Abstract
Blunt and penetrating traumatic injuries may result in acute or subacute vascular injuries. These injuries to solid organs and extremity vessels are often managed in a conservative fashion. Acuity and hemodynamic compromise may dictate a surgical course; however, interventional techniques first popularized in the early 1970s now offer a wide range of solutions principally using transcatheter arterial embolization. There are a wide range of materials and clinical scenarios for which embolization is appropriate. Embolic agents such as coils, Gelfoam, and particles may be used individually or in combination to stop or control bleeding. In this way, embolotherapy may prove to be the safest and most effective form of therapy. The purpose of this article is to review the indications for embolization in the trauma patient and to provide guidelines regarding techniques and material selection.
Keywords: Embolization, trauma, transcatheter arterial embolization
Historically, vessel ligation following surgical exploration was the standard for management of vascular injuries. This practice stemmed from extensive published series from World War II.1,2 With the development of surgical techniques to repair vascular injuries, vessel ligation and amputation became less frequent during the Korean and Vietnam wars.1,3 The acceptance of angiography in the diagnosis of vascular injuries over the years has evolved into the use of endovascular methods of treatment. Embolization for traumatic vascular injury was first described in the early 1970s, with the landmark reports by Rosch and Dotter4 in 1972, and Bookstein and Goldstein5 1 year later. These reports described selective embolization using autologous clot in gastroepiploic and renal branch arteries, respectively.
Because of a combination of recent trends toward conservative management of blunt and penetrating abdominal trauma, and with technological advances in coaxial catheter systems and microcatheters, the interventionalist is being more closely integrated into the trauma team. In this patient population, embolization techniques may prove to be the safest and most effective option to treat many solid organ and extremity vascular injuries.
Highly trained technologists, nurses, and interventionalists with dedicated angiography suites are now available around the clock. The team approach of care for trauma patients now usually includes an interventional radiologist. The interventionalist's role in managing traumatic vascular injuries has been promulgated in the surgical, trauma, and radiology literature and in some cases has supplanted early surgical intervention. New interventional techniques of embolization can be the definitive and often the only intervention required, or might provide preoperative stabilization needed for surgery. Successful treatment by embolization of traumatic vascular injuries ranges as high as 87 to 100%, depending on the location of injury.6,7,8,9,10,11,12 In addition, angiographically identified injury correlates highly with injuries diagnosed at surgery. In one study, 93% of 77 patients with major arterial injuries were visualized on angiography.13 If both definitive diagnosis and therapy can be achieved in the angiography suite, embolization will spare the patient a certainly more invasive procedure, with it own attendant risks and complications.
Many studies discuss nonoperative management of blunt abdominal injuries, and the increasing use of this strategy is ubiquitous in the literature. Nonoperative management, with only monitoring and noninvasive imaging, should be distinguished from nonoperative management with angiography and embolization. For the purposes of this article, conservative management will indicate that the patient undergoes neither surgery nor embolization.
Renal, hepatic, splenic, and extremity traumatic vascular injuries will be discussed in this article. The well-known application of embolization in managing pelvic fracture will be discussed elsewhere in this issue. Similarly, head and neck injuries amenable to interventional techniques will not be discussed here.
PATIENT POPULATION
The trauma patient often has sustained multiple significant solid organ or extremity vascular injuries. In most cases, patient selection for angiography is no longer based on proximity alone; other factors such as clinical evaluation and physical examination by the trauma team, hemodynamic stability, and blunt versus penetrating trauma all factor into the decision to pursue angiography. In one study of 279 trauma patients undergoing angiography, all 77 with major vascular injuries had the vascular abnormality identified within 5 cm of fracture fragments, bullet fragments, and entrance or exit wounds marked with lead markers.13 Imaging studies that have already been performed, such as plain films and computed tomography (CT), may guide the interventionalist once the decision to perform an angiogram has been made.
Trauma patients differ from other patients undergoing angiography in several ways (Table 1). As a group, these patients are usually younger with previously normal vessels; as a result, their arteries tend to be smaller and spasm more easily. Cardiovascular instability, common in trauma patients with vascular and solid organ injuries, drives the interventionalist to rapidly find and possibly treat injured vessels in a time frame different from that of other cases. The trauma patient with hemodynamic compromise due to hypotension and hypovolemia will have high cardiac output that may require increased rate and volume of contrast to achieve adequate arterial opacification. The increased volume of contrast theoretically carries an increased risk of contrast nephropathy, but recent studies with nonionic contrast suggest that this risk is minimal.14 In addition, patients have often undergone contrast-enhanced CT prior to angiography, thereby increasing their overall contrast load. Renoprotective agents such as fenoldopam and N-acetyl-cysteine need to be administered several hours before contrast is administered. This additional time is not typically available in the trauma setting and therefore these agents are not routinely used.
Table 1.
Clinical Issues Specific to Trauma Patients
| Previously normal vessels: tend to be smaller in size and spasm easily |
| Hemodynamic compromise: high cardiac output |
| Higher contrast volumes |
| Coagulopathy |
Altered clotting mechanisms in trauma patients are common. These patients frequently have a progressive coagulopathy, largely due to hypothermia and the administration of packed red cells and volume expanders without concomitant administration of plasma.14,15 Achieving hemostasis before the coagulopathy ensues is helpful to the interventionalist. The presence of a coagulopathy directs the type of embolic agent used and identifies which vessel should be embolized. In particular, catheter-directed embolization with coils requires a functioning coagulation pathway to obtain vessel thrombosis, given that the actual vascular occlusion is caused by thrombosis superimposed on the coil fibers. In the presence of a coagulation disorder, coils may not represent the best-suited embolic agent.
GENERAL PRINCIPLES OF EMBOLIZATION
In the trauma patient, angiography must be performed expeditiously. No matter how quickly the procedure is conducted, it must be understood that embolization requires a significant amount of both time and contrast. For the clinical settings in which either time or contrast must be limited, alternate treatment modalities should be considered.
Digital subtraction angiography (DSA) provides fast and accurate diagnoses. Conventional cut-film angiography is acceptable and may be superior with bowel and chest injuries in which motion might degrade the images; however, DSA has become the standard for trauma angiography. DSA decreases the procedure time—especially important in this patient population—and decreases contrast load. When compared with cut-film angiography, DSA also decreases the radiation dose on an image-by-image basis.
