Abstract
Massive hemoptysis is a frightening and potentially life-threatening clinical event. Patients with chronic inflammatory lung diseases such as bronchiectasis, sarcoidosis, tuberculosis, and cystic fibrosis develop markedly hypertrophied and fragile bronchial arteries that may lead to clinically significant hemoptysis. Surgical intervention is hazardous and often impossible in these patients with diffuse parenchymal lung disease. Superselective catheterization of the bronchial arteries feeding the affected areas followed by particulate embolization has proven to be an effective treatment for the control of bleeding. With modern microcatheters and guidewires, bronchial artery embolization is safe and well tolerated by patients. Because this treatment does not directly influence the primary underlying disease, recurrent episodes of bleeding are likely, which will require additional embolization procedures. In patients who have undergone prior bronchial artery embolization, the dominant feeding arterial supply often originates from nonbronchial systemic collateral vessels.
Keywords: Hemoptysis, embolization, bronchial artery, chronic inflammatory lung disease, cystic fibrosis
Massive hemoptysis (defined as bleeding greater than 240 to 300 mL/24 hours) carries a 50 to 85% mortality rate when treated conservatively. Death is most often due to asphyxiation from the aspiration of blood, leading to airway obstruction. Less commonly, death may be caused by exsanguination and acute hypotension.1 Recurrent bouts of moderate hemorrhage (three or more bouts of 100 ml of blood per day within a week) are now also considered a major hemorrhagic event. In addition, chronic or slowly increasing hemoptysis is considered an indication for transcatheter therapy.2 Because of the poor outcome associated with conservative therapy alone, many centers have instituted more aggressive therapeutic maneuvers. Although surgical resection may be curative for those individuals with focal disease, most patients presenting with major hemoptysis have diffuse chronic lung disease and limited pulmonary reserves. This group is considered to contain unacceptable surgical candidates. In patients who are surgical candidates, preoperative bronchial artery embolization to control the acute hemorrhage might be beneficial.
CLINICAL CONSIDERATIONS
Severe hemoptysis is typically seen in patients with a long history of chronic inflammatory lung disease. Tuberculosis (often associated with aspergillosis) likely is the most common worldwide etiology. Other etiologies, including sarcoidosis, cystic fibrosis (CF), and other types of bronchiectasis, also are common etiologies in most published series. In the setting of chronic bronchial inflammation, the bronchial arteries markedly hypertrophy and are at risk for hemorrhage.
Recurrent hemoptysis requires therapy because it can be very debilitating by precluding routine postural drainage of other lung regions. Approximately 1% of CF patients will have at least one episode of major hemoptysis per year. The majority of these patients will be at least 16 years of age. Patients with CF will often have crescendo bleeds and are likely to have recurrent bleeds following therapy.2,3,4
The source of bleeding must be defined clearly and hemoptysis must be differentiated from bleeding from the upper airways or alimentary tract. The possibility of a foreign body aspiration must always be entertained. Once hemoptysis has been established, a multidisciplinary approach involving pulmonary medicine, thoracic surgery, and interventional radiology is optimal. All medications that might contribute to bleeding should be stopped. A coagulation profile should be obtained and a sputum sample sent for culture and sensitivity, including bacteria, mycobacterium, and fungi.
A chest x-ray is required to identify any acute radiographic changes in the lung fields, localize the site of bleeding, and discover other potential causes of bleeding, such as a foreign body or cavity with mycetoma. If no localizing signs are present, a review of recent chest computed tomography (CT) might identify areas of severe bronchiectasis or new infiltrate, which would help to determine the site. Bronchoscopy might help localize the site of bleeding but might be nondiagnostic in the setting of severe bleeding. When performed early in the patient's management, Saumench et al5 identified the hemorrhage in 91% of cases compared with only 50% when performed later in the clinical course. Patients who have undergone previous bronchial artery embolization procedures should not be excluded from additional palliative embolization procedures. Bleeding can arise from recanalized vessels or hypertrophied nonbronchial systemic collaterals.
