is not expected to produce any changes in the cross bridge mechanics as such but simply to render a fraction of the muscle fibres inactive (Pedersen et al. 2005). Thus, we did expect that high K+ would reduce isometric force, and power, but not affect Vmax and curvature of the FV relation. This was largely confirmed by our findings. We did note that incubation at 10 mm K+ induced slightly larger relative decrease in Pmax than in isometric force indicating that some other aspect of the FV curve was affected. However changes in Vmax and curvature, which could account for this discrepancy, did not reach statistical significance. Nevertheless, it can be concluded that high extracellular K+ concentrations produce mostly similar relative changes in Pmax and Fmax.
Protective effect of lactic acid and CO2 in high K+
In 2001 it was shown that intracellular acidification protects muscle against the effects of depolarization by high [K+]o on excitability and isometric force (Nielsen et al. 2001). This effect was later shown to be related to a pH sensitive block of Cl− channels (Pedersen et al. 2004), which preserves excitability by reducing the short-circuiting Cl− current during action potentials. We found in the present study that this protective effect of lactic acid extends into the realm of dynamic muscle function as well, as indicated by the complete recovery of Pmax in 10 mm K+ with addition of lactic acid.
In experiments using 24 and 53% CO2 the protective effect in 10 mm K+ was somewhat dampened, presumably due to the co-existence of detrimental effects of reduced pH on muscle dynamic function due to changes at the cross-bridge level. It was evident, however, that even with severe acidification (53% CO2) the positive effects on excitability outweighed the negative effects caused by reduced cross-bridge function in whole muscles incubated at 10 mm K+.
Implications for muscle fatigue
A number of factors have been suggested to act as causative agents in the development of muscle fatigue including high K+ and acidification. Our results here suggest that lactic acidosis up to an extracellular concentration of 24 mm, which in the used preparation corresponds to a pHi of less than 6.8 (Nielsen et al. 2001), does not per se cause significant loss of power. Likewise, acidification with 24% CO2, which has been shown to reduce pHi to approximately 6.85 (Nielsen et al. 2001), had little depressing effect on maximal muscle power. On the contrary, the results show that at this level of intracellular acidification, it actually recovered the dynamic contractile function when muscles were depressed after depolarization by exposure to elevated extracellular K+. These results are in line with and extend earlier observations on the effects of K+ and lactic acid on maximal isometric contractions in isolated muscles (Nielsen et al. 2001; de Paoli et al. 2007). After intense exercise to exhaustion in vivo, interstitial [K+] is generally reported in the range of 8–12 mm (Mohr et al. 2004; Street et al. 2005). At the time of exhaustion the intracellular pH is reduced to a level close to 6.8 in studies using biopsy measurements (Bangsbo et al. 1996; Mohr et al. 2004). Together, these results indicate that moderate acidification is of limited importance for exercise-induced fatigue. However, in studies using magnetic resonance spectroscopy, more pronounced intracellular acidification (down to pHi of 6.4) has been observed during contractile activity in cat fast and slow twitch muscles (Adams et al. 1991) and human muscles (Nielsen et al. 2002). Furthermore, in our experiments use of 53% CO2 led to both a loss of power in normally polarized muscles and a less efficient recovery of force in depolarized muscles, suggesting that severe acidification to a level somewhere below an intracellular pH of 6.8 will induce detrimental effects on dynamic muscle function.
The importance for muscle fatigue of the ability of acidification to recover maximal power when muscles are depolarized by exposure to increased extracellular K+ is for two reasons difficult to estimate. Firstly, although studies on isolated muscles from our laboratory could not find any evidence of pH-related effects on the excitation-induced muscular K+-loss (Broch-Lips et al. 2007), studies on humans have reported an increased muscle K+ loss in acidified subjects (Street et al. 2005), indicating that acidification could lead to faster and more severe depolarization of muscles during exercise. Anyway, since the present study was done on resting muscles, where contractility was tested by short contractions (0.8 and 1.2 s) with 3 min rest intervals, the contribution of the excitation-induced loss of K+ from the muscle fibres to the extracellular K+ concentration was minimal. A possible effect of acidosis on muscle fibre K+ loss would, therefore, most likely not show up in these experiments.
Secondly, it is clear from our results that when muscles were exposed for 30 min to extracellular concentrations of K+ that were comparable to the levels of extracellular K+ observed in muscles of intensely working humans (Juel et al. 2000; Mohr et al. 2004; Street et al. 2005), their maximal power was significantly reduced. However, the depolarization occurring after a fast increase in extracellular K+ is dramatically slowed down by Cl− currents that tend to maintain the resting membrane potential at the equilibrium potential for Cl− (Hodgkin & Horowicz, 1959; Dutka et al. 2008). For that reason the depolarization induced by elevated extracellular K+ during brief intense exercise is most likely to be much smaller than the depolarization induced by prolonged exposure of muscles to the same level of extracellular K+, indicating that elevated K+ is of limited importance for exercise induced fatigue during exercise of short duration (for review see Allen et al. 2008). However, the present findings still indicate that in events of severe muscle depolarization, e.g. after prolonged elevations in extracellular K+ or during extensive build-up of extracellular K+, acidosis may have a role as a safety mechanism that tends to maintain muscle excitability. Moreover, the findings indicate that in such incidences, the positive effects of acidification will dominate over the negative effects even at a large reduction of pHi.
