Abstract
This article outlines the use of liquid acrylic adhesives in the management of congenital vascular malformations. Specifically, the chemical features of cyanoacrylates, including the physical and chemical properties, exovascular use of cyanoacrylates, and the techniques for use of these agents, are discussed.
Keywords: Cyanoacrylates, vascular malformation, congenital disease
There are few clinical conditions in which the old dictum “first, do no harm” is more applicable than in the management of vascular malformations. These lesions are generally encountered in young, otherwise healthy patients, and the lesions themselves often follow a fairly benign clinical course even when untreated. There are also a wide range of lesions that appear superficially similar but are biologically and prognostically quite distinct, including hemangiomas, hemangioendotheliomas, arteriovenous malformations (AVMs), cavernous venous malformations, lymphatic malformations, vascular tumors, and arteriovenous fistulas. Some of these lesions will involute spontaneously and require no treatment, others grow with the individual, and still others progress rapidly and may have life-threatening consequences. It is essential to first make the proper diagnosis, and only then consider the therapeutic options.
Not all AVMs require treatment, and even when treatment is appropriate, complete eradication of the lesion is not always necessary or possible. Embolization has assumed a primary role in the treatment of arteriovenous malformations during the last 25 years. Early on, it was often considered only as an adjunct to surgery or as a preoperative measure. Experience has shown that many lesions can be well treated or controlled with embolization alone, and surgical resection is now performed primarily for very localized lesions. There are many embolization techniques and a long list of agents and devices that have been used in the treatment of these lesions. It has long been recognized that proximal occlusion or ligation of vessels feeding an AVM is worse than useless, resulting in rapid recurrence of the lesion while sacrificing future direct access to the malformation. Efforts have been directed at finding agents that will penetrate and obliterate the nidus of the lesion with maximum permanence and minimal toxicity. At present, the ideal agent does not exist, and each of the available agents represents a compromise. Liquid acrylic adhesives were first used clinically nearly 40 years ago, and have only recently been reintroduced in the United States.
On the basis of our experience in treating more than 600 patients with vascular malformations over the last 25 years, we have found cyanoacrylates to be an imperfect but extremely valuable agent in managing these difficult problems.
EMBOLIZATION OF VASCULAR MALFORMATIONS
Congenital vascular malformations constitute a wide range of anatomic lesions, ranging from low-flow (primarily venous) lesions to rapidly shunting high-flow arteriovenous lesions causing high output states. They can occur in any part of the body and tend to grow at the same rate as the individual, occasionally showing periods of rapid expansion but virtually never involuting spontaneously. Only symptomatic lesions require treatment because incidentally discovered malformations usually follow a benign course; most of these patients will need only reassurance and conservative management. Absolute indications for treatment include hemorrhage, pain, mass effect causing functional disturbance, and high output states.
High-flow lesions are associated with a fairly complex physiology, involving enlargement of feeding vessels, enlargement of draining veins, vessel tortuosity, venous hypertension, distal ischemia due to stealing proximally, and increased cardiac output due to left to right shunting. All of these phenomena are the same as those seen in arteriovenous fistulae (AVF), whether congenital or acquired. The significance of this similarity is that the same pitfalls that have been well known in treating AVF for more than 100 years apply to the management of high-flow AVMs. Simple proximal ligation of an artery feeding an AVF results not only in failure, but also in significant worsening of the clinical problem due to recruitment of new collateral arterial feeders. Over time, this collateral recruitment can be extensive enough to resemble a complex congenital AVM both anatomically and physiologically, and can become as difficult to cure. The key to curing the fistula is to isolate and interrupt the actual site of communication between artery and vein; in a previously untreated fistula, this usually can be accomplished. The goal in treating a high-flow malformation is the same, but it is much more difficult (or impossible) to achieve because of the complexity of the blood supply. Nevertheless, every effort must be made to eradicate the actual nidus of arteriovenous communication, rather than occluding feeding vessels. Whether feeding arteries are ligated meticulously in a surgical skeletonization procedure lasting many hours, or occluded using multiple embolization coils, the result will be the same—rapid recurrence and more difficult subsequent treatment.
EMBOLIC MATERIALS
Numerous embolic materials and devices have been introduced during the last 30 years, but few have the capability of penetrating and occluding the nidus of an AVM (Table 1). Characteristics of the ideal embolic material include its natural radiopacity and a repeatable chemical behavior. It should be fluid enough to be delivered through the smallest microcatheter and should cause a sufficient inflammatory response to cause a permanent occlusion of the embolized vascular structure. It should not have any adverse effects on surrounding normal tissue and should not have any carcinogenic or teratogenic potential. Finally, it should be easily available and, if possible, inexpensive.1
Table 1.