BRONCHIAL ARTERY ANATOMY
The bronchial arteries typically arise from the thoracic aorta at the T3 to T8 levels and also supply the bronchi, vagus nerve, posterior mediastinum, and esophagus. Eighty percent of arteries arise from the T5 to T6 level. There are many bronchial artery anatomic variations described. The more common combinations include a single right intercostobronchial (ICB) trunk (Fig. 1) with single left bronchial artery, single right ICB truck, and single left bronchial artery arising from a common trunk (Fig. 2), and a single right ICB trunk with two left bronchial arteries. Two bronchial arteries can be seen on either the right or left. Left ICB trunks have not been identified, whereas the right bronchial artery frequently shares origins with an intercostals artery.6 As many as 20% of bronchial arteries have anomalous origins other than the aorta. Aberrant origins include the subclavian, thyrocervical, internal mammary, innominate, pericardiophrenic, superior intercostals, abdominal aorta, and inferior phrenic arteries.7,8,9 Bronchopulmonary arterial anastomoses can be quite prominent in patients with chronic inflammation or pulmonary hypertension. The pulmonary parenchyma may receive arterial blood supply from transpleural systemic collateral to the bronchial circulation via intercostals, mammary, phrenic, thyrocervical, axillary, and subclavian arteries.10
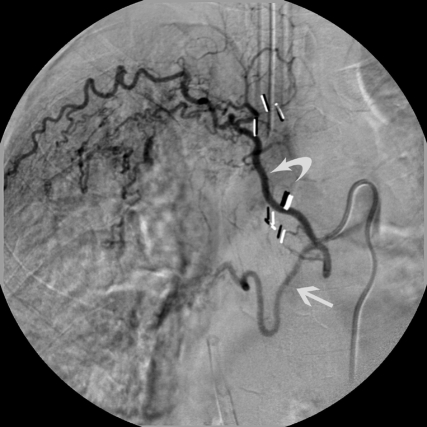
Figure 1.
Common bronchial-intercostal trunk. In another patient, both the right bronchial artery and superior intercostal artery arise from a common trunk. The bronchial artery (straight arrow) follows the course of the mainstem bronchus, whereas the intercostal artery follows the undersurface of the rib (curved arrow).
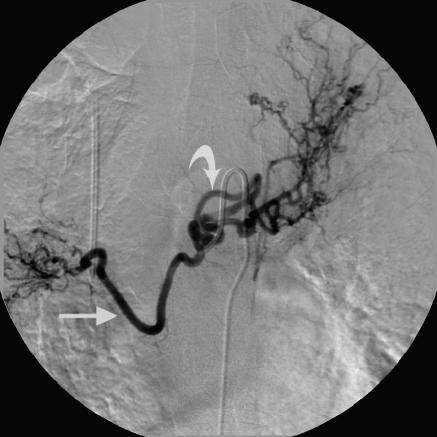
Figure 2.
Common bronchial trunk. Both the left and right bronchial arteries are opacified from a common injection. Note the enlargement and tortuosity of both the right (straight arrow) and left bronchial arteries (curved arrow).
When bronchial artery angiography and embolization is performed, consideration must be given to the arterial supply to the spinal cord. The dominant arterial supply is from the anterior spinal artery. This artery receives contributions from the intracranial segments of the vertebral arteries and also the anterior radiculomedullary branches of the intercostals and lumbar arteries.11 As many as six to eight contributing branches to the anterior spinal artery might exist. Each takes the characteristic course that resembles a hairpin loop. The largest anterior medullary branch (the artery of Adamkiewicz) has a variable origin but most commonly arises at the T8 to L1 level. In ∼5% of cases, the major supply of the anterior spinal artery arises from a right ICB trunk. The left bronchial arteries very rarely contribute the anterior spinal artery. The right superior intercostals and right bronchial artery might share a common trunk that sends a branch to supply the anterior spinal artery.
ANGIOGRAPHIC AND EMBOLIZATION TECHNIQUE
Prior to the procedure, a brief neurological exam is performed to establish a baseline. This will be repeated throughout the course of the embolization procedure. Some authors have suggested the use of somatosensory evoked potentials to monitor spinal cord ischemia during the procedure.12 With the current equipment, this is unnecessary.
A preliminary descending thoracic aortogram can be performed as a roadmap to the bronchial arteries. However, in patients who have not undergone prior embolization procedures, the authors often proceed directly to selective catheterization. If, however, successful cannulation is not promptly achieved, a thoracic aortogram is performed. Some authors advocate thoracic aortograms following embolization when selective catheterization is performed initially.13 Five or six French sheaths are commonly used, through which selective catheters are placed. Reverse-curved catheters (Mikaelson, Simmons I, SOS Omni) are initially used in our laboratory. Forward-looking catheters (such as Cobra, HIH, and RC,) can also be effective. Typically 4, 5, or 5.5 Fr catheters are routinely used.