Standard arterial access using the Seldinger technique should be achieved through a common femoral artery approach using the side opposite any unilateral pelvic or lower extremity injury. Given extensive injuries and location of those injuries, as well as the presence of orthopedic fixation devices, axillary or brachial arterial access might be required. Sheaths are not absolutely required; however, many angiographers use them because multiple catheter changes often are needed. In addition, should the catheter become occluded or embolic agent deployment compromised, arterial access can be maintained if a sheath is used.
The diagnostic angiogram must be complete, not only to identify injured vessels but also to document the presence of collaterals and variant anatomy. A normal aortogram does not exclude vascular injury, and therefore selective catheterization of potential bleeding sources must be performed. Findings of vascular injury on angiography include contrast extravasation, occlusion, intimal irregularity, pseudoaneurysm (PSA), arteriovenous malformation, and dissection.
Careful selection of catheters is required once the decision to perform embolization is made. End-hole catheters should be used to decrease the risk of nontarget embolization. Selection of a catheter or coaxial system with an inner diameter and taper large enough to prevent occlusion by the embolic material is important. Coaxial systems and microcatheters allow superselection of injured vessels and hence more selective embolization. Such superselective embolization techniques allow rapid hemostasis in an injured vessel while preserving as much tissue as possible.
As opposed to actively bleeding vessels, PSAs require slightly different considerations. If the vessel is nonvital, it might be sacrificed. However, many vessels cannot be completely embolized. Unlike aneurysms or well-developed PSA, coils placed within the acutely traumatic PSA should be avoided because theoretically, this might lead to expansion and/or rupture of the injured vessel. Use of a covered stent, while becoming more common in certain endovascular repairs, will not be discussed here (they are limited in where and when they can be used). When considering embolization of PSAs, so-called “back-door bleeding” is the most critical technical consideration. To prevent retrograde flow in the embolized vessel from collaterals, the vessel must be occluded both distal and proximal to the injury.17,18 If care is not taken in performing distal embolization, bleeding may occur in retrograde fashion via collateral blood flow. Should retrograde bleeding occur, re-embolization usually cannot be performed through the same vessel because of the more proximal embolization. Terminal vessel PSAs do occur, and if confirmed with scrupulous selective angiography, this technique to prevent back-door bleeding does not need to be employed.
Arteriovenous fistulae (AVF) represent another common injury identified in both visceral and extremity trauma. Penetrating extremity trauma has a high association with AVF formation. As in PSA embolization, retrograde bleeding is of great concern. AVF, in which extensive collaterals may have developed, is the one circumstance in which glue can be used. As one might imagine, considerable skill and personal experience are necessary when using glue.18 Care must be taken with coil embolization of AVFs because high flow through these fistulae might cause migration of the coils. Finally, direct percutaneous puncture of an AVF may be necessary in rare circumstances if access cannot be achieved via a transvascular approach.
An occluded vessel demonstrated at the time of the initial angiogram might need to be embolized. Almost universally, in the setting of trauma, vessels are occluded by thrombus. The underlying vascular injury may be transection, spasm, or dissection, but once the vessel is occluded superimposed thrombosis is nearly always the cause. As mentioned previously, trauma patients have altered coagulation profiles that might disallow reformation of thrombus once the initial clot has lysed. Therefore, a vessel that initially appears stable may be the source of delayed bleeding.
Embolization Agent Choice and Technique
Embolic agent selection is guided principally by two considerations: size of the vessel to be embolized and permanence of the desired occlusion. Personal preference of the angiographer also dictates choice of agents, as does the extent of hemorrhage. For many reasons, Gelfoam (Upjohn Co., Kalamazoo, MI) is the workhorse for trauma embolization procedures (Table 2).
Table 2.
Benefits of Gelfoam
| Readily available |
| Inexpensive |
| Temporary |
| Easy to work with |
| Leads to mechanical obstruction |
| Can be injected though a microcatheter |
Gelfoam is inexpensive, readily available, and temporary, with recanalization typically occurring as early as 2 to 3 weeks postprocedure. A temporary occlusive agent is adequate to stop active extravasation in the trauma population, to stop the hemorrhage, and to allow the artery to heal itself. Gelfoam can be used in several ways. An embolic pledget (also described as a torpedo) measuring from 1 to several millimeters in longitudinal dimension can be mixed with contrast and injected. Gelfoam particles, which measure from 50 to 450 μm, are not frequently used because of the risk of distal embolization and subsequent ischemia. In our experience, Gelfoam slurry is the preferred product for trauma embolization.
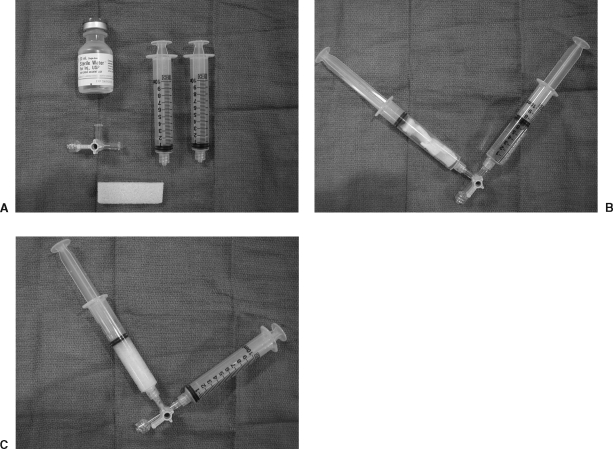
The Gelfoam sponge is torn into several pieces, then back-loaded into a syringe. Saline and Gelfoam are mixed into a slurry by sequential mixing of the two through a three-way stopcock connected to two syringes. Enough Gelfoam and saline are used to gain the consistency of pudding (Fig. 1).
Figure 1.
Preparation of Gelfoam slurry. (A) The tools needed for Gelfoam slurry embolization: two syringes, 5 to 10 mL of fluid, and a Gelfoam sponge. (B) The Gelfoam is torn into multiple pieces and back-loaded into one of the syringes. The other syringe is filled with 5 to 10 mL of fluid, and both syringes are attached to a three-way stopcock. (C) After using a to-and-fro motion between the two syringes, the Gelfoam slurry should have the consistency of pudding.