Embolic Agents Used for Congenital AVM Embolization
| Agents | Resorption | Occlusion Characteristics | Vascular Reaction | Toxicity |
|---|---|---|---|---|
| From Kunstlinger et al.31 | ||||
| Particulate | ||||
| Clots | Resorbable | Lysis, fragmentation, distal migration | No | No |
| Tissue | Theoretically unresorbable | Fragmentation, distal migration | No | |
| Gelfoam | Resorbable | Fragmentation, distal migration | Acute necrotizing arteritis | No |
| Oxycel | Resorbable | — | — | — |
| Ivalon | Nonresorbable | Distal migration | Inflammatory reaction, fibrosis | No |
| Fluids | ||||
| Alcohol | Nonresorbable | Variable | Inflammatory reaction | Potential |
| Silicone | Nonresorbable | Viscosity dependent | No | No |
| Cyanoacrylates | Slowly resorbable | Variable | Inflammatory reaction | ? |
Coils, balloons, Gelfoam pledgets (Upjohn, Kalamazoo, MI), and other macroscopic occluding devices are equivalent to an internal ligation, and therefore have little or no use in the management of these lesions. Particles and microspheres could theoretically penetrate and occlude the nidus if perfectly sized, but in reality this is rarely achieved. The particles must be small enough to penetrate the nidus, but not so small that they pass through to the venous side. Irregularly shaped particles, such as standard polyvinyl alcohol (PVA), incompletely occlude vessels, and recanalization around the particles tends to occur within a matter of weeks. Microspheres have the theoretical advantage of completely occluding the cylindrical vessel lumen, possibly reducing the recanalization phenomenon. Although the effect of small particles and microspheres is usually temporary, they may be useful preoperatively and they do not sacrifice future access, as is the case with ligation or coils.
Liquids have unique properties that would seem to make them the ideal agents for penetrating and occluding the nidus of a vascular malformation. Liquid embolic agents fall into two broad categories: sclerosants and occlusive or casting agents.
Sclerosants include agents such as absolute ethanol and sodium tetradecol. Both of these agents are used primarily by direct injection in the treatment of venous malformations, where they are fairly effective in inciting thrombosis, endothelial damage, and shrinkage of the lesion. Absolute ethanol also is used intraarterially in the treatment of high-flow arteriovenous malformations, where it can be effective in occluding the nidus of the lesion. However, ethanol carries significant risks; it is directly tissue toxic and thus can cause skin sloughing, mucosal necrosis, muscle necrosis, and permanent nerve injury. The distribution of this agent must therefore be carefully controlled to avoid any of these complications, and the fact that the agent itself is radiolucent adds to the problem. Another reported complication of ethanol use is acute cardiovascular collapse, which can occur if a significant volume of ethanol reaches the pulmonary circulation. Careful physiologic monitoring with precautionary placement of Swan Ganz catheters and limitation in the total volume of ethanol used have been recommended to reduce this risk.
Two other types of liquid used in AVM embolization are occlusive casting agents, which may be adhesive or nonadhesive. The nonadhesive types include such materials as liquid silicone and ethylene vinyl alcohol copolymer (EVAL); because of their viscosity and lack of vessel wall adherence, they can be somewhat difficult to control. The only adhesive agent currently available in the United States is N-butyl cyanoacrylate (Trufill, Cordis, Inc., Miami Lakes, FL), which was approved by the U.S. Food and Drug Administration (FDA) for neurointerventional use in September 2000.
CYANOACRYLATE
Cyanoacrylates are the main liquid adhesives used for endovascular procedures, especially for embolization of high-flow AVM. Chemically synthesized in 1949 by Ardis,2 the adhesive properties were accidentally discovered by Coover during the course of an experiment.3 Eastman 910 Monomer, marketed by Eastman Corporation (Rochester, NY), was the first commercially available cyanoacrylate. This had a single-carbon ester group and was a methyl cyanoacrylate. Isobutyl (four carbon), N-butyl (four-carbon ester), and 2-hexyl (six-carbon ester) became available later and became the standard of care for treatment of cerebrovascular AVM.
