Abstract
Oxidative stress is often associated to inactivity-mediated skeletal muscle atrophy. Glutathione is one of the major antioxidant systems stimulated, both at muscular and systemic level, by activation of oxidative processes. We measured changes in glutathione availability, oxidative stress induction and the extent of atrophy mediated by 35 days of experimental bed rest in vastus lateralis muscle of healthy human volunteers. To assess muscle glutathione synthesis, we applied a novel single-biopsy and double-tracer ([2H2]glycine and [15N]glycine) approach based on evaluation of steady-state precursor incorporation in product. The correlations between the traditional (multiple-samples, one-tracer) and new (one-sample, double-tracer infusion) methods were analysed in erythrocytes by Passing–Bablok and Altman–Bland tests. Muscle glutathione absolute synthesis rate increased following bed rest from 5.5 ± 1.1 to 11.0 ± 1.5 mmol (kg wet tissue)−1 day−1 (mean ±s.e.m.; n= 9; P= 0.02) while glutathione concentration failed to change significantly. Bed rest induced vastus lateralis muscle atrophy, as assessed by pennation angle changes measured by ultrasonography (from 18.6 ± 1.0 to 15.3 ± 0.9 deg; P= 0.01) and thickness changes (from 2.3 ± 0.2 to 1.9 ± 0.1 cm; P < 0.001). Moreover, bed rest increased protein oxidative stress, as measured by muscle protein carbonylation changes (from 0.6 ± 0.1 to 1.00 ± 0.1 Oxydized-to-total protein ratio; P < 0.04). In conclusion, we developed in erythrocytes a new minimally invasive method to determine peptide synthesis rate in human tissues. Application of the new method to skeletal muscle suggests that disuse atrophy is associated to oxidative stress induction as well as to compensatory activation of the glutathione system.
Introduction
Glutathione kinetics can be assessed by primed-continuous infusion of stable isotopic amino acid precursors and gas chromatography – mass spectrometry (GC-MS) analyses (Darmaun et al. 2005; Biolo et al. 2008). The standard equation to calculate peptide synthesis rate considers the increase in isotopic product enrichment after achievement of steady state condition for isotopic precursor. This requires a single isotopic precursor infusion and at least two separate biological samples reflecting different infusion and incorporation times. Multiple in vivo muscle sampling can limit studies on protein and peptide turnover, due to possible effects on muscle physiology and to clear ethical implications. In this study we applied a novel method involving infusions of two different isotopes of the same amino acid as precursors ([2H2]glycine and [15N]glycine) and a single muscle biopsy. The reliability of the method was tested in erythrocytes within the same experimental frame.
Reactive oxygen species (ROS) production plays a detrimental role on biological substrates but the activity of antioxidant systems can limit consequences of ROS synthesis. Oxidative stress, in fact, is determined by the balance between ROS synthesis and efficiency of antioxidant systems and has been recently recognized as a pathogenetic factor of muscle wasting in selected diseases (Moylan & Reid, 2007). Physical inactivity, which is normally associated to muscle atrophy (Biolo et al. 2005), was previously demonstrated to enhance muscle ROS production (Lawler et al. 2003). In skeletal muscle, excess ROS production can upregulate nuclear factor-κB activity, in turn enhancing protein degradation by the ubiquitin-proteasome system (Kramer & Goodyear, 2007). Glutathione is an important antioxidant at whole body level (Dobrowolny et al. 2008). Among other factors, glutathione is deeply involved in muscle to neutralize ROS activity after physical exercise (Powers & Lennon, 1999). Its action is principally mediated by a reaction catalysed by glutathione peroxidase, leading to oxidized glutathione disulfides (Lu, 2000). Glutathione is, in fact, a thiol tripeptide synthesized in two separate biochemical reactions from glycine, glutamate and cysteine as precursor amino acids (Lu, 2000). Physiological conditions associated to increased ROS production, such as overfeeding and strenuous exercise, may lead to increased glutathione availability (Ji et al. 1992; Biolo et al. 2008). Otherwise, glutathione depletion is known to characterize several pathologies linked to oxidative stress, such as liver cirrhosis (Altomare et al. 1988), chronic obstructive pulmonary disease or acute respiratory distress syndrome (Anderson, 1997) and cardiovascular pathologies (Morrison et al. 1999). Thus, kinetic assessment of the glutathione peptide pool is fundamental to monitor the efficiency of the antioxidant response. Nonetheless, glutathione kinetics was previously measured only in human red blood cells (Darmaun et al. 2005; Biolo et al. 2008) and in rat skeletal muscle (Malmezat et al. 2000) but never before in human muscles. Unloading was previously shown to affect activity of antioxidant systems (Banerjee et al. 2003). In this study we aimed to assess in human volunteers the impact of physical inactivity on glutathione synthesis of atrophying muscle. To achieve this we applied our novel method to monitor the peptide synthesis rate in a single biopsy taken before and after 35 days of experimental bed rest, a reliable model to study human physical inactivity (Biolo et al. 2005). Additionally, in each muscle biopsy, oxidative stress damage on muscle proteins was assessed determining carbonylation levels.
Methods
Subjects
Ten healthy male subjects (age 24.1 ± 2.9 years; body mass index 23.4 ± 2.6 kg m−2) were selected to investigate the effects of experimental bed rest on muscle redox balance and glutathione synthesis rate. The project was approved by the ethical committee of the University of Ljubljana and the experimental protocol was in accordance to the Declaration of Helsinki (2004) as well as to the US Code of Federal Regulations – Protection of Human Subjects (2001). Written informed consent was obtained from each subject upon enrolment. All volunteers were physically active before the admission to the Valdoltra Hospital, University of Primorska, Ankaran-Capodistria, Slovenia (July 2007). Their body mass was stable for 3 months before the studies. Routine medical and laboratory analyses were performed to exclude chronic diseases. None of the subjects were regularly taking any medication.
Protocol
Subjects were admitted to the Valdoltra Orthopaedic Hospital (Slovenia) 3 days before the beginning of the experimental bed rest, for dietary and environmental adaptation period (Ambulatory period). An eucaloric diet was tailored to each subject by an expert dietician in order to maintain energy balance through the whole study period. To reach this aim, resting energy expenditure (REE) was calculated for each individual according to the FAO/WHO equations (Muller et al. 2004) and dietary energy requirements were designed for each subject multiplying REE by a factor of 1.4 or 1.1 in ambulatory (adaptation) or physically inactive (bed rest) conditions, respectively. The diet contained approximately 60% of energy from carbohydrate, 25% from fat, and 15% from protein. Dietary frequency and relative macronutrient content were planned to be the same during the 35 day bed rest period and during the pre-bed rest adaptation period. Subjects received every day three main meals (breakfast, lunch and dinner) and three snacks. All foods were exactly weighed for each participant, and volunteers were asked to consume the complete meal.
Energy balance control
Energy balance achievement and maintenance in each subject (n= 10) were controlled once per week through changes in whole body fat mass as assessed by bioimpedance analysis (BioScan 916s, Maltron, UK) (Dumler & Kilates, 2005). Bioimpedance measurements were performed according to manufacturer's instructions (four-electrode measurement system). Bioimpedance analysis was performed on all subjects and energy intake was adjusted according to weekly changes in fat mass (Biolo et al. 2008).
Metabolic testing
In the morning of the last day of the ambulatory period (baseline) and of the 33rd day of bed rest (day 33), a metabolic test with stable isotope tracer infusions and a single vastus lateralis muscle biopsy was performed in order to assess glutathione synthesis rate of muscle and red blood cells (Fig. 1). In the morning of the seventh bed rest day (day 7) we performed a metabolic study only with stable isotope tracer infusions to detect glutathione kinetics in red blood cells (Fig. 1). In each study day, two subjects were analysed. After a 12 h over-night fast (postabsorptive state) blood samples were taken before starting infusions, in order to assess in erythrocytes by GC-MS baseline natural enrichments of [2H2]glycine, [15N]glycine, l-[2H2]glutathione and l-[15N]glutathione. At 07.00 or 08.00 h (t0), a polyethylene catheter was inserted into an antecubital vein and a [2H2]glycine primed constant infusion (priming dose 26.5 μmol kg−1; infusion 26.5 μmol kg−1 h−1) was started and maintained throughout 7 h, i.e. until 14.00 or 15.00 h. Four hours after the beginning of [2H2]glycine infusion (11.00 or 12.00 h), a [15N]glycine primed constant infusion (priming dose 26.5 μmol kg−1; infusion 26.5 μmol kg−1 h−1) was started and maintained for 3 h. Arterialized blood samples were collected from a second polyethylene catheter inserted in a contralateral heated wrist vein after 3 h (t3) and 7 h (t7) from the beginning of [2H2]glycine infusion. The last blood sample (t7) was taken at the end of [2H2]glycine (7 h) and [15N]glycine (3 h) infusions. Infusions performed on day 7 in volunteer no. 8 failed to be completed. Thus, glutathione kinetics in red blood cells were assessed on 10 subjects at baseline and after bed rest, while only on nine subjects at day 7.
Figure 1. Metabolic test.