Making Gelfoam slurry can be quickly accomplished without significant time limitations. The slurry is injected slowly under fluoroscopic guidance through the catheter until forward flow is reduced, but not to the point of cessation. This will ensure that embolic material does not reflux into nontarget vessels. If Gelfoam is injected until complete stasis is achieved, the risk of nontarget embolization is high. The Gelfoam slurry forms a “cast” of the vessel into which it is injected, causing a more proximal occlusion than that seen with particulate agents.
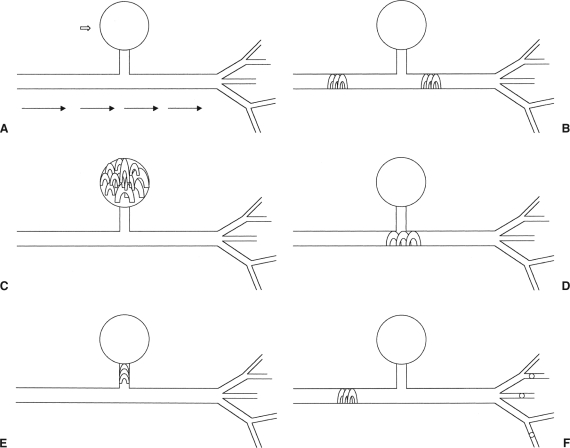
Coils are also commonly used for trauma embolotherapy, most often when a permanent occlusion is desired. Care should be taken to select a coil that reforms to the size of the vessel without forward movement and more distal embolization. In this way coils provide rapid control of bleeding, particularly in larger vessels. The appropriate number of coils within the coil pack should be selected to occlude the vessel and stop flow without causing retrograde propagation of clot into adjacent vessels. With traumatic AVF and PSA formation, coils are often selected for embolization because of the precise control necessary during deployment to ensure there is no antegrade or retrograde migration. Treatment of PSA with coils can be performed using the sandwich technique of coils and Gelfoam as described below; alternatively, PSA can be packed with coils or a single coil can be placed across the PSA origin or directly into the PSA neck. If coils cannot safely be placed beyond the neck of the PSA, particulate embolization can be performed distal to the neck to decrease the risk of retrograde filling of the PSA, followed by coil embolization of the feeding vessel (Fig. 2).
Figure 2.
Methods of pseudoaneurysm embolization. A pseudoaneurysm (PSA; open arrow) is noted arising from an artery (A). Flow is from left to right, and after the PSA origin the artery bifurcates into multiple arteriolar branches. The sandwich technique (B) gives both distal and proximal control of the PSA, preventing retrograde filling of the PSA from the distal branches. The PSA itself can be packed with multiple coils (C); conversely, the origin (D) or the neck (E) can be embolized. If distal control cannot be safely obtained, particles can be injected beyond the PSA origin, followed by proximal coil embolization (F).
Most available coils have thrombogenic fibers attached to their metal frame. Consideration of ongoing coagulopathy in many trauma patients as described previously can dictate the use of multiple agents. Specifically, occlusion with coils might be incompletely effective or lead to delayed bleeding in a coagulopathic patient. This occurs because of the lack of thrombosis superimposed on the coil fibers, or due to the lysis of the initial clot and the inability to form another clot on the coil. In our experience, a so-called Gelfoam sandwich works well in these circumstances. Coils are placed as a strut, followed by Gelfoam to cause a mechanical occlusion; two or three layers are placed in such a fashion. A dual-lumen occlusion balloon catheter can be used to decrease proximal embolization of Gelfoam and coils when using this technique. In addition, this balloon occlusion technique limits antegrade forward flow, aiding in thrombosis and increasing control of embolic agents. This might be especially important during AVF embolization where flow can be high.
Particulate embolization includes polyvinyl alcohol and tris-acryl gelatin microspheres (Embospheres, BioSphere, Rockland, MA). These agents embolize distally until they occlude a vessel smaller than the particle size. Typically, particles are not the agent of choice in the trauma setting. However, in splenic and renal trauma where distal selective embolization might be needed but catheter access is limited to the main splenic artery, this agent might be chosen.7,19 Particles might also be used where distal control cannot be achieved by placement of Gelfoam or coils.18 By injecting large particles proximal to a PSA, antegrade flow carries these particles beyond the PSA and causes occlusion of potential retrograde feeding vessels. Proximal control can then be gained in the usual fashion with coil embolization.
Sclerosing agents can be used with care in the trauma setting but their utility is limited to one indication. In our experience, absolute alcohol might be the agent of choice when rapid hemostasis is required because of multiple bleeding sites from a solid organ that can be safely sacrificed, such as with a shattered kidney. As with other uses of sclerosants for embolization, balloon occlusion catheters should always be used to prevent reflux of the sclerosant into adjacent vessels. Following injection, suction is aggressively placed on the syringe to remove whatever alcohol remains before the occlusion balloon is deflated. By this method, rapid hemostasis will be achieved at the expense of organ parenchyma.
Specific Clinical Scenarios
Embolization can be performed in the trauma patient in several scenarios. Embolization might be indicated when diagnostic angiography demonstrates a vascular injury in an unstable patient despite emergent surgery, in a patient initially managed conservatively who later becomes unstable, or following an angiogram performed as a targeted screening tool days or weeks after an injury in a stable patient because of the suspicion of posttraumatic vascular injuries. In addition to categorizing embolization procedures on the basis of their time course, embolization might be discussed according to the vessel or organ targeted. The discussion below focuses on organ-specific embolization procedures in the trauma patient.