Until recently, however, there was no FDA-approved agent for use in the United States. Trufill n-butyl cyanoacrylate (n-BCA) was approved by the FDA in 2000. Histoacryl (n-BCA; B. Braun, Melsungen, Germany) was used prior to Trufill n-BCA. A new agent, Neuracryl M (Prohold Technologies, El Cajon, CA) is currently under evaluation. This consists of a 2-hexyl-cyanoacrylate compound (Neuracryl M1) mixed with an esterified fatty acid and gold particles to retard polymerization and provide radiopacity.4
n-BCA has been shown to be equivalent to PVA as a preoperative embolic agent for treatment of cerebral AVM as determined by percent of nidus reduction and number of feeding vessels embolized.5 In this trial, n-BCA was safer, with fewer vessel perforations and incidences of subarachnoid hemorrhage. However, this study did not evaluate for permanence and durability of embolization with n-BCA as compared with PVA, nor did it enroll patients with lesions that could have been treated with embolization or embolization and stereotactic surgery.
A study comparing Avitene (Davol, Inc., Cranston, RI), cyanoacrylate, and PVA for embolization revealed that Avitene produced the mildest tissue response but resulted in relatively early endothelialization and recanalization. Cyanoacrylates were longer lasting but were associated with more acute and chronic inflammation and vessel wall changes. PVA foam/ethanol mixture had intermediate properties.6
Chemistry of Cyanoacrylates
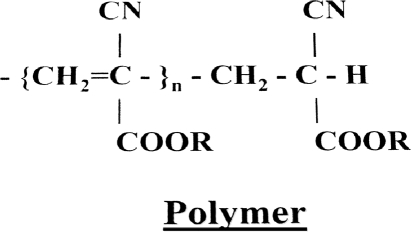
Monomeric cyanoacrylate structure includes a two-carbon ethylene group. The B carbon has two hydrogens attached to it, which contributes to the electric activity of the molecule. The A carbon has a cyano group and an ester function, called a carbonyl (Fig. 1). Various hydrocarbons can perform the carbonyl function. The hydrocarbons contribute to the name of the cyanoacrylate: isobutyl cyanoacrylate, n-BCA, or 2-hexyl cyanoacrylate. Upon contact with an anion, such as hydroxyl moiety in water or various anions in blood, a stable highly reactive carbanion is formed by bonding of the anions to the B carbon of the monomer. With this, the A carbon becomes negatively charged and contributes further negative anions to adjacent B carbons, thus initiating polymerization (Fig. 2). The longer the hydrocarbon at the R position, the slower the rate of polymerization, the less heat released during polymerization, and the lower the histotoxicity.1,7 Because even minute quantities of water can initiate polymerization, manufacturers add small amounts of free radical or anionic scavengers such as hydroquinone or other phenolic molecules.
Figure 1.
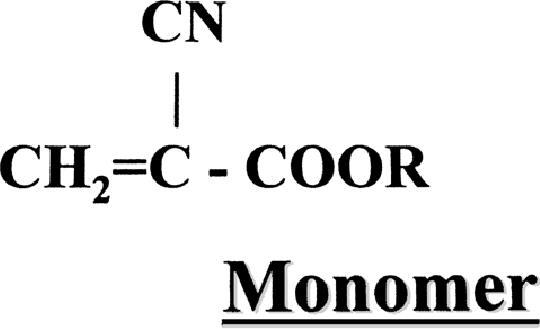
Basic structure of a cyanoacrylate monomer.6
Figure 2.
Polymerization in presence of an OH- or free electron to produce a stable but highly reactive molecule.6
Physical and Chemical Properties
The cyanoacrylates are typically clear, colorless, low-viscosity liquids that spread rapidly and polymerize quickly upon contact with negatively charged anions. They have an acrid, penetrating smell. They are stable at body temperature and degrade at temperatures higher than 165°C.
Exovascular Use of Cyanoacrylates
Cyanoacrylates were investigated for a variety of surgical functions including incisional wound closure, skin grafts, and tubular organ anastomosis.8,9 However, although the hemostatic properties were acceptable, the tensile strength of the materials was found to be lacking.10 Cyanoacrylates have been used in ophthalmic surgery for stromal melting of the cornea, for sealing corneal perforations and lacerations, and for repair of conjunctival fistulae.11 They have been used for skin grafting in plastic and reconstructive surgery,12 and in reconstruction of ossicular disruption in otologic surgery.13 Uses noted in dentistry and oral surgery include surface dressing and pulp capping techniques.14
Endovascular Use of Cyanoacrylates
Initially, isobutyl 2-cyanoacrylate, the first clinically used cyanoacrylate monomer, was injected relatively blindly into AVMs through flow-directed catheters.15 However, the degree of nidus penetration and catheter adhesion could not be judged.