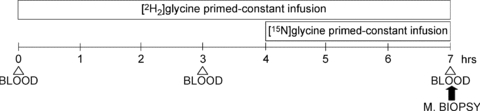
BLOOD, blood sample; M. BIOPSY, vastus lateralis muscle biopsy. The metabolic study was scheduled to apply and validate the novel one-sample and double-tracer approach (see Methods) for glutathione kinetics assessment in a single muscle biopsy. A [2H2]glycine primed (26.5 μmol kg−1) infusion (26.5 μmol (kg h)−1) was started at the beginning of the study and a second primed (26.5 μmol kg−1) infusion (26.5 μmol kg−1 h−1) of [15N]glycine was started 4 h later. Enrichments of precursors ([2H2]glycine and [15N]glycine) and of products (l-[15N]glutathione and l-[2H2] glutathione) were measured in the single final biopsy (7th hour). l-[15N]Glutathione enrichment reflects short term tracer incorporation (3 h) while l-[2H2]glutathione enrichment reflects long term incorporation (7 h). We then calculated muscle glutathione kinetics applying the one-sample, double-tracer equation based on the difference between measured product enrichments (changes over time). For validation of the approach, isotopic enrichments of [2H2]glycine and l-[2H2]glutathione were measured in two different blood samples taken 3 and 7 h after the metabolic study began. The traditional equation was applied to calculate glutathione synthesis rate in red blood cells. Blood samples were drawn at baseline, day 7 and day 33 while muscle biopsies were taken at baseline and day 33.
Muscle sampling
At baseline and day 33, after local anaesthesia, at t7 (i.e. at 14.00 or 15.00 h), a muscle biopsy was taken from the vastus lateralis in sterile conditions using conchotome forceps according to standard techniques; muscle fibres were immediately cleaned from visible fat or connective tissue and accurately dried to remove blood with a sterile gauze. Quality of the procedure was monitored by microdissection microscope. Samples (averaging in mass 120 mg) were immediately frozen in liquid nitrogen and stored at −80°C. At the end of muscle biopsy procedure, both infusions were stopped and catheters removed. Muscle sampling at day 33 failed to be completely performed in subject no. 2.
Glutathione kinetics and concentration in muscle and erythrocytes
Glutathione kinetics and concentration evaluations in muscle could be performed only on nine subjects. The procedure for GC-MS analysis of muscle glutathione and glycine isotopic enrichments was adapted from Lyons et al. (2001 and Biolo et al. (2008). Total glutathione concentrations were determined using GC-MS and the internal standard technique. Defrosted biopsies were weighted, homogenized in 500 μl sulphosalicylic acid (SSA) (6.5%) and known amounts (6.4 nmol final) of [glycine-13C215N]glutathione (Cambridge Isotope Laboratories, Andover, MA, USA) were added as internal standard. After centrifugation, 1 ml of ice-cold dithiothreitol (DTT, 20 mm in 1 m acetic acid) was added to supernatant. Proteins were precipitated with 400 μl of SSA 30% and 15 min centrifugation at 10,000 g at 4°C. The supernatant was transferred to an ion exchange column (AG 50W-X8 cation exchange resin, Bio-Rad, Hercules, CA, USA), washed with Milli-Q water (5 ml ×2). Glutathione was eluted using NH4OH (3 m, 4 ml). Frozen, lyophilized samples reacted with 500 μl of DTT solution (20 mm in 0.5 m acetic acid) at 100°C and dried again in nitrogen flow. After reaction with 300 μl of HCl/methanol solution (250 μl of 36% HCl in 7.5 ml methanol), incubation for 30 min at 80°C and drying under nitrogen flow, samples further reacted with 50 μl N-methyl-N-tert-butyl-dimethysilyl-trifluoroacetamide (MTBSTFA) and 50 μl of acetonitrile for 40 min at 90°C before injection into a gas chromatography mass spectrometer (HP 5890, Agilent Technologies, Santa Clara, CA, USA).
The GC-MS analysis of glutathione and glycine were carried out using a fixed silica capillary column (HP-5MS 25 mm ID, 0.25 μm film thickness). Column temperature was programmed at 10°C min−1 from 160 to 300°C (detector temperature was 260°C) and helium was used as the carrier gas. The derivative was measured under an electron impact ionization by selective ion monitoring at a nominal mass-to-charge ratio (m/z) of 363, 364 and 365 for glutathione enrichment, and of 218, 219 and 220 for glycine enrichment. To measure [glycine-13C215N]glutathione (internal standard) enrichments the derivative was measured at a nominal m/z ratio of 363 and 366.
A direct assessment of natural (before infusion beginning) isotopic enrichments in muscle could not be performed for reasons related to the study design. To estimate values of natural isotopic enrichments in muscle biopsies, red blood cell samples collected at t0 in each subject were analysed. Repeated measures were performed on specific amounts of red blood cells selected in order to provide glutathione chromatographic peak areas comparable with those obtained in muscle biopsies (t7). Mean values were chosen as representing baseline natural isotopic enrichments in muscle. We adopted such approach because the whole body pool of amino acids, as precursors of glutathione, is characterized by a rapid turnover and by a high exchange rate across cell membrane. Thus, in a fasting state condition, it is difficult to hypothesize different distributions of natural isotopic enrichments of precursors in separate cell types. Moreover, background glycine isotopic enrichments in circulating erythrocytes were previously demonstrated, in animals, to approximate enrichments measured in other tissues (bone marrow) (Hibbert et al. 2001). Moreover, the use of background enrichment of blood protein to assess muscle protein turnover has been directly validated in humans (Heys et al. 1990). To assess glutathione concentrations in muscles a standard calibration curve was generated. A known amount of [glycine-13C215N]glutathione (internal standard) was added to prepared solutions containing different concentrations (serial dilution) of unlabelled glutathione (Sigma-Aldrich Co., St Louis, MO, USA). Glutathione and [glycine-13C215N]glutathione isotopic enrichments were measured monitoring appropriate mass-to-charge ratios following the same extraction and derivatization procedure adopted for muscle biopsies. Ratios between measured enrichments (m/z 363 and 366) allowed standard calibration curve assessment. By interpolation analysis unlabelled glutathione concentrations could be measured in muscle biopsy samples.
Glutathione synthesis rate measurements in red blood cells were performed in 10 subjects before and after bed rest while analyses at day 7 could be performed only in nine subjects. Procedures to determine glutathione kinetics in red blood cells were adapted from previous work (Biolo et al. 2008).
Protein carbonylation
To directly assess muscle protein oxidation level, about 20 cryosections (12 μm) of each biopsy were solubilized at 4°C in 0.01% tetrafluoroacetic acid containing protease inhibitors, 5 mm EDTA and 2%β-mercaptoethanol. The Oxyblot Oxidized Protein Detection Kit (Chemicon/Millipore; Billerica, MA, USA) can detect carbonyl groups formed in protein side chains as a consequence of oxidation. Derivatization using 2,4-dinitrophenylhydrazine was performed for 15 min following the manufacturer's instruction on 6 μg of protein (Vescovo et al. 2008). Protein final mixture was separated by electrophoresis on 10% SDS polyacrylamide gel. Proteins transferred to nitrocellulose membranes were stained by Red Ponceau and scanned. Carbonylation level of actin and tropomyosin, as identified by standard Western blot analysis were detected by blots incubation with anti-4-dinitrophenyl hydrazine antibody followed by chemiluminescence development. Actin was chosen as one of the most abundant proteins in muscle while tropomyosin was chosen principally considering its important physiological role during fibre contraction. Densitometry was performed on scanned autoradiographic films using an NIH image system (ImageJ). To allow the comparison of oxidation level between different samples we defined the oxidative index (Oxy RP−1) as the ratio between the densitometric values of the Oxyblot bands (oxidation level) and Red Ponceau stained bands (protein content). This value directly reflects the degree of myofibrillar protein oxidation. On each gel two standard samples (one positive and one negative control) were always loaded. The Oxyblot analysis was performed at day 33 in only nine subjects because, as mentioned above, muscle sampling in subject no. 2 failed to be completely performed.
Muscle thickness and architecture
To monitor muscle atrophy progression we assessed before (Baseline) and after (Day 33) bed rest thickness of the vastus lateralis muscle in the supine position, by ultrasound imaging using portable ultrasound (Mylab25, Esaote, Genova, Italy) fitted with a 7–10 MHz linear probe (Reeves et al. 2004). Sagittal ultrasound images (Fig. 2) were obtained at 50% of muscle length measured along the mid-sagittal axis, after identification of the proximal and medial bone insertions of muscle. Muscle thickness was expressed in centimetres, as the vertical distance between muscle superficial and deep aponeuroses at an equidistant point from right and left borders of the image. Muscle thickness could not be measured in subject no. 8. Vastus lateralis muscle architecture was measured in the supine position using realtime B-mode ultrasonography (ATL-HDI 3000, Bothell) with a 40 mm, 7.5 MHz linear-array probe. Scans were taken before and after bed rest with knee joint in anatomical position (passively fully extended). Measurements were performed at 50% of muscle length (previously estimated with ultrasound), in the midsagittal plane. To ensure that all scanning measurements were taken in the same anatomical location, the ultrasound probe was positioned in the midsagittal plane, orthogonal to the mediolateral axis and its positioning was marked on acetate paper using moles and small angiomas (which may be assumed to maintain a fixed position) as reference points (Fig. 2). In each ultrasound image obtained at rest, the fascicular path was determined as the interspaces between echoes coming from the perimysial tissue surrounding the fascicle (Fig. 2). Pennation angle was measured using Matlab (The MathWorks Inc., Natick, MA, USA). Pennation angle was calculated as the angle between the fascicle and the deep aponeurosis of the muscle. In each scan, the average length and pennation angle of three fascicles were used for analysis (de Boer et al. 2008). Pennation angle was measured on all subjects.
