Abstract
Native arteriovenous fistulae are the best form of hemodialysis access. Every effort should be made to preserve and maintain these valuable access sites. The typical fistula is created from the distal radial artery to an adjacent forearm vein. Thrombosis is a relatively rare occurrence but when it occurs, surgical declotting is not as successful as percutaneous approaches. Thus, the interventional radiologist needs to be prepared to evaluate and declot fistulae. As the percentage of fistulae increases in the general dialysis population, radiologists expect to see the demand for percutaneous declotting increase. This article reviews approaches to declotting and maintaining fistulae. Common procedural complications and their treatment or follow-up are also reviewed.
Keywords: Arteriovenous fistula, dialysis, shunts, veins, transluminal angioplasty
Hemodialysis can be performed using native arteriovenous fistulae, synthetic loop grafts, and large-bore central venous catheters. Native arteriovenous fistulae are the best and most durable access for hemodialysis. The Brescia-Cimino native fistula was first described in 1966 and is a surgically created anastomosis of the distal radial artery and adjacent forearm vein.1 Compared with synthetic grafts, patency is much longer, averaging 58 to 70 months for native fistulae vs. 18 to 22 months for synthetic grafts. In addition, the infection rate is lower, averaging one per 200 years for a native fistula vs. one per 13.5 years for synthetic grafts.2 Large-bore central venous catheters, both tunneled and temporary, have extremely high complication rates consisting of thrombosis and infection, and should only be used when grafts and fistulae cannot be fashioned. Temporary and tunneled catheters have an infection rate 32.6 and 13.6 times higher than native fistulae and grafts, respectively.3 Each episode of catheter sepsis has an associated 5 to 10% risk of death. Thus, if a patient has a native arteriovenous fistula, every effort should be made to preserve it.
In the past, some interventional radiologists considered native fistulae too difficult to declot successfully.4 However, recently there have been a large number of small and large series describing native fistula declotting, with success rates varying from 73 to 100%.5,6,7,8,9,10,11,12,13,14,15 Nearly all current published literature focuses on percutaneous interventional techniques as the preferred method to restore flow to thrombosed or malfunctioning native fistulae. If interventional techniques fail, surgery is not precluded. Only a few surgeons will insist on performing surgery initially to restore or improve fistula flow,16 and even fewer interventional radiologists will oblige them.17 Therefore, in most hospitals, the interventional radiologist will be asked to perform native fistula declotting and needs to be aware of the common techniques used for declotting, maintenance, and managing complications.
NATIVE FISTULA DECLOTTING
Preprocedural Workup
Checking the coagulation profile is useful but elevated values rarely preclude fistula declotting. Concern about obtaining adequate hemostasis after declotting is no longer a consideration with the advent of purse-string sutures.18 However, knowledge of the prothrombin time (PT), partial thromboplastin time (PTT), and platelet count is very important when adjusting the heparin dose during the procedure.
The only true contraindication to fistula declotting is active infection, which is extremely rare in native fistulae. Fever is usually unrelated to fistula thrombosis and arm tenderness usually reflects thrombosis, not infection of the access site. Arm discomfort should not be interpreted as infection and should not preclude fistula declotting. Only if the fistula is clearly weeping infected material or pus is aspirated from within the fistula should the procedure be aborted.
Patients in need of acute dialysis should receive temporary hemodialysis catheters and be declotted the following day. Such patients typically are unable to lay flat because of fluid overload and may not tolerate the additional fluid and contrast used during the declotting procedure. Finally, any patients with significant chest pain and electrocardiographic (ECG) changes should be evaluated for myocardial infarction. Again, this is a relatively rare occurrence but does occur at a significant frequency in this patient population.
Fistula Access
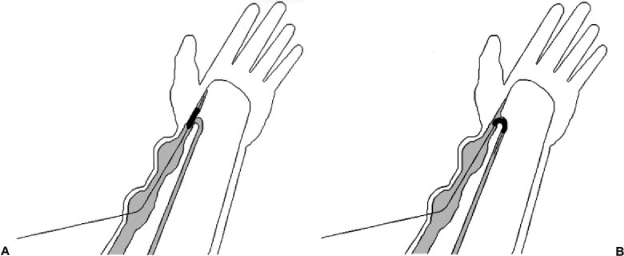
Typically, the fistula will have large, dilated venous segments that are used for needle puncture and these areas offer the easiest access to the fistula. Access should be done in a crossed fashion so that the entire length of fistula can be declotted (Fig. 1A). Fistulae are predominantly venous structures. Therefore, if access is difficult, an elastic tourniquet is tied around the upper arm to distend the veins and fistula. The tourniquet is placed near the axilla because a tourniquet on the distal forearm may not allow flow through patent collaterals and the fistula may not distend adequately. After tourniquet application, communicating collateral veins will distend the fistula so that access is facilitated.
Figure 1.
(A) Crossing catheters are placed in dilated segments of the fistula and the entire length of the fistula is crossed. (B) Third wire (broken line) placed in more central segment of cephalic vein to cross stenosis that could not be crossed from the opposite direction.