A variety of agents to make these agents radiopaque as well as to delay polymerization within the delivery catheters were evaluated. Powdered metals, including tantalum and tungsten, and iodized oils such as Pantopaque (Amersham Health, Princeton, NJ), Ethiodol (Savage Laboratories, Melville, NY), and lipiodol, were used to make the adhesive radiopaque.16 Tantalum powder can cause slow initiation of polymerization because it contains free electrons on the surface; adding a small amount of oil to the metal obviates the initiation of polymerization. Large variations in particle size can occur if the powdered metal is not carefully prepared.6 Iodized oils aid in opacification of the agent as well as prolong the polymerization time. Dilution with oily media produces a markedly degraded adhesive that formed small, nearly spherical droplets.6 Glacial acetic acid can also be used to delay polymerization.17
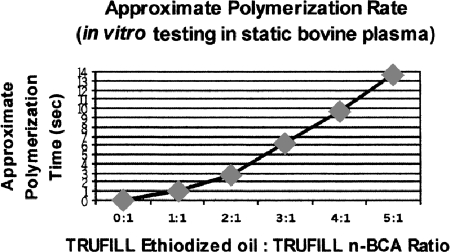
Ethiodol is the most widely used oily agent used in the United States, and is used commonly in Europe for embolizations. It is mixed in ratios varying from 1:1 to 1:4 (oil-to-glue ratio). No significant differences in either vessel wall injury or recanalization of embolized vessels has been reported with the 1:1 or 1:4 mixture.18 Fig. 3 demonstrates different polymerization times of n-BCA depending on the ratio of Ethiodol to n-BCA.
Figure 3.
Chart showing prolongation of polymerization time with increasing proportions of ethiodized oil to n-butyl cyanoacrylate (n-BCA) adhesive.
Tissue Interactions
Cyanoacrylates have varying degrees of tissue toxicity based on the release of formaldehyde during polymerization. Smaller side-chain esters such as methyl and ethyl cyanoacrylates are more toxic than are the longer side-chain esters such as isobutyl and normal butyl cyanoacrylates.6 Cyanoacrylates incite an acute inflammatory response in the vessel wall and surrounding tissue, progressing to a chronic and granulomatous process in ∼1 month.19,20,21
Glue embolization may result in permanent occlusion.22 However, partial embolization can result in recanalization, especially if the nidus of the lesion has not been embolized.19,23,24 In animal laboratory studies, sarcomas have been reported following administration of large doses of isobutyl 2-cyanoacrylate;25,26,27,28,29 however, no malignancies have been reported in humans with any of the cyanoacrylates.6,19,30 Isobutyl cyanoacrylate was withdrawn from the market by the manufacturer after the reports of the sarcomas in laboratory animals, and has been replaced by n-BCA since the late 1980s.
TECHNIQUE
The technique of using polymerizing adhesives in the treatment of AVMs is a combination of art and science. There are several variables in the process, including the mixture of components, the delivery system used, the volume and speed of injection, and the nature of the vascular bed that is being embolized. Significant experience is required to achieve safe and effective results. Some experience can be gained through in vitro models, but the behavior of these agents in biological systems is quite different; even after considerable operator experience, the result of each deposition is still somewhat unpredictable. The goal of the embolization procedure is selective obliteration of the nidus of the malformation, and this goal often is not completely achieved even in experienced hands.
The currently available adhesive, n-BCA, is relatively nonviscous, rapidly polymerizes on contact with any ionic medium or surface, and is completely radiolucent. The primary measure usually taken to modify some of these properties consists of combining the adhesive with an oily contrast agent (Ethiodol), which slows the polymerization time, increases viscosity, and makes the mixture radiopaque. The proportion of glue to oil can be varied, generally in the range of 1:1 up to 5:1. As the proportion of oil is increased, the polymerization time will be prolonged and the viscosity will be increased. On the bench top, changing these proportions will have fairly predictable effects on the behavior of the mixture; however, in the body the results are much less reproducible because of the number of variables in the system. The addition of more oil in an effort to slow polymerization and theoretically allow more complete penetration of the nidus might be more than counterbalanced by the increase in viscosity. In our experience, becoming comfortable with the behavior of one or two mixtures (1:1 or 2:1) reduces the number of variables and increases the predictability of the result. A third component, tantalum powder, is sometimes added to the mixture to increase radiopacity, but we rarely use this agent in our practice for several reasons. First, it adds further to the viscosity of the mixture and may cause clumping. Second, the black powder may cause permanent skin discoloration if the embolized vessel supplies a skin surface. Finally, the Ethiodol by itself provides more than adequate radiopacity in clinical use.