Figure 2. Ultrasound imaging of pennation angle.
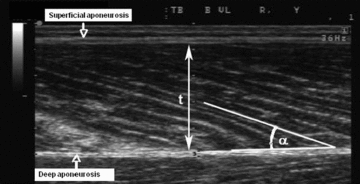
Typical ultrasound image of the human vastus lateralis muscle obtained in the sagittal plane using a linear 7.5 MHz probe. Muscle fibres fascicles are clearly visible as the structures stretching from the superficial and deep aponeuroses. t, muscle thickness; α, pennation angle.
Calculations
In red blood cells, enrichments of [15N]glycine were calculated as tracer-to-tracee ratios as follows:
| (1) |
where E15N-Gly is [15N]glycine enrichment, PAR is peak areas ratio between areas measured for m/z values indicated in brackets, ti is one of the sampling times after steady state achievement (t3 or t7) and t0 is the time of sampling before isotope infusion beginning (for natural enrichments). To calculate [2H2]glycine enrichment in red blood cells, correcting the influence of [15N]glycine infusion, the following equation was applied:
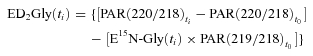 |
(2) |
where ED2-Gly is enrichment of [2H2]glycine and the other definitions are the same as in eqn (1). In muscle biopsies, enrichments of [15N]glycine were calculated as follows:
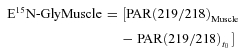 |
(3) |
where Muscle refers to measurements performed in muscle biopsies and the other definitions are the same as in eqns (1) and (2). Similarly, in muscle biopsies, enrichments of [2H2]glycine were calculated as follows:
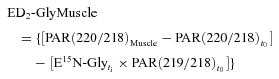 |
(4) |
where definitions are the same as in eqns (1), (2) and (3). Noteworthy, in eqns (3) and (4), t0 measurements were performed in red blood cells (see eqn (2) and eqn (1)). Enrichments of l-[15N]glutathione were assessed as follows:
| (5) |
where E15N-GSH is enrichment of l-[15N]glutathione and other definitions are the same as in previous equations. Enrichments of l-[2H2]glutathione were assessed as follows:
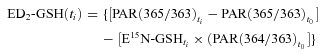 |
(6) |
where ED2-GSH is enrichment of l-[2H2]glutathione and other definitions are the same as in previous equations. To calculate glutathione fractional synthesis rate (FSR) by stable isotope tracers and using enrichment data obtained in a single tissue sample we have developed the following equation:
 |
(7) |
where ED2GSH(t7) and E15N-GSH(t7) are respectively l-[2H2]- and l-[15N]glutathione enrichments at the seventh hour of the metabolic study; ED2-Gly(t7) and E15N-Gly(t7) are respectively precursor enrichments of [2H2]- and [15N]glycine at the seventh hour of the metabolic study; D2-Gly inf. duration and 15N-Gly inf. duration are respectively duration of [2H2] and [15N]glycine tracer infusions. To express FSR as % day−1, 100 and 24 coefficients were applied. The present equation relies on two parallel and separate infusions of different isotopes of the same precursor, [2H2]glycine and [15N]glycine, started at the beginning of the metabolic study (t0) and 4 h later, respectively. Precursor enrichments at steady state ([2H2]glycine and [15N]glycine) and product enrichments after 3 h (l-[15N]glutathione) and 7 h (l-[2H2] glutathione) of infusion were measured in a single biological sample taken at the end of the metabolic study period (7 h). So, we obtained the evaluation of product enrichment changes over time (the core parameter for FSR assessment) as difference between two single differently labelled product enrichments measured within only one biological sample. In this method l-[15N]glutathione enrichment, in fact, reflects short term tracer incorporation as [15N]glycine infusion is started 3 h before the single final biological sampling. On the contrary, l-[2H2]glutathione enrichment reflects long term tracer incorporation as [2H2]glycine infusion is started 7 h before the single final biological sampling. By this approach, glutathione FSR can be calculated over a net 4 h incorporation period, as difference between differently labelled product enrichments measured at the same time point. Additionally, each product enrichment is normalized by precursor isotopic tracer enrichment at steady state. To validate the new equation, the traditional approach was applied to calculate glutathione FSR within the same experimental frame. We calculated glutathione FSR in red blood cells taking into account isotopic enrichments of l-[2H2]glutathione measured after achievement of the steady state condition for [2H2]glycine precursor enrichment in three different biological samples taken 3 h (t3) and 7 h (t7) after metabolic study beginning.
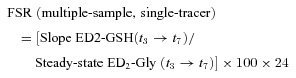 |
(8) |
where Slope ED2-GSH (t3→t7) is the slope of l-[2H2]glutathione product enrichments measured at t3 and t7, Steady-state ED2-Gly (t3→t7) is the enrichment of [2H2]glycine precursor at steady state. To express FSR as % day−1, 100 and 24 coefficients were applied. The absolute synthesis rate (ASR) was calculated as the product of FSR and glutathione (GSH) concentrations in studied tissues: ASR = FSR ×[GSH].
Statistical analysis
Data are presented as means ±s.e.m. In order to detect significant changes mediated by 5 weeks of bed rest we applied Student's t test for paired samples. P= 0.05 was chosen as the threshold for statistical significance. The relationship between variables was analysed by bivariate correlation using Spearman's test. Statistical analysis was performed using SPSS statistical software (v. 12; SPSS, Inc., Chicago, IL, USA). To validate the new ‘one sample’ method, glutathione FSR absolute values or pooled changes from baseline to day 7 and to day 33 measured in erythrocytes by ‘traditional’ approach were correlated to the same measurements performed by the ‘one sample’ approach. Regression line analysis of such correlations was performed by the Passing–Bablok test. In addition, the Altman–Bland plot was applied to further validate the two methods. These analyses were performed by MedCalc (v. 11.2.1.0; MedCalc Software; Mariakerke, Belgium).
Subjects inclusion in correlation analyses
Infusions performed on day 7 in volunteer no. 8 were stopped for technical reasons. Thus, correlation analysis between absolute values of FSR measurements performed in red blood cells by one sample and standard approaches, was carried out on 29 values. Consequently, correlation analysis between FSR changes assessed by both methods was performed only on 18 values.
Muscle sampling in subject no. 2 failed to be completely performed because of technical problems at day 33, and thus protein carbonylation levels were measured on nine subjects. Muscle thickness failed to be measured in subject no. 8, so that only eight subjects were included in correlation analyses between muscle thickness changes and protein carbonylation changes.
Results
Baseline body weight (72.8 ± 3.3 kg) displayed a bed rest mediated significant reduction (−2.3 ± 0.2 kg, P= 0.005) after 33 days. As assessed by bioimpedance, baseline fat free mass (59.6 ± 2.0 kg) was significantly (P < 0.001) reduced after 33 days of bed rest (−2.9 ± 0.4 kg). In contrast, fat mass measured before bed rest failed to change significantly during the experimental period (from 13.8 ± 2.0 kg at baseline to 13.1 ± 1.8 kg at day 33, P= 0.06).
Glutathione synthesis in erythrocytes: method validation
Steady state of [2H2]glycine enrichment in red blood cells was achieved after 3 h of primed-continuous infusion and maintained for the following 4 h (non-significant difference of [2H2]glycine enrichments at the seventh hour vs.[2H2]glycine enrichments at the third hour) (Fig. 3A). The mean value of steady state [2H2]glycine enrichment at the end of bed rest was significantly greater when compared to the pre-bed rest period (P < 0.05). In all three phases (baseline, day 7 and day 33) [15N]glycine enrichment mean values measured at the end of the infusion (7th hour) were greater (P < 0.05) than the corresponding [2H2]glycine enrichment values. Increases of l-[2H2]glutathione enrichments over time (in all three study phases) due to [2H2]glycine incorporation into the final product are displayed in Fig. 3B. To validate the single biopsy method against the traditional one to assess muscle glutathione synthesis rate, calculations were performed by both eqns (7) and (8) in blood samples drawn during three metabolic tests (baseline, day 7 and day 33 of bed rest). Glutathione FSR (% day−1) values measured in red blood cells at baseline, day 7 and day 33 by the ‘traditional’ approach (eqn (8)), despite an apparent tendency to a constant overestimation, showed no statistical difference from values measured by the ‘one-sample’ method (eqn (7)) (Fig. 4). Independently from the calculations used, no significant bed rest mediated changes in glutathione FSR were observed in red blood cells, either at early time points (day 7) or at the end of the experimental period (day 33) (Fig. 4). Pooled absolute values of glutathione FSR measured in each study phase by ‘traditional’ and ‘one-sample’ approaches were displayed to be in a significant (P < 0.001) and direct correlation (n= 28) (Fig. 5A). Passing–Bablok regression analysis of correlation between absolute glutathione FSR values measured in each study phase by ‘traditional’ and ‘one-sample’ approaches shows intercept A and slope B values are contained in their relative confidence interval (Table 1, regression analysis 1). An Altman-Bland plot of the same data set (Fig. 5A) shows only two measurements are outside the confidence interval. Equally, pooled FSR changes from baseline to day 7 and to day 33 measured by ‘traditional’ and ‘one-sample’ approaches were shown to be in a significant (P < 0.001) direct correlation (n= 19) (Fig. 5B). Passing–Bablok regression analysis of correlation between glutathione FSR changes from baseline to day 7 and to day 33 measured in each study phase by ‘traditional’ and ‘one-sample’ approaches shows intercept A as well as slope B values are included in their relative confidence interval (Table 1, regression analysis 2). An Altman–Bland plot of the same data set (Fig. 5B) shows only one measurement is outside the confidence interval.