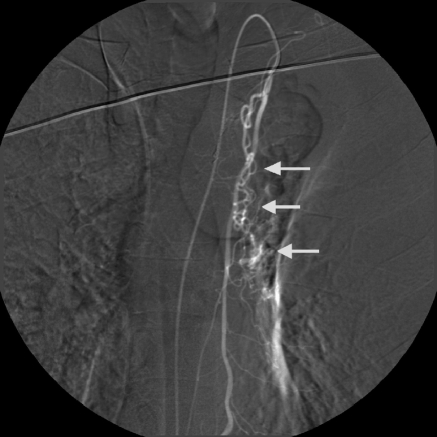
The left main stem bronchus serves as a convenient fluoroscopic landmark for the general location of the bronchial arteries. The catheter is direct lateral or anterolateral for the right bronchial and more anterior for the left. A selective bronchial arteriogram must be performed prior to any embolization. One must be sure not to occlude the artery during the selective injection, especially on the right, because this may result in spinal cord ischemia if spinal artery branches are present. Careful review of the run is required to identify the bronchial artery correctly, and to document angiographic abnormalities and the presence of anterior spinal artery contributions. The bronchial artery will have branches that follow the course of the main stem bronchus, whereas the intercostals artery will travel laterally along the undersurface of the rib. Abnormal angiographic appearances that support a site of bleeding include tortuosity, hypertrophy, hypervascularity, aneurysms, extravasation, and bronchial artery to pulmonary artery or vein shunting14,15,16,17,18,19 (Figs. 3 and 4). The bronchial artery injection may elicit a coughing response, although this is far less common with the use of the newer nonionic and iso-osmolar contrast agents. If the site of hemorrhage is known, all abnormal bronchial arteries to that region should be embolized. However, if the site of bleeding cannot be localized, any abnormal bronchial artery should be treated. If abnormal bronchial arteries cannot be identified, a continued search for additional bronchial arteries (including aberrant arteries) and nonbronchial systemic collateral supply must be performed (Figs. 3 and 5). The presence of significant hypertrophied nonbronchial systemic collaterals is particularly common in patients having undergone prior embolization procedures.
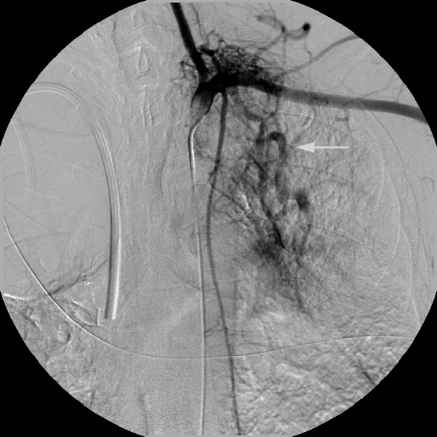
Figure 3.
Nonbronchial systemic arterial supply with pulmonary venous shunting. Selective left subclavian arteriogram demonstrates left apical hypervascularity arising from thyrocervical collateral. Note the prominent opacification of pulmonary veins due to shunting (arrow).
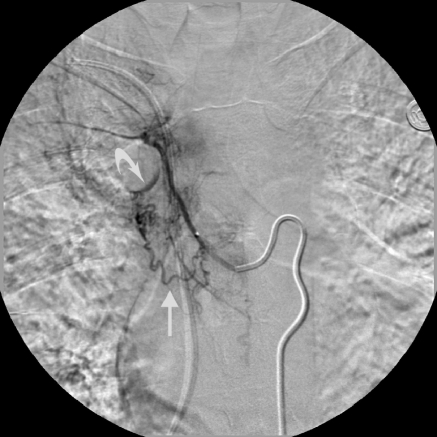
Figure 4.
Enlargement and tortuosity responsible for bleeding. Combined right intercostal and right bronchial artery trunk. The right bronchial artery demonstrates marked hypervascularity, enlargement, and tortuosity (straight arrow) with a focal area of extravasation (curved arrow). This vessel was embolized with PVA particles.
Figure 5.
Nonbronchial systemic arteries. Selective left internal mammary arteriogram shows collateral supply (arrows) to left hilum in a patient who has undergone previous bronchial artery embolization.
Recurrent hemoptysis in these patients following successful bronchial artery embolization with chronic inflammatory lung disease is not uncommon. The offending vascular supply in this situation includes a recanalized bronchial artery, a bronchial artery not previously embolized (aberrant or nonaberrant), or nonbronchial system collateral. Systemic nonbronchial collaterals include branches of the internal mammary artery, thyrocervical trunk, subclavian artery, axillary artery, and the inferior phrenic artery.