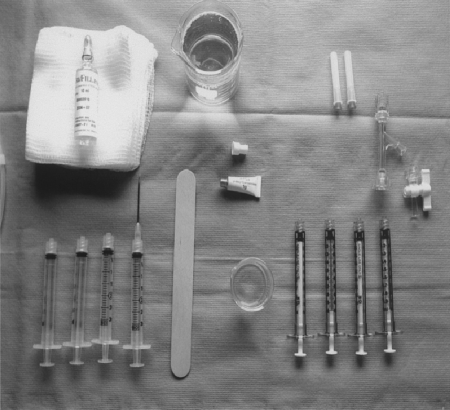
Using n-BCA requires scrupulous attention to detail, including the process of mixing the components. The oil and n-BCA mixture is relatively unstable, often hardening spontaneously after 15 minutes to 1 hour; therefore, the mixture is generally prepared at tableside just before clinical use. Given that the adhesive polymerizes immediately on contact with any ionic medium, including blood, saline, contrast, and tissue, the agent must be prepared in an isolated area, generally a separate sterile table (Fig. 4). This area should be considered a separate nonionic environment, with no contact between this area and the regular angiographic table. New gloves should be donned and all components required for the glue preparation (syringes, beakers, sponges, etc.) should be kept away from the standard angiographic supplies. Eye protection should be employed whenever acrylic adhesives are being used. Nonheparinized 5% dextrose in water, a nonionic solution, is used in place of normal saline flush. The glue and oil combination is mixed immediately just prior to use.
Figure 4.
Example of glue table, which is kept separate from main angiographic table to maintain nonionic environment. The glue is supplied in a 1-cm3 tube (center) and is mixed with oily contrast (upper left). A solution of 5% dextrose in water is used to flush the system and to inject boluses of adhesive. Generally 1-cm3 syringes are used for delivery, as a small amount of glue will occlude a fairly large vascular bed.
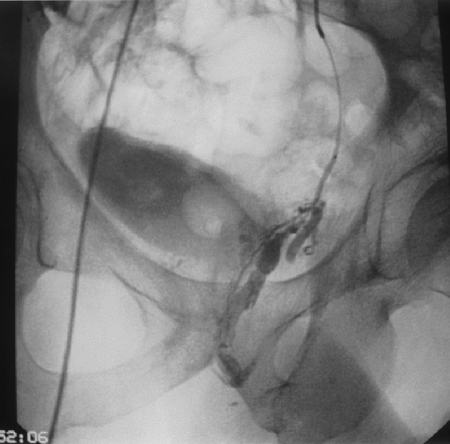
The delivery system is another key component of adhesive embolization. Virtually all of these embolizations are performed using coaxial microcatheter systems, generally through an outer selective angiographic catheter (Fig. 5). Microcatheters not only allow very distal delivery of the agent close to the nidus, but they also provide an important safety margin in the event glue adheres to the catheter tip during embolization. We generally place the outer catheter close to or within the branch being embolized, so that any adherent material can be peeled off the microcatheter as it is withdrawn into the outer catheter, preventing any stray material from entering a nontarget vessel. The presence of the outer catheter also maintains access to the area being embolized; multiple microcatheters are often needed during a complex embolization. Depending on the technique used, only one or two depositions can generally be made through a given microcatheter before the lumen becomes occluded.
Figure 5.
Coaxial microcatheter technique used during embolization of arteriovenous malformation supplied by left internal pudendal artery. Inner 3 Fr microcatheter allows distal deposition, whereas more proximal 5 Fr Cobra maintains purchase in proximal pudendal trunk and provides protection from nontarget embolization.
The actual technique of glue deposition is variable, depending on the vascular anatomy involved. The two general techniques are referred to as the push technique and the continuous column technique. The push technique (sometimes called sandwich or bolus technique) involves injecting a volume of glue smaller than the capacity of the microcatheter, ranging from 0.1 to 1.0 cm3, followed by an injection of 5% dextrose in sterile water solution, which expels the glue from the microcatheter and flushes it forward into the circulation. Although this volume of glue sounds quite small, especially relative to a large AVM, a small amount of glue creates a much larger cast because of the incorporation of blood elements. The average volume of adhesive mixture we generally inject on each deposition is from 0.2 to 0.5 cm3, and the total volume used during an entire procedure is often less than 2 cm3. The advantages of the push technique include good penetration of the nidus, preservation of the microcatheter lumen to allow more than a single deposition, and reduction of the likelihood of glue adhering to the catheter tip. The primary disadvantage is the limit to the volume of adhesive delivered on any given deposition.