Figure 3. Enrichments of isotopic tracers and products before, during and after bed rest in red blood cells.
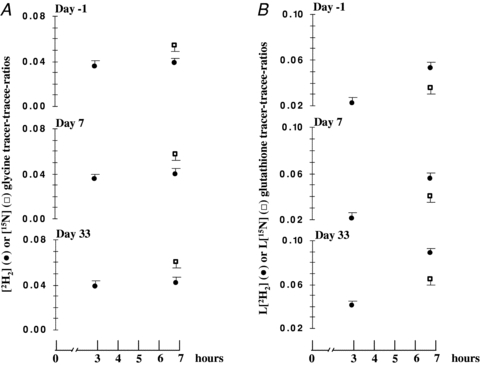
Red blood cells enrichments of isotopic tracers ([2H2] and [15N]glycine) and products ([2H2] and l-[15N]glutathione). A, steady state for [2H2]glycine (•) precursor pool is shown while steady state condition for [15N]glycine (□) pool is assumed. Diversity between tracer isotope steady state values is due to intrinsic metabolic differences between 2H2 and 15N isotopes. B, l-[2H2]glutathione (•) enrichment slope increase reflects linear tracer incorporation into glutathione products. Diversity between l-[2H2]glutathione and l-[15N]glutathione (□) product enrichments measured at the end of infusions (7th hour) is the result of different tracer incorporation times. [2H2]glycine was, in fact, infused for 7 h while [15N]glycine was infused for 3 h.
Figure 4. Bed rest impact on erythrocytes’ glutathione kinetics.
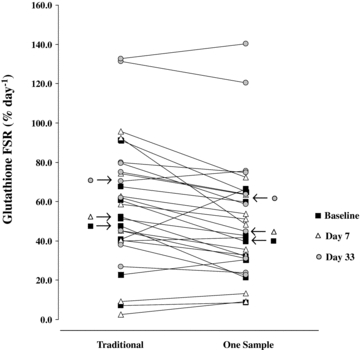
Red blood cells’ glutathione fractional synthesis rate (FSR) individual values measured by both the traditional and one sample approaches at selected study phases (Baseline, Day 7 and Day 33). Significant differences between FSR measured by traditional and novel one-sample approaches failed to be displayed in each study phase. No significant influence of bed rest on FSR values was shown, regardless of the equation applied.
Figure 5. Method validation.
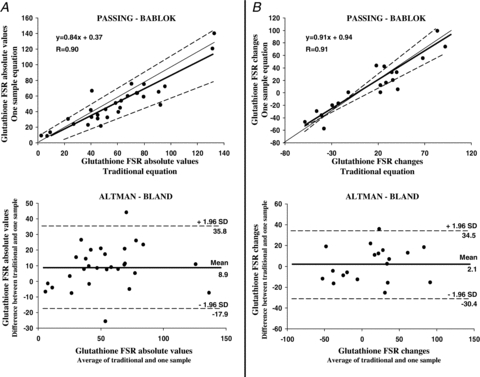
Traditional and one-sample approaches validation by Altman–Bland method and by analysis of linear regression of correlations by Passing–Bablok approach, applied to measurements of glutathione kinetics in erythrocytes. A, glutathione fractional synthesis rate (FSR) absolute values (% day−1) measured in red blood cells by ‘traditional’ and by ‘one-sample’ approach are in positive and significant (P < 0.001) linear correlation (Passing–Bablok plot, upper part) (n= 29). As evidenced in Table 1 slope and intercept are contained in their relative confidence intervals as analysed by Passing–Bablok test. Altman–Bland plot (lower part) of glutathione FSR absolute values shows only two measurements are outside the confidence interval and that data distribution across the mean can be considered as satisfactory. B, pooled changes of glutathione FSR (% day−1) from baseline to day 7 and to day 33 measured by ‘standard’ approach are in significant and positive (P < 0.001) linear correlation with analogous changes in glutathione FSR measured by the ‘one-sample’ approach (Passing–Bablok plot, upper part) (n= 19). As evidenced in Table 1, slope and intercept are contained in their relative confidence intervals as analysed by Passing–Bablok. An Altman–Bland plot of glutathione FSR changes shows only one measurement is outside the confidence interval and that data distribution across the mean can be considered as satisfactory. In Passing–Bablok plots regression lines are represented as continuous thick lines, identity lines (x=y) as continuous thin lines while limits of confidence intervals are dashed lines. In Altman–Bland plots the mean value line is represented by a continuous thick line while limits of confidence intervals are dashed lines.
Table 1.
Passing–Bablok regression analysis comparing ‘traditional’ and ‘one-sample’ approaches to measure red blood cell glutathione FSR
| Regression analysis 1 | ||
| Variable X | Variable Y | |
| Absolute glutathione FSR values (traditional equation) | Absolute glutathione FSR values (one-sample equation) | |
| Value | 95%c.i. | |
| Intercept A | −0.02 | from −11.32 to 7.01 |
| Slope B | 0.86 | from 0.68 to 1.04 |
| Cusum test for linearity | No significant deviation from linearity (P > 0.05) | |
| Regression analysis 2 | ||
| Variable X | Variable Y | |
| Glutathione FSR changes (traditional equation) | Glutathione FSR changes (One-sample equation) | |
| Value | 95%c.i. | |
| Intercept A | 0.95 | from −9.10 to 1.47 |
| Slope B | 0.91 | from 0.75 to 1.20 |
| Cusum test for linearity | No significant deviation from linearity (P > 0.05) | |
Table shows results of two separate regression line analyses performed by the Passing–Bablok method (see Fig. 5). Regression analysis 1 was performed to compare absolute glutathione fractional synthesis rate (FSR) values measured by ‘traditional’ approach (X variable) with the same values measured by the ‘one-sample’ (Y variable) approach. Regression analysis 2 was performed to compare changes from baseline to day 7 and to day 33 of glutathione FSR measured by the traditional equation (Variable X), with the same values measured by one-sample equation (Variable Y). Both analyses show inclusion in the relative confidence interval (95%c.i.) of each Intercept A and Slope B value characterizing obtained regression lines. Additionally, in both analyses linear distribution was confirmed by an appropriate test.
Bed rest induced atrophy and oxidative stress in skeletal muscle
In vastus lateralis both muscle thickness and fibre pennation angle were found to be significantly decreased from baseline ambulatory condition at bed rest day 33 (Fig. 6). In addition, muscle protein carbonylation levels were shown to be significantly increased after 33 days of bed rest by Oxyblot analysis (Fig. 6) (Dalla Libera et al. 2009). Muscle protein carbonylation changes mediated by 33 days of bed rest were inversely correlated to vastus lateralis thickness changes induced during the same experimental period (Fig. 7).
Figure 6. Bed rest effects on vastus lateralis muscle atrophy and oxidative stress.
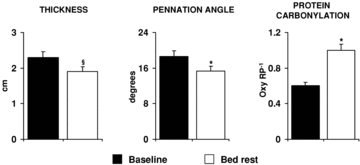
Values of vastus lateralis thickness, fibre pennation angle and protein carbonylation measured before (Baseline) and after 33 days of bed rest (Bed rest) are shown. Muscle thickness and fibre pennation angle were determined in supine position by ultrasonography approaches. Protein carbonylation was determined by Oxyblot analysis. Oxy RP−1, ratio between quantified oxidized proteins and Red Ponceau stained total protein. §P < 0.001 vs. Baseline; *P < 0.05 vs. Baseline. Statistical analysis was performed by Student's t test.
Figure 7. Correlations between bed rest mediated changes in muscle atrophy and redox markers in vastus lateralis.
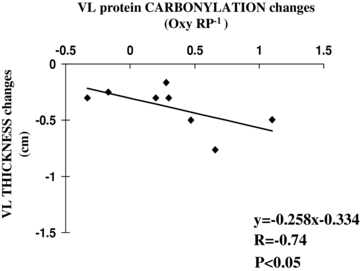
The figure shows a significant linear indirect correlation between protein carbonylation and vastus lateralis (VL) muscle thickness changes mediated by bed rest. Relationship between variables was analysed by bivariate correlation using Spearman's test. Continuous line represents the regression line.
Bed rest upregulated muscle glutathione synthesis
Values of tracer-to-tracee ratios (TTRs) for [15N] and [2H2]glycine as well as for l-[15N]- and l-[2H2]glutathione measured in muscle biopsy (Fig. 1) at the end of isotopic infusions are shown in Table 2. Bed rest failed to significantly affect the enrichment of each precursor ([15N]- and [2H2]glycine) as well as the enrichment of each isotopic product (l-[15N]- and L-[2H2]glutathione). In each study phase, [15N]glycine enrichment was found to be significantly higher than [2H2]glycine. Glutathione fractional synthesis rate (FSR) displayed a tendency to be increased during bed rest (Table 3). Glutathione concentrations did not change significantly during bed rest. Nevertheless, glutathione absolute synthesis rate (ASR) was significantly upregulated by bed rest at day 33 when matched to baseline (Table 3).