RENAL EMBOLIZATION
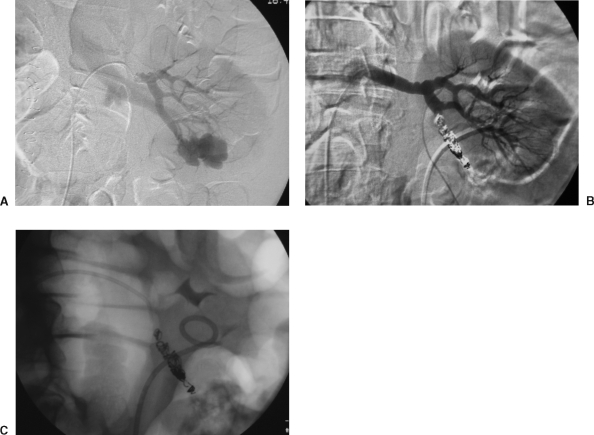
Blunt abdominal trauma as a cause of renal vascular injury is second only to penetrating trauma from stabbing and gunshot wounds. These injuries often result in immediate or delayed posttraumatic hemorrhage, PSA with or without AVF, or AVF alone. Specifically, PSA and AVF are commonly found days or weeks following the initial traumatic event (Fig. 3). However, PSA might present acutely with mass effect, hematuria, or rupture with extracapsular hemorrhage.
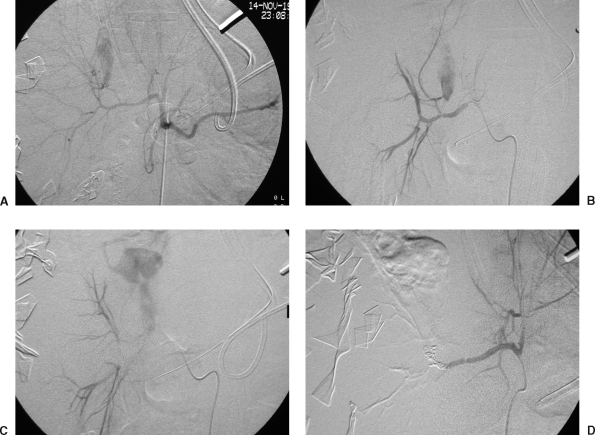
Figure 3.
Renal artery embolization in a 35-year-old male, status post stab wound to the abdomen 6 days earlier. He presented with a decreasing hematocrit level and bleeding from his Foley catheter. (A) Selective left renal artery injection demonstrates a large pseudoaneurysm (PSA) arising from the lower lobe arterial distribution. Note also the early appearance of the renal vein, indicating a concomitant AVF. (B) Selective left renal artery injection following coil embolization of the lower lobe artery, demonstrating complete cessation of flow to the lower lobe arterial distribution. The PSA and AVF are no longer visualized. Of import, note the persistent flow to the remainder of the kidney. (C) Unsubtracted image demonstrating the coil pack postembolization. Figure courtesy of Jan Durham, M.D.
In addition to accidental trauma, renal embolization also might be helpful when treating iatrogenic renal injuries from percutaneous biopsy, nephrostomy, or nephrolithotomy.6
It is generally accepted that hemodynamically unstable patients with renal injuries—and all solid organ injuries with instability—will usually undergo immediate laporotomy.7,20,21,22 In the hemodynamically stable patient, vascular injuries should be considered on the basis of proximity of injury, screening contrast-enhanced abdomen and pelvis CT findings (e.g., retroperitoneal hematoma), and gross or microscopic hematuria. Most blunt renal injuries are diffuse capsular and parenchymal injuries; in a hemodynamically stable patient these most often are managed conservatively. These injuries correspond to Renal Injury Scale Grade I and II.7,20,21 Blunt trauma may occasionally lead to PSA formation in main and branch renal arteries while penetrating trauma from stabbing and gunshot wounds leads to direct vascular injury with extravasation and delayed AVF and PSA formation.5,23 Currently, high-grade injuries (grades III to V) and penetrating injuries are more likely to undergo angiography. Contrast-enhanced CT is the accepted modality for the initial evaluation of suspected renal injury because of its high sensitivity and specificity. Excretory urography, which was commonly used in the past, is rarely used today.
There is a trend toward obtaining angiography before surgery because this provides diagnostic information as well as possible definitive treatment, especially with penetrating injuries.10,24 Recent literature suggests that with correct patient selection, grade III to V injuries with evidence of uncontrolled bleeding can be treated effectively with embolization, achieving the goals of hemostasis and maximizing preservation of renal parenchyma.6,7,20,21,24,25 In a recent series of nine consecutive patients with renal vascular injuries following blunt trauma, Dinkel et al7 demonstrated effective control of bleeding in three patients with frank extravasation and six patients with PSA and/or AVF. Superselective embolization was performed wherever possible. In a separate study, Hagiwara et al20 demonstrated that of 21 patients with grade III or greater renal injury on CT, seven of eight patients with extravasation on angiography were successfully embolized. Furthermore, shock index (heart rate divided by systolic blood pressure) and average rate of fluid resuscitation improved significantly following embolization.
Before the development of coaxial systems and microcatheters, superselective angiography and embolization were not possible. As a result, more proximal embolization was performed, leading to increased renal parenchymal loss. Superselective technique is now the standard. Fisher et al6 showed less than 30% loss of parenchyma in 12 of 15 consecutive renal embolizations in trauma patients. Unlike splenic and hepatic arterial beds, renal arteries are end arteries and therefore occlusion of vessels proximal to the site of the injury will achieve hemostasis without retrograde bleeding. Rarely, parenchymal enhancement can be seen from collateral capsular arterial branches; therefore, any embolization should be performed proximal to the origin of these vessels. Packing of a PSA neck, or sandwiching a PSA or AVF with proximal and distal control, is not required because of the end arterial supply of the kidney and lack of potential retrograde filling.
SPLENIC INJURIES
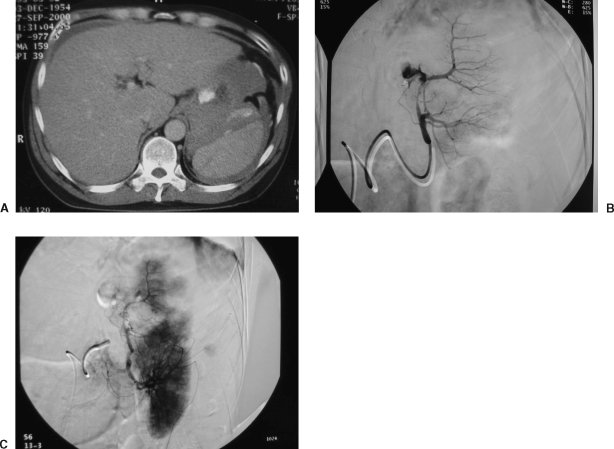
Traumatic injuries to the spleen often are amenable to embolization. Embolotherapy spares the patient a more invasive procedure and compared with splenectomy, targeted embolization saves tissue; it also spares the patient from the risks of postsplenectomy sepsis by maintaining splenic immune function (Fig. 4). As is the standard for other visceral injuries from blunt abdominal trauma, traumatic splenic injuries are commonly managed conservatively. Generally speaking, hemodynamic stability dictates whether immediate surgery or expectant management with or without transarterial embolization will be the course of treatment.8,9,27 However, multiorgan injuries and extremes of age might complicate nonoperative management alone. Contrast-enhanced spiral CT is an effective and accepted way of identifying splenic injuries and guiding the trauma team toward angiogram and possible embolization. However, the true value for identifying vascular injury or predicting surgical versus conservative management remains controversial.8,28
Figure 4.