A stable catheter position is required for any embolization. The authors now routinely use coaxial catheterization utilizing the initial diagnostic catheter with its tip in the bronchial artery origin as the outer catheter. There now are many available microcatheters that will easily exit a 0.038-in. tapered diagnostic catheter. For routine coaxial catheterization, the authors use a dual-pressurized flush system located between the groin sheath and outer catheter and between the outer and inner catheters. For difficult or prolonged cases, a third flush between the inner catheter and micro guidewire is useful. The microcatheter should be placed beyond the intercostals artery (in the case of an ICB truck), and any vessels feeding the anterior spinal artery. The authors routinely placed the microcatheter at least 1 to 2 cm beyond the origin of the bronchial artery to be sure it is distal to any anterior radiculomedullary branches that might not be identified on initial arteriography. Tanaka et al20 demonstrated the value of superselective catheterization with a microcatheter. They documented improved hemorrhage control when using superselective versus selective catheterization techniques. The authors also tended to use smaller particle sizes when performing the embolization through the microcatheter. This might also be responsible for the improved results.
Ideally, distal embolization should be performed but should not affect the capillary bed of the bronchus. Particles greater than 200 to 250 μm should be used to avoid tissue ischemia and neurologic damage. Currently, polyvinyl alcohol (PVA) particles in the size range of 300 to 500 μm are commonly used with good results. Other embolic agents used include Gelfoam (Upjohn, Kalamazoo, MI) pledgets (1 to 2 mm), Gelfoam slurry, thrombin, and glue.3,4,15,18,19,21,22,23 The authors believe the recently approved embospheres and spherical PVA also will be effective in this application. Proximal occlusion with large particles or coils should be avoided if possible. Proximal occlusion affords only very temporary relief because collateral pathways readily develop. Very small particles (less than 200 μm) or liquid embolic agents should always be avoided because these cause tissue infarction.
Careful review of the initial diagnostic arteriogram for the presence of bronchial artery to pulmonary artery or vein shunts is of critical importance. Particles, which traverse shunts into the pulmonary artery circulation, will cause small pulmonary emboli and those that enter the pulmonary venous circulation could result in catastrophic systemic emboli. In these cases, larger particles should be chosen, and in the case of very large shunts, coils may be indicated.
RESULTS
The value in the immediate control of hemoptysis has been well documented. Rabkin et al14 published the largest series to date in 1987, reporting on 306 patients. Immediate control of the hemoptysis was achieved in 91% of patients. Uflacker et al6 and Remy et al24 achieved immediate control in their series in 77 and 84%, respectively. Guo25 supported the optimistic results in 1992 by reporting results in the patients with bronchial artery extravasation; they found control of hemoptysis in 93, 87, and 79% of patients at 1 week, 1 month, and 3 months following bronchial artery embolization, respectively. More recently, Goh et al26 published the results of a 6-year review in which they demonstrated an overall success rate of 82%.
Bronchial artery embolization has been particularly effective and clinically useful in the management of hemoptysis in patients with CF. In combining the series by Fellows et al4 and Cohen et al,3 31 of 33 patients (94%) had immediate control of bleeding. In 1998, Brinson et al27 reported on 13 CF patients undergoing 28 embolization procedures. Nine required only one embolotherapy session, whereas four required multiple (two or three) sessions to control the hemorrhage during a single hospital admission. Three of the 13 patients have returned with recurrent bleeds. All recurrent bouts of hemorrhage have been successfully controlled with bronchial artery embolization. The time interval between recurrent bouts of hemoptysis in this CF population following control with embolization averaged 20 months (range, 6 to 37 months). The early control of major and moderate bouts of hemoptysis in young patients with CF is particularly valuable as lung transplantation programs become more prevalent. Bronchial artery embolization does not appear to interfere with subsequent lung transplantation.
COMPLICATIONS
Spinal cord ischemia and transverse myelitis are the most feared and recognized complications. They are, fortunately, extremely rare. The use of nonionic contrast agents has significantly reduced, if not eliminated, the risks of transverse myelitis. With the current use of nonionic contrast media, particulate embolic agents greater than 200 μm and modern microcatheters or micro guidewires, nontarget embolization should be quite rare.
There have been two cases of bronchial infarction following the use of liquid embolic agents.14,28 A bronchoesophageal fistula has also been reported in the combination of esophageal and bronchial ischemia secondary to the use of fine particulate materials.29,30 These types of agents are no longer being used for this embolization indication. Chest pain and dysphagia commonly occur with selective (versus superselective) embolization within the first week following the procedure. These symptoms are secondary to the interruption of the blood supply to the posterior mediastinum and midportion of the esophagus. This is less common when distal superselective catheterization is used.