Table 2.
Muscle glycine and glutathione tracer-tracee ratios at the end of infusions
| Baseline | Bed rest | |
|---|---|---|
| [15N]Glycine | 0.0571 ± 0.0040 | 0.0581 ± 0.0062 |
| l-[15N]Glutathione | 0.0138 ± 0.0025 | 0.0103 ± 0.0036 |
| [2H2]Glycine | 0.0421 ± 0.0027 | 0.0411 ± 0.0027 |
| l-[2H2]Glutathione | 0.0273 ± 0.0021 | 0.0317 ± 0.0052 |
Muscle glycine and glutathione tracer-to-tracee ratios measured in vastus lateralis muscle are shown. Data were derived from isotopic enrichments measured by gas chromatography–mass-spectrometry analyses (see Methods) performed in muscle biopsies taken at the end of metabolic tests performed in ambulatory conditions (Baseline) and after 33 days of bed rest (Bed rest) on nine subjects (see Fig. 1). When matched to baseline, bed rest values of tracer-to-tracee ratios failed to display significant differences. Data are presented as means ±s.e.m. Statistical analysis was performed by Student's t test.
Table 3.
Bed rest effect on vastus lateralis muscle glutathione kinetics parameters
| Glutathione | Baseline | Bed rest | P |
|---|---|---|---|
| FSR (% day−1) (n= 9) | 268 ± 61 | 408 ± 47 | 0.07 |
| Concentration (mmol (kg wet tissue)−1) (n= 10) | 2.3 ± 0.2 | 2.7 ± 0.1 | 0.30 |
| ASR (mmol (kg wet tissue)−1 day−1) (n= 9) | 5.5 ± 1.1 | 11.0 ± 1.5 | 0.02 |
Glutathione fractional synthesis rate (FSR), concentration and absolute synthesis rate (ASR) measured in vastus lateralis by metabolic tests (Fig. 1) in ambulatory conditions (Baseline) and after 33 days of bed rest (Bed rest). Values were derived from isotopic enrichments measured by gas chromatography–mass spectrometry analyses performed in muscle biopsies taken at the end of metabolic tests. Concentration values were assessed by internal standard approach, while FSR and ASR were calculated by the novel one-sample, double-tracer approach. Data are presented as means ±s.e.m. Statistical analysis was performed by Student's t test.
Discussion
In the present work, we assessed the impact of prolonged physical inactivity on muscle glutathione synthesis applying, before and after 35 days of bed rest, a novel method involving a single biopsy and a double infusion of two isotopic glycine precursors. We showed that in atrophied muscles, bed rest significantly enhanced glutathione availability as well as fibre oxidative damage.
Novel single-sample method to determine peptide synthesis by constant tracer infusions
The core parameter for traditional determination of peptide FSR is the assessment of product enrichment changes over time during continuous infusion of isotopic precursor tracer (Wolfe, 2004). When precursor incorporation into product is linear, FSR can be determined evaluating at least two single product enrichments in two separate biological samples taken at different times. We measured in a single tissue sample muscle glutathione synthesis rate applying a modified precursor–product approach. Isotopic glycine was chosen as the precursor tracer (Jahoor et al. 1995), and the linearity of incorporation into glutathione as final product was shown previously (Biolo et al. 2008). Moreover, the distribution of the present values of l-[2H2]glutathione enrichments measured in blood samples taken at the third and seventh hour of infusion as well as at an intermediate point failed to deviate from linearity (data not shown). Our novel method was based on performing two parallel and separate infusions of different isotopes, [2H2]glycine and [15N]glycine, starting at different times (4 h shift) (Fig. 1). In this way, as evidenced in the Calculations section, difference between l-[15N]glutathione and l-[2H2]glutathione enrichments measured in a single final biological sample, allowed FSR assessment. In order to validate our ‘one-sample double tracer’ approach to measure glutathione kinetics in human muscle, we assessed in human red blood cells glutathione FSR in two different ways: the traditional approach (eqn (8)) and a novel approach (eqn (7)). To calculate for method validation glutathione FSR in red blood cells by the traditional approach, we considered labelled product enrichments measured at the third and at the seventh hour of a single precursor infusion ([2H2]glycine). As the novel approach was designed for muscle assay by a single final muscle biopsy, for method validation in red blood cells calculations involved only the final blood sample but both tracer infusions ([2H2]glycine and [15N]glycine; see Calculations). Values of glutathione FSR obtained in red blood cells by ‘traditional’ and ‘one-sample’ methods were not significantly different (Fig. 4) and were shown to be in high and significant correlation (Fig. 5): this evidence suggests the novel method is in accordance with the traditional one. Passing–Bablok regression line analysis of correlations between absolute values of glutathione FSR obtained by ‘traditional’ and ‘one-sample’ methods strengthens the validation of the new technical protocol (Fig. 5A). This statistical approach, in fact, can quantitatively describe parameters validating a new method when matched to a standard one, with no assumptions regarding sample distribution (Passing–Bablok, 1983). Inclusion of intercept A value in the confidence interval demonstrates, in fact, that no constant difference between the two methods can be evidenced. Similarly, B slope value belonging to its relative confidence interval underlines that there are not significant proportional differences between the two methods. It is worth noting that the data distribution fails to significantly deviate from linearity. Even though these results strongly suggest the reliability of our novel approach, also the Altman–Bland test was performed on the analysed data set. The Altman–Bland confidence interval ranges from –17.9 to 35.8% day−1 and data are well distributed suggesting no proportional errors. Moreover, the presence of only two measurements outside the confidence interval can be considered as a satisfactory condition. A constant bias seems to appear from localization of the mean value line, but this effect can derive from the fact that the dataset was constituted by repeated measures during the bed rest period.
We further analysed the reliability of our new approach (Fig. 5B) by statistical tests comparing pooled FSR changes from baseline to day 7 and to day 33 measured by the traditional approach, with the same changes measured by the one-sample equation. Similarly to data analysis performed on absolute values of glutathione FSR, Passing–Bablok and Altman–Bland tests were performed. The Passing–Bablok test confirms that the ‘one-sample’ method applied to bed rest mediated changes is not plagued by a proportional or constant error. Additionally, in analogy with the abovementioned analysis, no significant deviation from linearity of data distribution was observed. In parallel, the Altman–Bland plot shows only one measurement is outside a sufficiently narrow confidence interval including well distributed values. Thus, taken together, our results suggest that our ‘one-sample’ method can be reliably utilized in vivo to assess glutathione FSR. The impossibility, for ethical reasons, of directly matching glutathione FSR measures performed by both methods in serial blood samples with the same values assessed in serial muscle biopsies represents an unavoidable limitation potentially plaguing the application to muscle.
Absolute values of tracer steady-state enrichments in red blood cells were different: [15N]glycine enrichment was found to be higher than [2H2]glycine. These two isotopic amino acid were infused at the same rate and publications comparing 2H and 15N labelled glycine are at present lacking: thus such a discrepancy between measured steady-state enrichments cannot be directly explained. Nevertheless, in a previously published work (Yang et al. 1984), infusion of [15N]alanine isotopic tracer induced a higher steady state enrichment than [2H]alanine infusion, even though the two tracers were administered at the same infusion rate. As alanine shares with glycine analogous metabolic pathways of synthesis and utilization, published evidence for alanine tracers can support our present data regarding glycine tracers. However, it is very important to underline that this peculiar feature characterizing [15N]glycine versus[2H2]glycine fails to affect reliability of our FSR estimation as, in order to exclude direct influences of absolute values of precursor enrichments, ratios between isotopic product and related tracer enrichments were considered in the equation. Finally, the strength of our method mainly stems from the abovementioned validation performed against the well assessed traditional one by separate statistical analyses. It is worthy of note that two reliable assumption were necessary to build our new equation and method. (i) While achievement of steady-state conditions after 3 h for [2H2]glycine enrichments was previously published (Biolo et al. 2008) and directly assessed (data not shown), the steady state condition was assumed for [15N]glycine after an equal time and rate of infusion. This seems to be acceptable by itself but in addition [15N]glycine was previously published to reach a steady state in plasma within, or even before, the third hour of infusion in similar conditions (Cryer et al. 1986). (ii) Different labelling of isotopic precursors was reasonably considered not to affect tracer uptake into the respective final product: isotopic labelling per se is, in fact, known not to influence general tracer incorporation in products (Wolfe, 2004).
The utility to assess protein or peptide FSR in a single sample stems from drawbacks linked to multiple sample collection. During investigations on small animals, the first tissue biopsy can lead to the animal's death while in bigger animals or in humans, multiple tissue sampling can lead to inflammatory process activation. For the same reasons and for clear ethical implications, complex metabolic studies requiring multiple tissue sampling are impossible to perform during surgery in human subjects. Approaches aimed to measure peptide FSR in a single biological sample were previously published. Dudley et al. (1998) employed for the first time a multiple-tracer and single-sample method. The protocol was based on six staggered and overlapping isotopomer infusions. FSR was obtained designing a posteriori an enrichment curve. Protocol validation was indirectly performed by comparing plasma free amino acid turnover rates. An analogous technique aimed at measuring muscle protein fractional breakdown rate (FBR) and FSR in a single muscle biopsy has been also proposed by Zhang et al. (2002). In this method, three pulse tracer injections of three different isotopic amino acid precursors were staggered at different time points. The authors demonstrated that three different enrichments assessed in a unique final muscle biopsy allowed FBR evaluation. Differently from previous publications, we directly validated in human subjects a new single sample method involving only two separate isotopic infusions to reliably assess a peptide FSR: our approach simplifies calculations and reduces technical workloads as well as economic expense.