Splenic embolization in a 45-year-old unrestrained driver in a motor vehicle collision. (A) Contrast-enhanced computed tomography scan demonstrates a large splenic laceration and perisplenic hematoma with active contrast extravasation. (B) Selective splenic angiogram demonstrates active contrast extravasation from the proximal portion of the upper lobe artery. (C) Postembolization angiogram demonstrates cessation of flow to the actively bleeding branch. Note the segmental perfusion abnormalities: these are secondary to Gelfoam embolization. Because of persistent bleeding, a single coil was placed in the proximal bleeding vessel. Note also the uncompromised perfusion to the lower lobe branch distribution.
Grade I and II injures are managed conservatively. The trauma team will use triage imaging and hemodynamic status of the patient to guide the decision for angiography. Angiography addresses the issue of acuity of perisplenic or subcapsular fluid collections identified on imaging studies.8,23 As with other organ systems, the interventionalist has the opportunity to definitively treat the patient as well as provide a diagnosis. Recently, Shanmuganathan et al28 showed 100% sensitivity for contrast extravasation on CT in predicting the need for splenic arteriography with subsequent endovascular treatment or splenic surgery. Six of these seven patients (86%) who had CT evidence of extravasation also had angiographic evidence, whereas one of seven (14%) had no angiographic evidence of extravasation (false-negative) but required splenectomy 12 hours later for continued bleeding. In two separate studies, Scalfani et al8 and Hiagawara et al9 have shown that splenic angiography can be used to diagnose and successfully embolize traumatic splenic vascular lesions with all grades of splenic injuries. Although splenic injury severity was greatest in patients who ultimately required embolization in these studies, CT appearance did not predict angiographic findings. It is accepted that the likelihood of vascular injury increases with increasing splenic injury severity score.8,9
The vascular injuries sustained in the spleen are no different from those in other organs. Active contrast extravasation from transmural arterial injury, as well as PSA and AVF, are common. The natural progression of untreated splenic PSA is not known. Although some injuries might spontaneously thrombose, the limited structural integrity of a PSA (adventitia and perivascular parenchyma) may lead to failure of nonoperative management in up to 67% of splenic PSAs.19 Therefore, even in the absence of contrast extravasation, PSAs typically are embolized whenever they are identified.
Although high splenic salvage rates are documented in the literature, the incidence of preservation of splenic function is not known.8,9,29 Interestingly, in one study, 12 of 13 patients (92%) who had a combination of selective and main splenic artery embolization demonstrated scintigraphic splenic uptake on sulfur colloid scan several weeks after the procedure.9 This emphasizes the intense collateralization available to the spleen as opposed to the end arterial supply of the kidney. As a result of this collateral flow, several options are available to the angiographer when considering splenic embolization. If diffuse bleeding is identified or suspected, selective distal embolization with Gelfoam may be performed with subsequent coil embolization of the proximal splenic artery. This combined embolization may help with oozing associated with this type injury8,30; theoretically, decreasing the pressure head behind the injured vessel will lead to more timely hemostasis and has been performed routinely in several series.8,9 Alternatively, selective embolization of a specific bleeding site without proximal embolization can be employed.
HEPATIC INJURIES
Hepatic injuries are common in blunt trauma. As with splenic and renal trauma, conservative management has become the standard of care, especially for blunt injuries.31,32 However, a recent published series of 1842 liver injuries shows angiography and embolization used to control bleeding increased from < 1% in the late 1970s to 9% in the late 1990s, mirroring the increasing use of conservative management with visceral injuries.31
As with most blunt abdominal traumatic injuries, contrast-enhanced CT usually demonstrates any significant hepatic injury. However, there is a paucity of information regarding the efficacy of CT in high-grade liver injuries. Extravasation of contrast during angiography is concordant with high-grade hepatic injuries, and these patients typically fail conservative management. Deep parenchymal bleeding from complex traumatic liver injuries is often amenable to embolization rather than surgery33 (Fig. 5). Although a retrospective study of 404 patients with blunt hepatic injury showed 14% with high-grade injuries, 66% of those who experienced failure after conservative management were those with organ injury scale IV and V injuries.34 Hagiwara et al35 demonstrated that of 28 patients with Mirvus grade III to IV hepatic injuries by CT, 15 had angiographic evidence of extravasation and all were successfully embolized. Furthermore, as with their splenic embolization study, the shock index, a common means of expressing hemodynamic stability in trauma patients, was significantly lower in these patients following embolization. This supports the role of early embolization in trauma patients, and may argue against the commonly held belief that all hemodynamically unstable patients should proceed to emergency laparotomy. Early embolization in blunt liver trauma also has been shown to decrease the volume of blood products and number of liver-related operations.26
Figure 5.
Blunt hepatic trauma in a 29-year-old male status post motor vehicle collision, with liver laceration and diaphragmatic injury by initial computed tomography scan. The patient was initially treated conservatively. Seven days later, the patient went to the operating room for an emergency laparotomy due to bleeding from his liver laceration; packing failed to control the bleeding, and the patient was transferred emergently to the angiography suite. (A) Diagnostic angiogram of the common hepatic artery demonstrating contrast extravasation from one of the right hepatic artery branches. Note the early bifurcation of the proper hepatic artery, with the left hepatic artery arising proximally. (B) Diagnostic angiogram of the right hepatic artery, early phase, demonstrating severe contrast extravasation from the proximal right hepatic artery. (C) Diagnostic angiogram of the right hepatic artery, late phase, showing large amount of contrast pooling from the proximal right hepatic arterial injury. (D) Postembolization angiogram demonstrating coil pack within the right hepatic artery. Gelfoam slurry failed to stop the extravasation. Figure courtesy of David Kumpe, M.D.