CONCLUSION
Major hemoptysis is a frightening and potentially fatal complication of a variety of chronic inflammatory conditions of the lung. The development of bronchial artery embolization techniques has revolutionized the approach to these patients. Persistent hemoptysis can be successfully controlled in as many as 90% of patients. Recurrent hemoptysis is due to the continued presence of acute on chronic inflammation and the recanalization of a previously embolized bronchial artery, bleeding from a site supplied by a nonembolized bronchial artery or bleeding from nonbronchial systemic collaterals. Recurrent hemoptysis also can be successfully controlled with embolization. Therefore, despite a prior history of bleeding and prior embolization procedures, no patient should be denied the opportunity for additional transcatheter therapy.
REFERENCES
- Wholey M H, Chamorro H A, Rao G, et al. Bronchial artery embolization for massive hemoptysis. JAMA. 1976;236:2501–2504. [PubMed] [Google Scholar]
- Tonkin I LD, Hanissian A S, Boulden T F, et al. Bronchial arteriography and embolotherapy for hemoptysis in patients with cystic fibrosis. Cardiovasc Intervent Radiol. 1991;14:241–246. doi: 10.1007/BF02578470. [DOI] [PubMed] [Google Scholar]
- Cohen A M, Doeshuk C F, Stern R C. Bronchial artery embolization to control hemoptysis in cystic fibrosis. Radiology. 1990;175:401–405. doi: 10.1148/radiology.175.2.2326467. [DOI] [PubMed] [Google Scholar]
- Fellows K E, Khaw K T, Schuster S, et al. Bronchial artery embolization in cystic fibrosis: technique and long-term results. J Pediatr. 1979;95:959–963. doi: 10.1016/s0022-3476(79)80283-8. [DOI] [PubMed] [Google Scholar]
- Saumench J, Excarrabill J, Padro L, et al. Value of fiberoptic bronchoscopy and angiography for diagnosis of the bleeding site in hemoptysis. Ann Thorac Surg. 1989;48:272–274. doi: 10.1016/0003-4975(89)90087-8. [DOI] [PubMed] [Google Scholar]
- Uflacker R, Kaemmerer A, Picon P D, et al. Bronchial artery embolization in management of hemoptysis: technical aspects and long-term results. Radiology. 1985;157:637–644. doi: 10.1148/radiology.157.3.4059552. [DOI] [PubMed] [Google Scholar]
- Tan R T, Mcgahan J P, Link D P, et al. Bronchial artery embolization in management of hemoptysis. J Interv Radiol. 1991;6:67–76. [Google Scholar]
- McPherson S, Routh W D, Nath H, et al. Anomalous origin of bronchial arteries: potential pitfall of embolotherapy for hemoptysis. J Vasc Interv Radiol. 1990;1:86–88. doi: 10.1016/s1051-0443(90)72509-2. [DOI] [PubMed] [Google Scholar]
- Cohen A M, Antoun B W, Stern R C. Left thyrocervical trunk bronchial artery supplying right ling: source of recurrent hemoptysis in cystic fibrosis. AJR Am J Roentgenol. 1992;158:1131–1133. doi: 10.2214/ajr.158.5.1566680. [DOI] [PubMed] [Google Scholar]
- Keller F S, Rösch J, Loflin T G, et al. Nonbronchial systemic collateral arteries: significance in percutaneous embolotherapy for hemoptysis. Radiology. 1987;164:687–692. doi: 10.1148/radiology.164.3.3615866. [DOI] [PubMed] [Google Scholar]
- Stoll J F, Bettmann M A. Bronchial artery embolization to control hemoptysis: a review. Cardiovasc Intervent Radiol. 1988;11:263–269. doi: 10.1007/BF02577032. [DOI] [PubMed] [Google Scholar]
- Schrodt J F, Becker G J, Scott J A, et al. Bronchial artery embolization: monitoring with somatosensory evoked potentials. Radiology. 1987;164:135–139. doi: 10.1148/radiology.164.1.3295987. [DOI] [PubMed] [Google Scholar]
- Chun H J, Byun J Y, Yoo S, et al. Added benefit of thoracic aortography after transarterial embolization in patients with hemoptysis. AJR Am J Roentgenol. 2003;180:1577–1581. doi: 10.2214/ajr.180.6.1801577. [DOI] [PubMed] [Google Scholar]