Muscle atrophy and oxidative status
Bed rest is a well recognized model of physical inactivity to study the consequences of muscle unloading on human volunteers (Stein & Wade, 2005). For such reasons we investigated the effect of a prolonged period of bed rest on muscle atrophy and architecture. We evidenced muscle wasting by whole body impedance analysis as changes in fat free mass. Moreover, we showed enhanced vastus lateralis muscle atrophy after prolonged bed rest (Tauler et al. 2006) in terms of decreased vastus lateralis thickness and fibre pennation angle. Pennation angle was previously demonstrated to be correlated to muscle shape. Ageing (Morse et al. 2005) and immobilization (Narici & Cerretelli, 1998; Reeves et al. 2002), in fact, were shown to negatively affect muscle architecture leading to a deep reduction of fibre pennation angle and to a concomitantly enhanced muscle atrophy (de Boer et al. 2007). For such reasons, the observed decrease in pennation angle was considered to occur in parallel with muscle atrophy.
Muscle unloading was demonstrated in animal models to increase reactive oxygen species production (Lawler et al. 2003) and, in parallel, oxidative stress was shown to activate proteolysis by the ubiquitin proteasome system (Powers & Lennon, 1999; Bar-Shai et al. 2008). Published results, obtained in in vitro or animal models suggested a link between physical inactivity, muscle oxidative stress induction and consequent atrophy (Powers et al. 2005; Bar-Shai et al. 2008). As muscle wasting is an important clinical feature of several chronic diseases and oxidative stress has been recognized as pathogenetic factor in such process (Moylan & Reid, 2007), we further investigated in humans the role of oxidative stress in muscle damage induction during inactivity. The increase in protein carbonylation we observed as a consequence of 35 days of bed rest by Oxyblot analyses can be associated with enhanced muscle fibre damage secondary to oxidative stress induction in vastus lateralis. Protein carbonylation, in fact, occurs as a consequence of ROS action on carbon groups leading to altered enzyme structure and activity (Stadtman, 2001) and quantification of carbonylated proteins was previously demonstrated to be a reliable marker of the occurrence of oxidative stress (Greilberger et al. 2008). Protein carbonylation is significantly associated with disease progression in muscle wasting patients with leukaemia (Ahmad et al. 2008). Proteins oxidatively modified by carbonylation are selectively degraded by the 20S core proteasome without ubiquitination (Grune et al. 2003) and there is evidence that, to avoid accumulation of damaged peptides, carbonylated proteins are more efficiently and rapidly scavenged by proteolytic degradation than their non-oxidized counterparts (Dukan et al. 2000; Bota & Davies, 2002; Grune et al. 2003): this suggests an association between upregulation of protein carbonylation and increased proteolysis, possibly leading to muscle atrophy. In this paper we show a linear inverse relationship between changes in protein carbonylation and vastus lateralis thickness, suggesting that oxidative damage by carbonylation in human muscle proteins could be one of the possible pathways leading to muscle atrophy. Nevertheless, carbonylation changes failed to relate to changes in fibre orientation, suggesting that other oxidative processes, such as for example reactive nitrogen species (Bar-Shai & Reznick, 2006), could be involved in regulation of this parameter. Otherwise, different mechanisms affecting muscle protein turnover, such as changes in insulin-like growth factor expression, could contribute to alterations of muscle morphology (Clemmons, 2009b). Further studies are required to understand pathways linking unloading and muscle architecture changes. Additional complexity in the relationships linking physical activity level and redox condition comes from published evidence showing strenuous exercise can induce fibre skeletal muscle damage (Close et al. 2004) by enhanced ROS production. In contrast, long term moderate training upregulated antioxidant systems leading to improved redox balance (Gomez-Cabrera et al. 2008).
When excessive ROS production occurs, antioxidant system activation is triggered (Pastore et al. 2003) in order to limit the final damage to biological substrates. Between several enzymic and non-enzymic mechanisms activated to reduce oxidative damage, the glutathione system is quantitatively an important antioxidant in muscle (Dobrowolny et al. 2008). This tripeptide is synthesized in order to scavenge hydroperoxides by self-oxidation and dimerization. Physiological conditions associated with increased ROS production may lead to increased glutathione availability (Ji et al. 1992; Biolo et al. 2008). Thus, kinetic assessment of glutathione pools effectively monitors oxidative stress onset. By the novel and validated one-sample and double isotopic tracer infusion approach, we assessed bed rest impact on muscle glutathione synthesis rate. Our data demonstrate that after 33 days of bed rest, muscle glutathione synthesis is significantly increased. This underlines that in humans, unloading can enhance glutathione antioxidant activity in muscle: such an effect can be strongly hypothesized to be a response to an increased reactive oxygen species production. Previous animal studies yielded conflicting results about the effect of physical inactivity on muscle glutathione regulation. Glutathione concentrations were, in fact, shown to be negatively affected in rat unloaded muscle (Ikemoto et al. 2002b) and activities of key enzymes in the glutathione system such as glutathione reductase and glutathione peroxidase were demonstrated to be increased (Sen et al. 1992), unaltered or downregulated (Tauler et al. 2006). Reasons leading to such a wide range of results can be ascribed to model and experimental design differences. Glutathione assays in muscle biopsies can be theoretically biased by the presence of red blood cells within explanted fibres. This is an intrinsic limitation for muscle studies but, in this work, its impact was strongly minimized by accurate cleaning of each biopsy. The glutathione FSR that we measured in red blood cells and in muscles were strongly different. A previous publication (Flaring et al. 2009) showed slightly different glutathione turnover rates in erythrocytes and in muscles, but another work (Malmezat et al. 2000) showed glutathione turnover rate in muscle can be three time higher than in erythrocytes. Moreover, additional publications showed that glutathione synthesis rate is markedly higher in muscles when compared to other tissues (Griffith & Meister, 1979) and that glutathione turnover is particularly low in red blood cells (Mortensen et al. 1956). Thus, the present glutathione FSR measurements performed in muscle and in red blood cells are in line with previous publications. Moreover, in this context, published values of glutathione concentrations in muscle are distributed over a quite wide range, starting from 0.5 mmol (kg wet tissue)−1 going up to 1.7–1.8 mmol (kg wet tissue)−1 (Griffith & Meister, 1979; Luo et al. 1998; Medved et al. 2004; Rutten et al. 2008). We measured in healthy subjects a muscle glutathione concentration around 2 mmol (kg wet tissue)−1: considering the range of published data, the concentrations we measured in healthy young subjects can be considered as reliable.
Upregulation of glutathione synthesis was here considered to be a consequence of previously occurring oxidative protein carbonylation in atrophying muscle after bed rest. Nevertheless statistically significant correlation between bed rest mediated changes of muscle protein oxidation and glutathione availability failed to be shown in this work: this probably was due to the reduced sample size but also to the possible involvement of other antioxidant systems as for example heme oxidase (Dalla Libera et al. 2009). Additionally, probably for the same abovementioned reasons, in the present work we failed to observe a reliable correlation between glutathione synthesis rate and muscle atrophy markers. Nevertheless, previous publications suggest that altered availability of glutathione could affect clinical conditions and muscle wasting in patients. Decreased glutathione levels, in fact, in sarcopaenic critically ill patients (Biolo et al. 2007a) can be considered as an inefficient antioxidant response to oxidative stress. Moreover, glutathione depletion was demonstrated to influence clinical outcome (Crimi et al. 2006), as well as symptoms and severity of selected pathologies (Najim et al. 2007). It is noteworthy that diseases downregulating muscle antioxidant systems are associated with cachexia (Laviano et al. 2007). Conversely, an increased bioavailability of glutathione precursors such as cysteine or N-acetyl-cysteine was previously found to ameliorate glutathione system scavenging action (Badaloo et al. 2002) and to reduce, in an animal model, muscle protein ubiquitination (Ikemoto et al. 2002a). Thus, dietary glutathione precursor supplementation is likely to ameliorate clinical conditions of muscle wasted critically ill patients. Results presented in this work propose that oxidative stress can be a physical inactivity mediated alteration leading to muscle atrophy, but other metabolic factors can affect body composition during immobility (Clemmons, 2009a).
Energy intake level is a critical factor for muscle mass maintenance control in bed resting patients. We previously demonstrated that negative (Biolo et al. 2007b) and positive (Biolo et al. 2008) energy balance can worsen muscle atrophy during bed rest, respectively affecting whole body protein turnover and systemic inflammation. Moreover, fat mass gain was shown to enhance in bed resting subjects erythrocyte glutathione synthesis while fat maintenance prevented this response (Biolo et al. 2008). In the present study diet was controlled to maintain a constant energy balance and glutathione evaluations were also performed in erythrocytes. Consistently with previous observations, erythrocyte glutathione synthesis failed to be affected by bed rest. In this study we could not directly display the effects of energy balance changes on muscle oxidative stress and antioxidant defence activation during inactivity.