The liver's dual blood supply allows for safe embolization of the hepatic artery. Once patency of the portal vein is proven, embolization can be performed anywhere in the hepatic artery. If small particles are used, embolization is safest if performed distal to the origin of the cystic artery. Arterial embolization in trauma patients has not been proven effective for bile duct and venous injuries; however, tamponade within the organ tends to limit venous bleeding. Delayed posttraumatic injures such as PSA, AVF, and arterial-biliary fistulae often occur. The suspected cause of these delayed injuries is unique to the liver. Specifically, spillage of bile leads to inflammation that may damage adjacent vessels, subsequently leading to delayed bleeding.35 Some conclude that a nuclear medicine hepatoiminodiacetic acid (HIDA) scan should be performed to rule out bile leaks in patients following embolization for high-grade hepatic injuries.35 Hepatic arterial-portal fistulae and hemobilia from fistulae between vessels and bile ducts also are unique to the liver and can be treated safely with embolization.26,36
EXTREMITY EMBOLIZATION
Traumatic vascular injury in the extremity is usually the result of a penetrating injury. Direct blunt trauma can also cause extremity vascular injury, especially in the setting of fractures and joint dislocations. Angiography has been shown to be sensitive in detecting vascular injuries of the extremity.37 Because of the historically low yield during surgical exploration, exclusion angiography is the standard for evaluating patients when vascular injuries are suspected. Unlike in visceral injury, physical examination has higher yield when evaluating the extremities. Hard clinical signs of vascular injury include active bleeding, diminished pulses, expanding or pulsatile hematoma, bruit or thrill, and evidence of peripheral ischemia.38,39 These findings all factor into the decision to perform angiography or to proceed directly to the operating room.
As with other traumatic injuries, angiography with possible endovascular therapies has become an important factor in managing suspected extremity vascular injury. Major findings include occlusion, extravasation, PSA, and AVF; in addition, intraluminal filling defects such as thrombi and intimal flaps can also be visualized. Minor findings include luminal narrowing, focal widening of the lumen, arterial deviation, and slow flow that may be encountered in the calf because of high compartment pressures. As opposed to visceral injuries in which CT plays a major diagnostic role, physical examination is vital in the diagnosis of vascular injury in the extremity.17 These physical examination findings help refine the algorithm for the decision to perform angiography and possible transarterial therapy, given that absence of physical examination findings is not universally accepted to exclude clinical vascular injury in the extremity. This is particularly true with injuries central to the hip or shoulder girdle. Noninvasive imaging techniques such as Doppler evaluation of vessels might be insensitive to extremity vascular injuries; it has been shown that Doppler interrogation can be falsely positive because of hemodynamic instability regardless of the presence vascular injury.40
Proximity injuries in the appendicular skeleton are controversial. In the current environment, proximity alone generally is not accepted as the sole criterion for angiography because of low yield on exploratory angiography and likely benign course of many clinically occult injuries.17,38,40,41 Exceptions to this rule include shotgun injuries and other injuries with large blast effects; these patients routinely undergo angiography regardless of clinical findings.14,16,35 Certain fractures or fracture dislocations involving the knee and elbow also routinely undergo angiography given the high association with vascular injury.17,40 Angiographic evaluation and possible endovascular therapy, however, is obviated by active external bleeding or expanding hematoma that is life threatening. Emergent surgical exploration is typically indicated in these patients.
Embolization is used to treat extravasation, PSA, and AVF as described previously with solid organ injuries. The principle of gaining proximal and distal control as close to the site of injury must be observed to minimize rebleeding from collateralization. Moreover, rapid hemostasis with minimal tissue compromise is the goal of therapy, and embolotherapy might prove impossible if the downstream organ cannot be embolized safely. However, there are several differences regarding extremity trauma when compared with visceral embolization. Extravasation can only be embolized in nonvital branch vessels. Parallel arteries of the calf and forearm as well as nonaxial proximal arteries (profunda femoral [PFA] and profunda brachial arteries) can usually be embolized safely.7,40 Actively bleeding major vessels, such as the superficial femoral, popliteal, and axillary arteries should not be embolized because of the risk of subsequent limb-threatening ischemia. Some injuries in main extremity arteries are amenable to percutaneous therapies and can be treated by the interventional radiologist. These injuries are more commonly being treated with endovascular covered stent placement that is beyond the scope of this discussion. In addition, major bleeding vessels such as the femoral artery and the axillary artery can be temporized with an occlusion balloon as an aid to surgical intervention.
When embolization is being considered, it is important to first document adequate collateral flow. Embolization is useful when surgically inaccessible actively bleeding vessels, such as the PFA, are identified.40,42 Underlying disease processes also must be considered. In young patients it is uniformly considered safe to sacrifice the PFA; in older patients, the PFA may represent the major vascular supply to the lower leg.17,42
Complications
The risks of trauma angiography and embolization are typical of other angiographic procedures. The most common of these potential complications include contrast reactions and vascular injury. In 1987, a published series of 279 consecutive angiograms performed for trauma resulted in a 5% complication rate.13 In this study, there were no clinical sequelae nor was surgical intervention required. In a recent prospective study of 100 consecutive patients undergoing embolization for bleeding in the abdomen and pelvis, 5 patients (5%) developed contrast nephropathy, with creatinine returning to baseline within 5 days in all patients. These patients received an average of 248 mL of nonionic, low-osmolality contrast administered during their embolization procedure.14 A separate study has shown minor complications in 4% of 137 patients undergoing angiography for abdominal trauma.10
Embolization can lead to a greater area of tissue loss than expected at the time of embolization. However, the greatest fear is nontarget embolization into distant or adjacent vessels. The true incidence of nontarget embolization is unknown, probably because of its rarity and the often silent nature of such an event. Several prospective embolization studies have reported no significant complications related to embolization in their series.7,8,9,10,35 The likelihood of nontarget embolization can be minimized with meticulous attention to detail. Superselective technique, frequent checking of catheter position, frequent interval angiograms to check for vascular stasis, and slow extrusion of embolic agent are key to minimizing such complications.