In the present study we mainly focused on a preparation containing predominantly slow twitch fibres. However, it is well known that fast twitch muscles are more likely to be acidified due to a higher glycolytic flux and they are more fatiguable and release more K+ during action potentials (Clausen et al. 2004). On the other hand, a higher K+ concentration is needed to induce force depression in fast twitch muscles (Cairns et al. 1997; Hansen et al. 2005) and Knuth et al. (2006) showed that the loss of power induced by pH reduction is only about half the magnitude in fast twitch fibres as the power loss in slow twitch fibres at 30°C. Therefore, although earlier studies have shown that lactic acid induces a protective effect on isometric force also in EDL (fast twitch muscles) depressed by high K+ (Hansen et al. 2005) it is quite likely that there are quantitative differences between fast and slow twitch muscles in the effects of K+ and pH on dynamic contractility.
In a study of dynamic muscle function in humans, a relative loss of power beyond the relative loss of force has been observed in fatigued muscles (Jones et al. 2006). This was related to an increased curvature during fatigue (decreased a/Fo). Such a change cannot be explained by the observed changes caused by high K+, but would be consistent with the change in curvature seen at 53% CO2. Furthermore, fatigue was associated with a slowing of the maximal shortening speed (Jones et al. 2006). Although our study shows that muscles were slowed by both high K+ and acidification with CO2 at force levels above 5% of Fmax, we could not demonstrate a general change in Vmax by these interventions. Therefore it seems likely that the large relative loss of power and reduced Vmax after fatigue observed by Jones et al. (2006) was related to other mechanisms than to high extracellular K+ and acidification of the muscles.
Conclusion
Dynamic performance of isolated rat soleus muscle is not compromised by lactic acid up to a concentration of 24 mm. Further acidification with CO2 reduces maximal power due to decreased capacity for isometric force development and changes in curvature of the FV relation. In addition, high extracellular [K+] inhibits maximal isometric force and maximal power, but not maximal contractile speed of muscles. Furthermore when muscles were influenced by high extracellular [K+], acidification exerts a positive effect on excitability that outweighs the negative effects and protects muscles against K+-induced loss of dynamic contractile function.
Acknowledgments
The authors thank Cuno Rasmussen, Vibeke Uhre and Annette Bache Nielsen for expert technical assistance. The study was supported by the Danish Ministry of Culture.
Glossary
Abbreviations
- EDL
extensor digitorum longus
- Fmax
maximal isometric force
- FV
force–velocity
- Lo
optimal length for isometric force development
- Pmax
maximal power
- Vmax
maximal shortening velocity
Author contributions
K.O., G.W.H. and O.B.N. contributed to conception and design of the experiments and collection, analysis and interpretation of data. K.O. and O.B.N. drafted the article and revised it critically for important intellectual content. All authors approved the final manuscript.
References
- Adams GR, Fisher MJ, Meyer RA. Hypercapnic acidosis and increased H2PO4− concentration do not decrease force in cat skeletal muscle. Am J Physiol Cell Physiol. 1991;260:C805–C812. doi: 10.1152/ajpcell.1991.260.4.C805. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol. 1996;495:587–596. doi: 10.1113/jphysiol.1996.sp021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar A, Pette D. Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Lett. 1988;235:153–155. doi: 10.1016/0014-5793(88)81253-5. [DOI] [PubMed] [Google Scholar]
- Broch-Lips M, Overgaard K, Praetorius HA, Nielsen OB. Effects of extracellular HCO3− on fatigue, pHi, and K+ efflux in rat skeletal muscles. J Appl Physiol. 2007;103:494–503. doi: 10.1152/japplphysiol.00049.2007. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Flatman JA, Clausen T. Relation between extracellular [K+], membrane potential and contraction in rat soleus muscle: modulation by the Na+-K+ pump. Pflugers Arch. 1995;430:909–915. doi: 10.1007/BF01837404. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Hing WA, Slack JR, Mills RG, Loiselle DS. Different effects of raised [K+]o on membrane potential and contraction in mouse fast- and slow-twitch muscle. Am J Physiol Cell Physiol. 1997;273:C598–C611. doi: 10.1152/ajpcell.1997.273.2.C598. [DOI] [PubMed] [Google Scholar]