Conclusions
We firstly applied a novel isotope tracer method to assess glutathione kinetics in human muscles. Our approach, involving a single muscle biopsy and infusion of only two separate isotopic amino acid tracers, can be considered as a significant technical improvement in the field of metabolic research, both for physiological and practical reasons. Within the same experimental frame, the technical protocol was tested against the traditional one matching, by correlations and specific regression line analyses, measurements performed only in red blood cells. Afterwards the novel one-sample method was applied to assess glutathione synthesis rate in muscle biopsies. A specific comparison between assessments performed in blood and muscle by both methods could not be performed owing to ethical concerns limiting serial biopsies. This could be a possible but unavoidable limitation to the application of the novel method in muscle.
In addition, we assessed the impact of prolonged inactivity on muscle oxidative stress development, glutathione antioxidant response and muscle atrophy. We showed that bed rest can induce oxidative damage in human vastus lateralis fibres and that this effect is paralleled by a significant muscle mass reduction. In addition, prolonged physical inactivity induced in muscles of healthy human volunteers a significant increase in glutathione availability that could be considered as an antioxidant response to a previously elicited ROS production. Such evidence suggests that redox imbalance in muscle can contribute to muscle atrophy during unloading but additional metabolic mechanisms must be taken into account to fully explain sarcopaenia mediated by unloading. Nevertheless these data and published evidence suggest that, along with other nutritional countermeasures, supplementation of glutathione precursors in inactive subjects could be an eligible strategy to minimize muscle atrophy.
Acknowledgments
We thank all the volunteers and the staff of the Valdoltra Orthopaedic Hospital (Koper, SLO). The authors acknowledge the valuable dietetic assistance of Mrs L. Vouk-Grbac and special thanks should be addressed to Mrs Olivera Rakovic Bošnjak for her precious nursing assistance during the entire experimental study. We finally acknowledge the excellent technical assistance of Mrs Mariella Sturma for performance of the analyses and Marta Maurutto for useful help during metabolic studies. This study was supported by grants from Italian Space Agency (ASI) OSMA 2006–2009.
Glossary
Abbreviations
- ASR
absolute synthesis rate
- FSR
fractional synthesis rate
- GSH
glutathione
- REE
resting energy expenditure
- ROS
reactive oxygen species
Author contributions
Study conception and design: F.A., S.M., I.B.M., R.P., G.B.; data analysis, collection and interpretation: F.A., L.D.L., J.R., S.M., M.J., L.G., G.B.; drafting of the article: F.A., S.M., G.B.; critical revision of the article: F.A., L.D.L., J.R., S.M., M.J., G.B., G.B., I.B.M., R.P. All authors approved the final version for publication.
References
- Ahmad R, Tripathi AK, Tripathi P, Singh S, Singh R, Singh RK. Malondialdehyde and protein carbonyl as biomarkers for oxidative stress and disease progression in patients with chronic myeloid leukemia. In Vivo. 2008;22:525–528. [PubMed] [Google Scholar]
- Altomare E, Vendemiale G, Albano O. Hepatic glutathione content in patients with alcoholic and non alcoholic liver diseases. Life Sci. 1988;43:991–998. doi: 10.1016/0024-3205(88)90544-9. [DOI] [PubMed] [Google Scholar]
- Anderson ME. Glutathione and glutathione delivery compounds. Adv Pharmacol. 1997;38:65–78. doi: 10.1016/s1054-3589(08)60979-5. [DOI] [PubMed] [Google Scholar]
- Badaloo A, Reid M, Forrester T, Heird WC, Jahoor F. Cysteine supplementation improves the erythrocyte glutathione synthesis rate in children with severe edematous malnutrition. Am J Clin Nutr. 2002;76:646–652. doi: 10.1093/ajcn/76.3.646. [DOI] [PubMed] [Google Scholar]
- Banerjee AK, Mandal A, Chanda D, Chakraborti S. Oxidant, antioxidant and physical exercise. Mol Cell Biochem. 2003;253:307–312. doi: 10.1023/a:1026032404105. [DOI] [PubMed] [Google Scholar]
- Bar-Shai M, Carmeli E, Ljubuncic P, Reznick AZ. Exercise and immobilization in aging animals: the involvement of oxidative stress and NF-κB activation. Free Radic Biol Med. 2008;44:202–214. doi: 10.1016/j.freeradbiomed.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Bar-Shai M, Reznick AZ. Reactive nitrogen species induce nuclear factor-κB-mediated protein degradation in skeletal muscle cells. Free Radic Biol Med. 2006;40:2112–2125. doi: 10.1016/j.freeradbiomed.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Biolo G, Agostini F, Simunic B, Sturma M, Torelli L, Preiser JC, Deby-Dupont G, Magni P, Strollo F, di Prampero P, Guarnieri G, Mekjavic IB, Pisot R, Narici MV. Positive energy balance is associated with accelerated muscle atrophy and increased erythrocyte glutathione turnover during 5 wk of bed rest. Am J Clin Nutr. 2008;88:950–958. doi: 10.1093/ajcn/88.4.950. [DOI] [PubMed] [Google Scholar]
- Biolo G, Antonione R, De Cicco M. Glutathione metabolism in sepsis. Crit Care Med. 2007a;35:S591–S595. doi: 10.1097/01.CCM.0000278913.19123.13. [DOI] [PubMed] [Google Scholar]
- Biolo G, Ciocchi B, Stulle M, Bosutti A, Barazzoni R, Zanetti M, Antonione R, Lebenstedt M, Platen P, Heer M, Guarnieri G. Calorie restriction accelerates the catabolism of lean body mass during 2 wk of bed rest. Am J Clin Nutr. 2007b;86:366–372. doi: 10.1093/ajcn/86.2.366. [DOI] [PubMed] [Google Scholar]
- Biolo G, Ciocchi B, Stulle M, Piccoli A, Lorenzon S, Dal MV, Barazzoni R, Zanetti M, Guarnieri G. Metabolic consequences of physical inactivity. J Ren Nutr. 2005;15:49–53. doi: 10.1053/j.jrn.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- Clemmons DR. Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol Metab. 2009a;20:349–356. doi: 10.1016/j.tem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Clemmons DR. Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol Metab. 2009b;20:349–356. doi: 10.1016/j.tem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Close GL, Ashton T, Cable T, Doran D, MacLaren DP. Eccentric exercise, isokinetic muscle torque and delayed onset muscle soreness: the role of reactive oxygen species. Eur J Appl Physiol. 2004;91:615–621. doi: 10.1007/s00421-003-1012-2. [DOI] [PubMed] [Google Scholar]
- Crimi E, Sica V, Williams-Ignarro S, Zhang H, Slutsky AS, Ignarro LJ, Napoli C. The role of oxidative stress in adult critical care. Free Radic Biol Med. 2006;40:398–406. doi: 10.1016/j.freeradbiomed.2005.10.054. [DOI] [PubMed] [Google Scholar]
- Cryer DR, Matsushima T, Marsh JB, Yudkoff M, Coates PM, Cortner JA. Direct measurement of apolipoprotein B synthesis in human very low density lipoprotein using stable isotopes and mass spectrometry. J Lipid Res. 1986;27:508–516. [PubMed] [Google Scholar]
- Dalla Libera L, Ravara B, Gobbo V, Tarricone E, Vitadello M, Biolo G, Vescovo G, Gorza L. A transient antioxidant stress response accompanies the onset of disuse atrophy in human skeletal muscle. J Appl Physiol. 2009;107:549–557. doi: 10.1152/japplphysiol.00280.2009. [DOI] [PubMed] [Google Scholar]
- Darmaun D, Smith SD, Sweeten S, Sager BK, Welch S, Mauras N. Evidence for accelerated rates of glutathione utilization and glutathione depletion in adolescents with poorly controlled type 1 diabetes. Diabetes. 2005;54:190–196. doi: 10.2337/diabetes.54.1.190. [DOI] [PubMed] [Google Scholar]
- de Boer MD, Maganaris CN, Seynnes OR, Rennie MJ, Narici MV. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. J Physiol. 2007;583:1079–1091. doi: 10.1113/jphysiol.2007.135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer MD, Seynnes OR, di Prampero PE, Pisot R, Mekjavic IB, Biolo G, Narici MV. Effect of 5 weeks horizontal bed rest on human muscle thickness and architecture of weight bearing and non-weight bearing muscles. Eur J Appl Physiol. 2008;104:401–407. doi: 10.1007/s00421-008-0703-0. [DOI] [PubMed] [Google Scholar]
- Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Bonconpagni S, Belia S, Wannenes F, Nicoletti C, Del Prete Z, Rosenthal N, Molinaro M, Protasi F, Fano G, Sandri M, Musaro A. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008;8:425–436. doi: 10.1016/j.cmet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Dudley MA, Burrin DG, Wykes LJ, Toffolo G, Cobelli C, Nichols BL, Rosenberger J, Jahoor F, Reeds PJ. Protein kinetics determined in vivo with a multiple-tracer, single-sample protocol: application to lactase synthesis. Am J Physiol Gastrointest Liver Physiol. 1998;274:G591–G598. doi: 10.1152/ajpgi.1998.274.3.G591. [DOI] [PubMed] [Google Scholar]