The continuous column technique consists of filling a syringe with adhesive and injecting continuously as the agent exits the catheter and forms a cast. The potential advantage of this technique is that larger volumes of glue can be used to make a more complete cast of the malformation. In practice, we have found this method more difficult to control, often producing a proximal cast with reflux of the adhesive until it surrounds the catheter tip, resulting in increased potential for nontarget embolization as the catheter is withdrawn. In addition, the microcatheter lumen is rarely preserved for more than a single deposition using this technique. Whichever technique is used, care should be taken to preserve the main vascular trunks leading to the malformation given the likelihood that future treatment will be required. Once the visible nidus has been occluded, there is nothing gained by more proximal occlusion, which only makes subsequent access more difficult.
COMPLICATIONS
Complications of acrylic adhesive use in the treatment of vascular malformations fall into two categories: those associated with the actual deposition of adhesive, and those related to the underlying anatomy. Nontarget embolization can occur because of insecure catheter position, reflux of the embolic agent, adherence of the adhesive to the catheter tip, and passage of the material through the malformation. As in any selective embolization, proper seating of the catheter is essential, and injection near the origin of the target vessel is to be avoided. The use of coaxial systems has already been described, and represents an important safeguard; the outer catheter position is equally important in the prevention of nontarget embolization. One complication that is disproportionately feared by new users of this agent is gluing the catheter in place. The tensile strength of the polymerized glue mixture is such that the microcatheter can be separated from the glue cast with a quick tug in nearly every case, although a small amount of the glue may adhere to the catheter tip. This adherent adhesive is usually visible because of the marked radiopacity of the mixture, thus allowing maneuvers to be undertaken that will prevent the loss of agent into nontarget vessels. Actual gluing of the catheter in place is generally encountered only in cerebral embolization, when the extremely fine microcatheters used may have less tensile strength than the glue cast, allowing catheter separation on withdrawal.
Complications can also result from specific anatomic factors in the circulation being treated. Liquid adhesives can pass completely through high-flow lesions, resulting in pulmonary embolization. Unlike particles and ethanol, the marked radiopacity of the glue mixture generally allows this problem to be appreciated immediately, so that the injection can be stopped before a large volume of the agent reaches the pulmonary circulation. In the adult patient, a small amount of adhesive reaching the lung is rarely of any clinical significance. We have seen this occur in several patients, none of whom showed any immediate or delayed change in oxygenation, cardiac rhythm, or pulmonary function studies. In the infant or small child, loss of this agent into the pulmonary circulation might have more significant consequences. Embolization of agent to the lungs is primarily associated with fistula-like large vessel arteriovenous connections, where extreme caution must be used; in some cases, proximal balloon occlusion may be required to control the flow of the agent. Interestingly, this event is rarely seen in the more common high-flow lesions with a complex nidus, even when angiographic shunting is almost instantaneous. In these instances, the adhesive is trapped in the complex vasculature of the nidus before shunting can occur. Test injections of various volumes of contrast agent in an effort to determine the volume of glue to be used are usually misleading, given that the early occlusion by the adhesive causes rapid and dynamic changes in the flow pattern not seen on a simple pre-embolization contrast injection. It is generally safer to underestimate, rather than overestimate, the volume that will be required.
Rarely, abrupt changes in the regional circulation following adhesive embolization can result in complications. This is more commonly seen in neuroembolization procedures, where the system is much more sensitive to flow alterations, including elevation of venous pressures. We encountered a similar situation in a patient with an extremely high-flow pelvic arteriovenous malformation causing high output heart failure; after an aggressive embolization procedure in which virtually every part of the nidus was occluded with adhesive, the patient immediately developed nearly exsanguinating hematuria, determined to originate from a ruptured distended vein in the urinary bladder. The assumption was that the abrupt and extreme change in regional flow somehow resulted in acute distension of a draining vein. The hematuria was ultimately controlled only by an emergency partial cystectomy. We now treat very extensive high-flow lesions in multiple stages, and have not encountered this type of complication again.
A distinct advantage of liquid adhesives over other liquid agents such as ethanol is the lack of acute or long-term tissue toxicity. The agent can be used safely in vessels supplying mucosal surfaces, skin, or nerves without fear of direct toxic effects, although ischemic complications can occur, particularly in the distal extremities. To our knowledge, no long-term adverse effects, including foreign body reactions or carcinogenicity, have been reported in more than 30 years of human clinical use.