- Chase PB, Kushmerick MJ. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988;53:935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Nielsen OB. Potassium, Na+,K+-pumps and fatigue in rat muscle. J Physiol. 2007;584:295–304. doi: 10.1113/jphysiol.2007.136044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Overgaard K, Nielsen OB. Evidence that the Na+-K+ leak/pump ratio contributes to the difference in endurance between fast- and slow-twitch muscles. Acta Physiol Scand. 2004;180:209–216. doi: 10.1111/j.0001-6772.2003.01251.x. [DOI] [PubMed] [Google Scholar]
- de Paoli FV, Ortenblad N, Pedersen TH, Jorgensen R, Nielsen OB. Lactate per se improves the excitability of depolarised rat skeletal muscle by reducing the Cl− conductance. J Physiol. 2010 doi: 10.1113/jphysiol.2010.196568. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paoli FV, Overgaard K, Pedersen TH, Nielsen OB. Additive protective effects of the addition of lactic acid and adrenaline on excitability and force in isolated rat skeletal muscle depressed by elevated extracellular K+ J Physiol. 2007;581:829–839. doi: 10.1113/jphysiol.2007.129049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplanches D, Mayet MH, Ilyina-Kakueva EI, Sempore B, Flandrois R. Skeletal muscle adaptation in rats flown on Cosmos 1667. J Appl Physiol. 1990;68:48–52. doi: 10.1152/jappl.1990.68.1.48. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka TL, Murphy RM, Stephenson DG, Lamb GD. Chloride conductance in the transverse tubular system of rat skeletal muscle fibres: importance in excitation–contraction coupling and fatigue. J Physiol. 2008;586:875–887. doi: 10.1113/jphysiol.2007.144667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Hansen AK, Clausen T, Nielsen OB. Effects of lactic acid and catecholamines on contractility in fast-twitch muscles exposed to hyperkalemia. Am J Physiol Cell Physiol. 2005;289:C104–C112. doi: 10.1152/ajpcell.00600.2004. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Horowicz P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DA, de Ruiter CJ, de Haan A. Change in contractile properties of human muscle in relationship to the loss of power and slowing of relaxation seen with fatigue. J Physiol. 2006;576:913–922. doi: 10.1113/jphysiol.2006.116343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R400–R406. doi: 10.1152/ajpregu.2000.278.2.R400. [DOI] [PubMed] [Google Scholar]
- Knuth ST, Dave H, Peters JR, Fitts RH. Low cell pH depresses peak power in rat skeletal muscle fibres at both 30°C and 15°C: implications for muscle fatigue. J Physiol. 2006;575:887–899. doi: 10.1113/jphysiol.2006.106732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Point: lactic acid accumulation is an advantage during muscle activity. J Appl Physiol. 2006;100:1410–1412. doi: 10.1152/japplphysiol.00023.2006. [DOI] [PubMed] [Google Scholar]
- Mohr M, Nordsborg N, Nielsen JJ, Pedersen LD, Fischer C, Krustrup P, Bangsbo J. Potassium kinetics in human muscle interstitium during repeated intense exercise in relation to fatigue. Pflugers Arch. 2004;448:452–456. doi: 10.1007/s00424-004-1257-6. [DOI] [PubMed] [Google Scholar]
- Nielsen HB, Hein L, Svendsen LB, Secher NH, Quistorff B. Bicarbonate attenuates intracellular acidosis. Acta Anaesthesiol Scand. 2002;46:579–584. doi: 10.1034/j.1399-6576.2002.460516.x. [DOI] [PubMed] [Google Scholar]
- Nielsen OB, de Paoli F, Overgaard K. Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol. 2001;536:161–166. doi: 10.1111/j.1469-7793.2001.t01-1-00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen OB, de Paoli FV. Regulation of Na+-K+ homeostasis and excitability in contracting muscles: implications for fatigue. Appl Physiol Nutr Metab. 2007;32:974–984. doi: 10.1139/H07-099. [DOI] [PubMed] [Google Scholar]
- Pate E, Bhimani M, Franks Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol. 1995;486:689–694. doi: 10.1113/jphysiol.1995.sp020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, de Paoli F, Nielsen OB. Increased excitability of acidified skeletal muscle: role of chloride conductance. J Gen Physiol. 2005;125:237–246. doi: 10.1085/jgp.200409173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG. Intracellular acidosis enhances the excitability of working muscle. Science. 2004;305:1144–1147. doi: 10.1126/science.1101141. [DOI] [PubMed] [Google Scholar]
- Sejersted OM, Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80:1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- Smith D, Green H, Thomson J, Sharratt M. Oxidative potential in developing rat diaphragm, EDL, and soleus muscle fibers. Am J Physiol Cell Physiol. 1988;254:C661–C668. doi: 10.1152/ajpcell.1988.254.5.C661. [DOI] [PubMed] [Google Scholar]
- Street D, Nielsen JJ, Bangsbo J, Juel C. Metabolic alkalosis reduces exercise-induced acidosis and potassium accumulation in human skeletal muscle interstitium. J Physiol. 2005;566:481–489. doi: 10.1113/jphysiol.2005.086801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lännergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]