Migration of coils is a rare complication. Again, the true incidence of coil migration is unknown. Several series have reported distant migration of a coil without significant sequelae.7,8 Intraluminal retrieval devices can be used to snare misplaced coils.
Postembolization syndrome (pain, leukocytosis, and pyrexia) is uncommon in the trauma population.21 Despite many clinical concerns, hypertension following partial renal embolization is rare.6,7 Similarly, postembolization infections and abscess formation seem uncommon. Such complications are purported to be minimal with superselective embolization, which is now considered the standard.19
CONCLUSIONS
The paradigm for management of traumatic injuries is shifting. Until the late 1970s, operative management was considered the only legitimate course for blunt and penetrating abdominal and extremity vascular injuries. Many studies suffer from the lack of inclusion of angiography and embolotherapy in evaluation of nonsurgical management of patients. A therapeutic alliance between trauma surgeons and interventional radiologists will advance the standard of care for trauma patients who might require embolization. Much work remains; specifically, although angiography has decreased the false-negative rate of surgical exploration, angiography itself can suffer the same problem. Ongoing investigation of noninvasive imaging and its correlation to angiographic findings will improve the yield and effectiveness of interventional techniques.
Continued vigilance on the part of the trauma and surgical communities to incorporate the interventionalist into the trauma team is required. Embolization represents a safe and effective technique for rapidly achieving hemostasis, probably even in some patients previously relegated to surgery because of hemodynamic concerns. Interventionalists welcome this complimentary role in trauma care that allows for not only definitive treatment of vascular injuries but also for selection of those patients who may ultimately experience failure of conservative management.
REFERENCES
- Rich N M, Baugh J H, Hughes C W. Acute arterial injuries in Vietnam: 1,000 cases. J Trauma. 1970;10:359–369. doi: 10.1097/00005373-197005000-00001. [DOI] [PubMed] [Google Scholar]
- DeBakey M E, Simeone F A. Battle injuries of the arteries in World War II: an analysis of 2,471 cases. Ann Surg. 1946;123:534–573. [PubMed] [Google Scholar]
- Inui F K, Shannon J, Howard J M. Arterial injuries in the Korean conflict: experiences with 111 consecutive cases. Ann Surg. 1954;17:850–857. [PubMed] [Google Scholar]
- Rosch J, Dotter C T, Brown M J. Selective arterial embolization: a new method for control of acute gastrointestinal bleeding. Radiology. 1972;102:303–306. doi: 10.1148/102.2.303. [DOI] [PubMed] [Google Scholar]
- Bookstein J J, Goldstein H M. Successful management of postbiopsy arteriovenous fistula with selective arterial embolization. Radiology. 1973;109:535–536. doi: 10.1148/109.3.535. [DOI] [PubMed] [Google Scholar]
- Fisher R G, Ben-Menachem Y, Whigham C. Stab wounds of the renal artery branches: angiographic diagnosis and treatment by embolization. AJR Am J Roentgenol. 1989;152:1231–1235. doi: 10.2214/ajr.152.6.1231. [DOI] [PubMed] [Google Scholar]
- Dinkel H, Danuser H, Triller J. Blunt renal trauma: minimally invasive management with microcatheter embolization—experience with nine patients. Radiology. 2002;223:723–730. doi: 10.1148/radiol.2233011216. [DOI] [PubMed] [Google Scholar]
- Sclafani S JA, Shaftan G W, Scalea T M, et al. Nonoperative salvage of computed tomography-diagnosed splenic injuries: utilization of angiography for triage and embolization for hemostasis. J Trauma. 1995;39:818–825. doi: 10.1097/00005373-199511000-00004. [DOI] [PubMed] [Google Scholar]
- Hagiwara A, Ukioka T, Ohta S, et al. Nonsurgical management of patients with blunt splenic injury: efficacy of transcatheter arterial embolization. AJR Am J Roentgenol. 1996;167:159–166. doi: 10.2214/ajr.167.1.8659363. [DOI] [PubMed] [Google Scholar]
- Velmahos G C, Chahwan S, Falabella A, Hanks S E, Demetriades D. Angiographic embolization for intraperitoneal and retroperitoneal injuries. World J Surg. 2000;24:539–545. doi: 10.1007/s002689910087. [DOI] [PubMed] [Google Scholar]
- Kaufman S L, Martin L G, Zuckerman A M, et al. Peripheral transcatheter embolization with platinum microcoils. Radiology. 1992;184:369–372. doi: 10.1148/radiology.184.2.1620829. [DOI] [PubMed] [Google Scholar]
- Scalfani J A, Cooper R, Shaftan G W, et al. Arterial trauma: diagnostic and therapeutic angiography. Radiology. 1986;161:165–172. doi: 10.1148/radiology.161.1.3763860. [DOI] [PubMed] [Google Scholar]
- Rose S C, Moore E E. Emergency trauma angiography: accuracy, safety and pitfalls. AJR Am J Roentgenol. 1987;148:1243–1246. doi: 10.2214/ajr.148.6.1243. [DOI] [PubMed] [Google Scholar]
- Vassiliu P, Sava J, Toutouzas K G, Velmahos G C. Is contrast as bad as we think? Renal function after angiographic embolization of injured patients. J Am Coll Surg. 2002;194:142–146. doi: 10.1016/s1072-7515(01)01138-3. [DOI] [PubMed] [Google Scholar]
- Ray C E, Waltman A C. General principles of embolization and chemoembolization. New York: Thieme; 2002. In: Vascular and Interventional Radiology: Principles and Practice, 1st ed. pp. 89–100.