CONCLUSION
The treatment of congenital vascular malformations is often difficult and frustrating for both patient and practitioner. Embolization has assumed a primary role in the management of these patients during the last 20 years, with steady improvements in imaging, instrumentation, and embolic agents. Despite these advances, the ideal embolic agent does not exist at present, with each currently available agent representing a compromise between safety, efficacy, and ease of use. In the case of high-flow lesions, it is clear that proximal occlusion should be avoided due to the rapid recruitment of collateral vessels. Thus, efforts have been directed at developing agents that will penetrate and occlude the nidus of the malformation. Particulate agents tend to be associated with fairly rapid recurrence of the malformation, even when the particles themselves are non-reabsorbable. The liquid agents have several characteristics that make them well suited to this application; we have had an extensive and generally positive experience using acrylic adhesives in the embolization of vascular malformations during the past 25 years. This agent's low viscosity, rapid polymerization, and minimal tissue toxicity, as well as the ability to make the agent radiopaque without impairing its occlusive properties, have made acrylic adhesives our agent of choice in treating these difficult lesions.
REFERENCES
- Kerber C W, Wong W. Liquid acrylic adhesive agents interventional Neuroradiology. Neurosurg Clin N Am. 2000;11:85–99. [PubMed] [Google Scholar]
- Ardis A E. U.S. Patent 2,467,926, 1949.
- Coover H W, Jr, Joyner F B, Shearer N H, Jr, et al. Chemistry and performance of cyanoacrylate adhesives. Soc Plast Eng J. 1959;15:413–417. [Google Scholar]
- Barr J D, Hoffman E J, Davis B R, Edgar K A, Jacobs C R. Microcatheter adhesion of cyanoacrylates, comparison of normal butyl cyanoacrylate to 2-hexyl cyanoacrylate. J Vasc Interv Radiol. 1999;10:165–168. doi: 10.1016/s1051-0443(99)70459-8. [DOI] [PubMed] [Google Scholar]
- The n-BCA trial investigators AJNR Am J Neuroradiol. 2002;23:748–755. [PMC free article] [PubMed] [Google Scholar]
- Schweitzer J S, Chang B S, Madsen P, et al. The pathology of arteriovenous malformations of the brain treated by embolotherapy. Neuroradiology. 1993;35:468–474. doi: 10.1007/BF00602835. [DOI] [PubMed] [Google Scholar]
- Toriumi D M, Raslan W F, Friedman M, Tardy M E. Histotoxicity of cyanoacrylate tissue adhesive: a comparative study. Arch Otolaryngol Head Neck Surg. 1990;116:546–550. doi: 10.1001/archotol.1990.01870050046004. [DOI] [PubMed] [Google Scholar]
- Galil K A, Schonfield I D, Wright G Z. Effect of butyl 2-cyanoacrylate on the healing of skin wounds. Can Dent Assoc J. 1984;50:565–569. [PubMed] [Google Scholar]
- Petrella E, Orlandini G, Poisetti P, et al. A new end to side anastomosis formed without sutures for hemodialysis fistulas. Nephron. 1975;14:398–400. doi: 10.1159/000180471. [DOI] [PubMed] [Google Scholar]
- O'Leary J A. Tissue adhesives and pelvic hemostasis: an evaluation of isobutyl 2-cyanoacrylate. J Surg Oncol. 1971;3:117–120. doi: 10.1002/jso.2930030204. [DOI] [PubMed] [Google Scholar]
- Fogle J A, Kenyon K R, Foster C S. Tissue adhesive arrests stromal melting in the human cornea. Am J Ophthalmol. 1980;89:795–802. doi: 10.1016/0002-9394(80)90168-3. [DOI] [PubMed] [Google Scholar]
- Wilkinson T S, Rybka F J. Experimental study of prevention of tip necrosis in ischemic z-plasties. Plast Reconstr Surg. 1971;47:37–38. doi: 10.1097/00006534-197101000-00008. [DOI] [PubMed] [Google Scholar]
- Smyth G DL, Kerr A G. Histoacryl (butyl cyanoacrylate) as an ossicular adhesive. J Laryngol Otol. 1974;88:539–542. doi: 10.1017/s0022215100079044. [DOI] [PubMed] [Google Scholar]
- Bhaskar S N, Frisch J, Margetis P M, Leonard F. Application of a new chemical adhesive in periodontic and oral surgery. Oral Surg. 1966;22:526–535. doi: 10.1016/0030-4220(66)90434-8. [DOI] [PubMed] [Google Scholar]
- Bank W O, Kerber C W, Cromwell L D. In: Moss A, Goldberg H, editor. Interventional Radiological Techniques: Computed Tomography and Ultrasonography. San Francisco: University of California Press; 1981. Rapidly polymerizing glue for the treatment of intracerebral arteriovenous malformations. pp. 83–92.