- Dukan S, Farewell A, Ballesteros M, Taddei F, Radman M, Nystrom T. Protein oxidation in response to increased transcriptional or translational errors. Proc Natl Acad Sci USA. 2000;97:5746–5749. doi: 10.1073/pnas.100422497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler F, Kilates C. Prospective nutritional surveillance using bioelectrical impedance in chronic kidney disease patients. J Ren Nutr. 2005;15:148–151. doi: 10.1053/j.jrn.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Flaring UB, Hebert C, Wernerman J, Hammarqvist F, Rooyackers OE. Circulating and muscle glutathione turnover in human endotoxaemia. Clin Sci (Lond) 2009;117:313–319. doi: 10.1042/CS20080462. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Greilberger J, Koidl C, Greilberger M, Lamprecht M, Schroecksnadel K, Leblhuber F, Fuchs D, Oettl K. Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer's disease. Free Radic Res. 2008;42:633–638. doi: 10.1080/10715760802255764. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979;76:5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grune T, Merker K, Sandig G, Davies KJ. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun. 2003;305:709–718. doi: 10.1016/s0006-291x(03)00809-x. [DOI] [PubMed] [Google Scholar]
- Heys SD, McNurlan MA, Park KG, Milne E, Garlick PJ. Baseline measurements for stable isotope studies: an alternative to biopsy. Biomed Environ Mass Spectrom. 1990;19:176–178. doi: 10.1002/bms.1200190314. [DOI] [PubMed] [Google Scholar]
- Hibbert JM, Sutherland GB, Jr, Wright LL, Jr, Wolfe LG, Wolfe KA, Gao SP, Gore DC, Abd-Elfattah AS. Measurement of hemoglobin synthesis rate in vivo using a stable isotope method. Anal Biochem. 2001;291:118–123. doi: 10.1006/abio.2001.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto M, Nikawa T, Kano M, Hirasaka K, Kitano T, Watanabe C, Tanaka R, Yamamoto T, Kamada M, Kishi K. Cysteine supplementation prevents unweighting-induced ubiquitination in association with redox regulation in rat skeletal muscle. Biol Chem. 2002a;383:715–721. doi: 10.1515/BC.2002.074. [DOI] [PubMed] [Google Scholar]
- Ikemoto M, Okamura Y, Kano M, Hirasaka K, Tanaka R, Yamamoto T, Sasa T, Ogawa T, Sairyo K, Kishi K, Nikawa T. A relative high dose of vitamin E does not attenuate unweighting-induced oxidative stress and ubiquitination in rat skeletal muscle. J Physiol Anthropol Appl Human Sci. 2002b;21:257–263. doi: 10.2114/jpa.21.257. [DOI] [PubMed] [Google Scholar]
- Jahoor F, Wykes LJ, Reeds PJ, Henry JF, del Rosario MP, Frazer ME. Protein-deficient pigs cannot maintain reduced glutathione homeostasis when subjected to the stress of inflammation. J Nutr. 1995;125:1462–1472. doi: 10.1093/jn/125.6.1462. [DOI] [PubMed] [Google Scholar]
- Ji LL, Fu R, Mitchell EW. Glutathione and antioxidant enzymes in skeletal muscle: effects of fiber type and exercise intensity. J Appl Physiol. 1992;73:1854–1859. doi: 10.1152/jappl.1992.73.5.1854. [DOI] [PubMed] [Google Scholar]
- Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-κB signaling in skeletal muscle. J Appl Physiol. 2007;103:388–395. doi: 10.1152/japplphysiol.00085.2007. [DOI] [PubMed] [Google Scholar]
- Laviano A, Meguid MM, Preziosa I, Rossi FF. Oxidative stress and wasting in cancer. Curr Opin Clin Nutr Metab Care. 2007;10:449–456. doi: 10.1097/MCO.0b013e328122db94. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Curr Top Cell Regul. 2000;36:95–116. doi: 10.1016/s0070-2137(01)80004-2. [DOI] [PubMed] [Google Scholar]
- Luo JL, Hammarqvist F, Andersson K, Wernerman J. Surgical trauma decreases glutathione synthetic capacity in human skeletal muscle tissue. Am J Physiol Endocrinol Metab. 1998;275:E359–E365. doi: 10.1152/ajpendo.1998.275.2.E359. [DOI] [PubMed] [Google Scholar]
- Lyons J, Rauh-Pfeiffer A, Ming-Yu Y, Lu XM, Zurakowski D, Curley M, Collier S, Duggan C, Nurko S, Thompson J, Ajami A, Borgonha S, Young VR, Castillo L. Cysteine metabolism and whole blood glutathione synthesis in septic pediatric patients. Crit Care Med. 2001;29:870–877. doi: 10.1097/00003246-200104000-00036. [DOI] [PubMed] [Google Scholar]
- Malmezat T, Breuille D, Capitan P, Mirand PP, Obled C. Glutathione turnover is increased during the acute phase of sepsis in rats. J Nutr. 2000;130:1239–1246. doi: 10.1093/jn/130.5.1239. [DOI] [PubMed] [Google Scholar]
- Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol. 2004;97:1477–1485. doi: 10.1152/japplphysiol.00371.2004. [DOI] [PubMed] [Google Scholar]
- Morrison JA, Jacobsen DW, Sprecher DL, Robinson K, Khoury P, Daniels SR. Serum glutathione in adolescent males predicts parental coronary heart disease. Circulation. 1999;100:2244–2247. doi: 10.1161/01.cir.100.22.2244. [DOI] [PubMed] [Google Scholar]
- Morse CI, Thom JM, Reeves ND, Birch KM, Narici MV. In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J Appl Physiol. 2005;99:1050–1055. doi: 10.1152/japplphysiol.01186.2004. [DOI] [PubMed] [Google Scholar]
- Mortensen RA, Haley MI, Elder HA. The turnover of erythrocyte glutathione in the rat. J Biol Chem. 1956;218:269–273. [PubMed] [Google Scholar]
- Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35:411–429. doi: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- Muller MJ, Bosy-Westphal A, Klaus S, Kreymann G, Luhrmann PM, Neuhauser-Berthold M, Noack R, Pirke KM, Platte P, Selberg O, Steiniger J. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am J Clin Nutr. 2004;80:1379–1390. doi: 10.1093/ajcn/80.5.1379. [DOI] [PubMed] [Google Scholar]
- Najim RA, Sharquie KE, Abu-Raghif AR. Oxidative stress in patients with Behcet's disease: I correlation with severity and clinical parameters. J Dermatol. 2007;34:308–314. doi: 10.1111/j.1346-8138.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- Narici M, Cerretelli P. Changes in human muscle architecture in disuse-atrophy evaluated by ultrasound imaging. J Gravit Physiol. 1998;5:73–74. [PubMed] [Google Scholar]
- Passing H, Bablok A new biometrical procedure for testing the equality of measurements from two different analytical methodsApplication of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983;21:709–720. doi: 10.1515/cclm.1983.21.11.709. [DOI] [PubMed] [Google Scholar]
- Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta. 2003;333:19–39. doi: 10.1016/s0009-8981(03)00200-6. [DOI] [PubMed] [Google Scholar]
- Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R337–R344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- Powers SK, Lennon SL. Analysis of cellular responses to free radicals: focus on exercise and skeletal muscle. Proc Nutr Soc. 1999;58:1025–1033. doi: 10.1017/s0029665199001342. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Narici MV. Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol. 2004;91:116–118. doi: 10.1007/s00421-003-0961-9. [DOI] [PubMed] [Google Scholar]
- Reeves NJ, Maganaris CN, Ferretti G, Narici MV. In-fluence of simulated microgravity on human skeletal muscle architecture and function. J Gravit Physiol. 2002;9:153–154. [PubMed] [Google Scholar]
- Rutten EP, Engelen MP, Gosker H, Bast A, Cosemans K, Vissers YL, Wouters EF, Deutz NE, Schols AM. Metabolic and functional effects of glutamate intake in patients with chronic obstructive pulmonary disease (COPD) Clin Nutr. 2008;27:408–415. doi: 10.1016/j.clnu.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Sen CK, Marin E, Kretzschmar M, Hanninen O. Skeletal muscle and liver glutathione homeostasis in response to training, exercise, and immobilization. J Appl Physiol. 1992;73:1265–1272. doi: 10.1152/jappl.1992.73.4.1265. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- Stein TP, Wade CE. Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S. doi: 10.1093/jn/135.7.1824S. [DOI] [PubMed] [Google Scholar]
- Tauler P, Aguilo A, Gimeno I, Fuentespina E, Tur JA, Pons A. Response of blood cell antioxidant enzyme defences to antioxidant diet supplementation and to intense exercise. Eur J Nutr. 2006;45:187–195. doi: 10.1007/s00394-005-0582-7. [DOI] [PubMed] [Google Scholar]
- Vescovo G, Ravara B, Dalla LL. Skeletal muscle myofibrillar protein oxidation and exercise capacity in heart failure. Basic Res Cardiol. 2008;103:285–290. doi: 10.1007/s00395-007-0692-x. [DOI] [PubMed] [Google Scholar]
- Wolfe RR. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. New York: Wiley-IEEE, Inc.; 2004. [Google Scholar]
- Yang RD, Matthews DE, Bier DM, Lo C, Young VR. Alanine kinetics in humans: influence of different isotopic tracers. Am J Physiol Endocrinol Metab. 1984;247:E634–E638. doi: 10.1152/ajpendo.1984.247.5.E634. [DOI] [PubMed] [Google Scholar]
- Zhang XJ, Chinkes DL, Wolfe RR. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am J Physiol Endocrinol Metab. 2002;283:E753–E764. doi: 10.1152/ajpendo.00053.2002. [DOI] [PubMed] [Google Scholar]