- Coldwell D M, Stokes K R, Yakes W F. Embolotherapy: agents, clinical applications, and techniques. Radiographics. 1994;14:623–643. doi: 10.1148/radiographics.14.3.8066276. [DOI] [PubMed] [Google Scholar]
- Hanks S E, Pentecost M J. Angiography and transcatheter treatment of extremity trauma. Semin Interv Radiol. 1992;9:19–27. [Google Scholar]
- Jackson J E, Mitchell A. Advanced vascular interventional techniques in the management of trauma. Semin Interv Radiol. 1997;14:139–150. [Google Scholar]
- Davis K A, Fabian T C, Croce M A, et al. Improved success in nonoperative management of blunt splenic injuries: embolization of splenic artery pseudoaneurysms. J Trauma. 1998;44:1008–1013. doi: 10.1097/00005373-199806000-00013. [DOI] [PubMed] [Google Scholar]
- Hagiwara A, Sakaki S, Goto H, et al. The role of interventional radiology in the management of blunt renal injury: a practical protocol. J Trauma. 2001;51:526–531. doi: 10.1097/00005373-200109000-00017. [DOI] [PubMed] [Google Scholar]
- Larsen D W, Pentecost M J. Embolotherapy in renal trauma. Semin Interv Radiol. 1992;9:13–18. [Google Scholar]
- Altman A L, Haas C, Dinchaman K H, Spirnak J P. Selective nonoperative management of blunt grade 5 renal injury. J Urol. 2000;164:27–31. [PubMed] [Google Scholar]
- Ray C E. Renal embolization. Semin Interv Radiol. 2001;18:37–45. [Google Scholar]
- Heyns C F, Vollenhoven P Van. Increasing role of angiography and segmental artery embolization in the management of renal stab wounds. J Urol. 1992;147:1231–1234. doi: 10.1016/s0022-5347(17)37524-9. [DOI] [PubMed] [Google Scholar]
- Corr P, Hacking G. Embolization in traumatic intrarenal vascular injuries. Clin Radiol. 1991;43:262–264. doi: 10.1016/s0009-9260(05)80252-1. [DOI] [PubMed] [Google Scholar]
- Wahl W L, Ahrns K S, Brandt M, Franklin G A, Taheri P A. The need for early angiographic embolization in blunt liver injuries. J Trauma. 2002;52:1097–1101. doi: 10.1097/00005373-200206000-00012. [DOI] [PubMed] [Google Scholar]
- Brasel K J, DeLisle C M, Olson C J, Borgstrom D C. Splenic injury: trends in evaluation and management. J Trauma. 1998;44:283–286. doi: 10.1097/00005373-199802000-00006. [DOI] [PubMed] [Google Scholar]
- Shanmuganathan K, Mirvis S E, Boyd-Kranis R, Takada T, Scalea T M. Nonsurgical management of blunt splenic injury: use of CT criteria to select patients for splenic arteriography and potential endovascular therapy. Radiology. 2000;217:75–82. doi: 10.1148/radiology.217.1.r00oc0875. [DOI] [PubMed] [Google Scholar]
- Haan J, Scott J, Boyd-Kranis R L, et al. Admission angiography for blunt splenic injury: advantages and pitfalls. J Trauma. 2001;51:1161–1165. doi: 10.1097/00005373-200112000-00023. [DOI] [PubMed] [Google Scholar]
- Kidney D D. In: Dyet JF, Ettles DF, Nicholson AA, Wilson S, editor. Textbook of Endovascular Procedures, 1st ed. Philadelphia: Churchill Livingston; 2000. The endovascular approach to trauma. pp. 313–327.
- Richardson D J, Franklin G A, Lukan J K, et al. Evolution in the management of hepatic trauma: a 25 year perspective. Ann Surg. 2000;232:324–330. doi: 10.1097/00000658-200009000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo E H, Platz A, Miller F B, Richardson J D, Polik H C. Non-operative management of blunt hepatic trauma. Br J Surg. 1998;85:461–468. doi: 10.1046/j.1365-2168.1998.00721.x. [DOI] [PubMed] [Google Scholar]
- Asensio J A, Demetriades D, Chahwan S, et al. Approach to the management of complex hepatic injuries. J Trauma. 2000;48:66–69. doi: 10.1097/00005373-200001000-00011. [DOI] [PubMed] [Google Scholar]
- Pachter H L, Knudson M M, Esrig B, et al. Status of nonoperative management of blunt hepatic injuries in 1995: a multicenter experience with 404 patients. J Trauma. 1996;40:31–38. doi: 10.1097/00005373-199601000-00007. [DOI] [PubMed] [Google Scholar]
- Hagiwara A, Yukioka T, Ohat S, et al. Nonsurgical management of patients with blunt hepatic injury: efficacy of transcatheter arterial embolization. AJR Am J Roentgenol. 1997;169:1151–1156. doi: 10.2214/ajr.169.4.9308480. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Hiramatsu K, Ido K, et al. Expanding role of emergency embolization in the management of sever blunt hepatic trauma. Cardiovasc Intervent Radiol. 1990;13:193–199. doi: 10.1007/BF02575473. [DOI] [PubMed] [Google Scholar]
- Snyder W H, Thal E R, Bridges R A. The validity of normal arteriography in penetrating trauma. Arch Surg. 1978;113:424–428. doi: 10.1001/archsurg.1978.01370160082013. [DOI] [PubMed] [Google Scholar]
- Frykberg E R, Dennis J W, Bishop K, Laneve L, Alexander R H. The reliability of physical examination in the evaluation of penetrating extremity trauma for vascular injury: results at one year. J Trauma. 1991;31:502–511. doi: 10.1097/00005373-199104000-00009. [DOI] [PubMed] [Google Scholar]
- Frykberg E R. Advances in the diagnosis and treatment of extremity vascular trauma. Surg Clin North Am. 1995;75:207–223. doi: 10.1016/s0039-6109(16)46584-9. [DOI] [PubMed] [Google Scholar]
- Reid S K, Kirchner T M, Pagan-Marin H. Arteriography and intervention in extremity trauma. Semin Interv Radiol. 1991;14:193–204. [Google Scholar]
- Francis H, Thal E R, Weigelt J A, Redman H C. Vascular proximity: is it a valid indication for arteriography in asymptomatic patients. J Trauma. 1991;31:512–514. [PubMed] [Google Scholar]
- Scalfani SJA, Shaftan G W. Transcatheter treatment of injuries to the profunda femoris artery. AJR Am J Roentgenol. 1982;138:463–466. doi: 10.2214/ajr.138.3.463. [DOI] [PubMed] [Google Scholar]