- Cromwell L D, Kerber C W. Modification of cyanoacrylate for therapeutic embolization: preliminary experience. AMJ Am J Roentgenol. 1979;132:799–801. doi: 10.2214/ajr.132.5.799. [DOI] [PubMed] [Google Scholar]
- Spiegel S M, Vinuela F, Goldwasser J M, Fox A J, Pelz D M. Adjusting the polymerazation of isobutyl-2 cyanoacrylate. AJNR Am J Neuroradiol. 1986;7:109–112. [PMC free article] [PubMed] [Google Scholar]
- Sadato A, Wakhloo A K, Hopkins L N. Effect of a mixture of a Low concentration of n-butylcyanoacrylate and Ethiodol on tissue reactions and the permanence of arterial occlusion after embolization. Neurosurgery. 2000;47:1197–1203. doi: 10.1097/00006123-200011000-00037. [DOI] [PubMed] [Google Scholar]
- Brothers M F, Kaufman J C, Fox A J, Devekis J P. N-Butyl 2-cyanoacrylate- substitute for IBCA in interventional neuroradiology: histopathological and polymerization time studies. AJNR Am J Neuroradiol. 1989;10:777–786. [PMC free article] [PubMed] [Google Scholar]
- White R I, Jr, Strandberg J V, Gross G S, Barth K H. Therapeutic embolization with long term occluding agents and their effects on embolized tissues. Radiology. 1977;125:677–687. doi: 10.1148/125.3.677. [DOI] [PubMed] [Google Scholar]
- Vinters H V, Galil K A, Lundie M J, Kaufman J C. The histotoxicity of cyanoacrylates: a selective review. Neuroradiology. 1985;27:279–291. doi: 10.1007/BF00339559. [DOI] [PubMed] [Google Scholar]
- Wikholm G. Occlusion of cerebral arteriovenous malformations with N-butyl cyano-acrylate is permanent. AJNR Am J Neuroradiol. 1995;16:479–482. [PMC free article] [PubMed] [Google Scholar]
- Gruber A, Mazal P R, Bavinski G, Killer M, Budka H, Richling B. Repermeation of partially embolized cerebral arteriovenous malformations: a clinical, radiologic, and histologic study. AJNR Am J Neuroradiol. 1996;17:1323–1331. [PMC free article] [PubMed] [Google Scholar]
- Rao V R, Mandalam K R, Gupta A K, Kumar S, Joseph S. Dissolution of isobutyl 2-cyanoacrylate on long term follow up. AJNR Am J Neuroradiol. 1989;10:135–141. [PMC free article] [PubMed] [Google Scholar]
- Brown L C, Mellick P W, Smith C D, et al. Carcinogenicity bioassay of isobutyl 2-cyanoacrylate (IBC) in fischer-344 rats. NTIS/AD-A222 000/2, 1990.
- Brown L C, Mellick P W, Smith C D, et al. Carcinogenicity bioassay of isobutyl 2-cyanoacrylate in fischer-344 rats. Report: ISS LAIR-433, Toxicology ser-187, Order No. AD-A22 2000; 1990. p. 1161.
- Brown L C, Smith C D, Lollini L O, et al. Carcinogenicity bioassay of isobutyl 2-cyanoacrylate(IBC) in fischer-344 rats-one year interim sacrifice report, Vol. 1. NTIS/AD-A195 913/9; 1988.
- Brown L C, Smith C D, Lollini L O, et al. Carcinogenicity bioassay of isobutyl 2-cyanoacrylate(IBC) in fischer-344 rats-one year interim sacrifice report, Vol. 2, part 1. NTIS/AD-A201 448/8; 1989.
- Brown L C, Smith C D, Lollini L O, et al. Carcinogenicity bioassay of isobutyl 2-cyanoacrylate(IBC) in fischer-344 rats-one year interim sacrifice report. Vol. 2, part 2. NTIS/AD-A197 679/4; 1988.
- Debrun G M, Aletich V, Ausman J I, Charbel F, Dujovny M. Embolization of the nidus of brain arteriovenous malformations with n-butyl cyanoacrylate. Neurosurgery. 1997;40:112–120. [PubMed] [Google Scholar]
- Kunstlinger F, Brunelle F, Chaumont P, Doyon D. Vascular occlusive agents. AJR Am J Roentgenol. 1981;136:151–156. doi: 10.2214/ajr.136.1.151. [DOI] [PubMed] [Google Scholar]