- Rabkin J E, Astafjev V, Gothman L N, et al. Transcatheter embolization in the management of pulmonary hemorrhage. Radiology. 1987;163:361–365. doi: 10.1148/radiology.163.2.3562815. [DOI] [PubMed] [Google Scholar]
- Bookstein J J, Moser K M, Kalafer M E, et al. The role of bronchial arteriography and therapeutic embolization in hemoptysis. Chest. 1977;72:658–661. doi: 10.1378/chest.72.5.658. [DOI] [PubMed] [Google Scholar]
- Osada H, Kawada T, Ashida H, et al. Bronchial artery aneurysm. Ann Thorac Surg. 1986;41:440–442. doi: 10.1016/s0003-4975(10)62706-3. [DOI] [PubMed] [Google Scholar]
- Harley J D, Killien F C, Peck A G. Massive hemoptysis controlled by transcatheter embolization of the bronchial arteries. AJR Am J Roentgenol. 1977;128:302–304. doi: 10.2214/ajr.128.2.302. [DOI] [PubMed] [Google Scholar]
- Muthuswamy P P, Akbik F, Franklin C, et al. Management of major or massive hemoptysis in active pulmonary tuberculosis by bronchial arterial embolization. Chest. 1987;92:77–82. doi: 10.1378/chest.92.1.77. [DOI] [PubMed] [Google Scholar]
- Vujic I, Pyle R, Hungerford G D, et al. Angiography and therapeutic blockade in the control of hemoptysis. Radiology. 1982;143:19–23. doi: 10.1148/radiology.143.1.7063726. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Yamakado K, Murashima S, et al. Superselective bronchial artery embolization for hemoptysis with a coaxial microcatheter system. J Vasc Interv Radiol. 1997;8:65–70. doi: 10.1016/s1051-0443(97)70517-7. [DOI] [PubMed] [Google Scholar]
- Heesch H A van, Tjan G T, Lampmann L E. Treatment of hemoptysis by embolization of the systemic arteries with isobutyl-2-cyanoacrylate: technique and long term results. J Interv Radiol. 1988;3:63–68. [Google Scholar]
- Mauro M A, Jaques P F. Transcatheter embolization with a Gelfoam slurry. J Interv Radiol. 1987;2:157–159. [Google Scholar]
- Vrachliotis T, Sheiman R G. Treatment of massive hemoptysis with intraarterial thrombin injection of a bronchial artery. AJR Am J Roentgenol. 2002;179:113–114. doi: 10.2214/ajr.179.1.1790113. [DOI] [PubMed] [Google Scholar]
- Remy J, Arnaud A, Fardou H, et al. Treatment of hemoptysis by embolization of bronchial arteries. Radiology. 1977;122:33–37. doi: 10.1148/122.1.33. [DOI] [PubMed] [Google Scholar]
- Guo J X. Arteriography and bronchial artery embolization for massive hemoptysis in 100 cases of tuberculous. Zhonghua Jie He He Hu Xi Za Zhi. 1992;15:77–78. [PubMed] [Google Scholar]
- Goh P YT, Lin M, Teo N, et al. Embolization for hemoptysis: a six-year review. Cardiovasc Intervent Radiol. 2002;25:17–25. doi: 10.1007/s00270-001-0047-1. [DOI] [PubMed] [Google Scholar]
- Brinson G M, Noone P G, Mauro M A, et al. Bronchial artery embolization for the treatment of hemoptysis in patients with cystic fibrosis. Am J Respir Crit Care Med. 1998;157(6 pt 1):1951–1958. doi: 10.1164/ajrccm.157.6.9708067. [DOI] [PubMed] [Google Scholar]
- Ivanick M J, Thorwarth W, Donohue J, et al. Infarction of the left main-stem bronchus: a complication of bronchial artery embolization. AJR Am J Roentgenol. 1983;141:535–537. doi: 10.2214/ajr.141.3.535. [DOI] [PubMed] [Google Scholar]
- Helenon C H, Chatel A, Bigot J M, et al. Fistule sophago-bronchique gauche apres embolization bronchique. Nouv Presse Med. 1977;6:4209. [PubMed] [Google Scholar]
- Munk P L, Morris D C, Nelems B. Left main bronchial-esophageal fistula: a complication of bronchial artery embolization. Cardiovasc Intervent Radiol. 1990;13:95–97. doi: 10.1007/BF02577360. [DOI] [PubMed] [Google Scholar]