Occasionally, ultrasound-guided puncture of the fistula may be beneficial, particularly if veins do not distend with the use of a tourniquet. Rarely, a transbrachial or radial artery puncture10,19,20 is required to help guide access into the fistula. An arterial puncture should be used as a last resort because the patient will be anticoagulated during the declotting procedure and obtaining hemostasis of the arterial puncture might be difficult. In these cases, only the 3-French inner-coaxial dilator from a Micropuncture kit (Cook Inc., Bloomington, IN) is placed in the artery and only an 0.018-in. wire is used. Larger wires and sheaths are not required and should not be used.
After the fistula is punctured in crossed fashion, all segments of the fistula need to be traversed with a wire before declotting begins. Each dilated segment of fistula should have a wire placed across it. If a segment of fistula cannot be crossed in one direction, a third puncture to approach the occlusion from the other direction often is required (Fig. 1B). Use of a 0.018-in. wire (V 18; Boston Scientific Corp., Natick, MA) is sometimes helpful to cross tight occlusions. Crossing 7-French sheaths (Merit Medical, South Jordan, UT) are then placed.
Patient Anticoagulation
In nearly all procedures, 5000 U of heparin is used. However, if the patient is larger than average, adjustment of the heparin dose can be made using activated coagulation time (ACT) values as a guide. In general, we aim for an ACT value of between 200 to 250 seconds. Occasionally, up to 10,000 U of heparin might be required to increase the ACT value adequately. If declotting the fistula is difficult or time consuming (i.e., greater than 1 hour, which is common), we will repeat an ACT during the procedure and give additional heparin to achieve a value of 200 to 250 seconds, usually striving for 250 or slightly higher in difficult cases.
If the patient is anticoagulated with coumadin, lowering the heparin dose is recommended. If the PT value is between 15 and 20, we give 3000 U of heparin. If the PT is above 20, 2000 U of heparin or less is used. If the patient is very frail, elderly, or severely hypertensive with normal PT values, we also tend to use less heparin, typically 3000 to 4000 U. If the platelets are less than 100, the heparin dose is reduced to 2000 U or less.
Heparin should be used in all cases because it decreases pulmonary vascular resistance. This is important because small pulmonary emboli occur during any declotting procedure. In addition, clearing of small amounts of residual clot within the fistula or pulmonary arteries will be facilitated with the use of adequate amounts of heparin. Adequate heparinization will also prevent the formation of clot within the fistula (and propagation of embolized clot in the pulmonary arteries), both during the procedure and shortly thereafter.
Sedation
A formal survey in our department found that declotting of fistulae and grafts, especially angioplasty of the veins, to be one of the most painful procedures performed. Therefore, we initially administer a bolus to the patient (though a patent central vein of the fistula) with 1 mg of midazolam hydrochloride (Versed; Hoffman-La Roche, Nutley, NJ) and 50 μg of fentanyl citrate (Sublimaze; Abbott Laboratories, North Chicago, IL), referred to as a round of sedation. In general, we have found that two rounds or a dose of 2 mg of midazolam hydrochloride and 100 μg of fentanyl to be an adequate dose for most patients prior to starting the procedure. Additional doses are usually too much for typical dialysis patients and less is often inadequate, unless the patient is frail or elderly. Adjustment of sedation above two rounds is done in half-rounds of 25 μg fentanyl and 0.5 mg midazolam hydrochloride.
Fistula Declotting
Multiple techniques to declot a fistula have been described.5,6,7,8,9,10,11,12,13,14,15 It does not matter what method is used as long as the radiologist is comfortable with the technique and does a good job clearing the thrombus. Clearing every bit of thrombus in a fistula is not as critical as it is in a synthetic graft. Synthetic grafts are more thrombogenic and therefore need optimal flow immediately after the procedure to remain patent. Fistulae are more forgiving to small amounts of residual thrombus, especially with adequate heparinization. If good antegrade flow is restored and no large amounts of thrombus are left, the fistula will function and will clear small amounts of residual clot over time. However, a large or obstructing ball-valve thrombus within the fistula would need to be cleared before the declotting procedure could be considered complete.
Resistant or Ball-Valve Thrombus
In many instances, thrombus will remain in the large dilated portions of the fistula, creating a ball-valve blockage. These can be cleared with many different techniques. An AngioJet thrombectomy catheter (Possis Medical, Minneapolis, MN) can be placed within the thrombus and external manual compression near the tip of the catheter can help the catheter engage the thrombus and remove it. Similarly, use of large catheters for percutaneous aspiration is very successful.7 Alternatively, the use of thrombolytic agents can help reduce a large thrombus burden. Often, angioplasty of the inflow and outflow will help clear some of the thrombus and over time the flow in the fistula slowly removes the remaining thrombus. Use of a pliable compliant occlusion balloon to dislodge small amounts of thrombus is also very helpful.8 Finally, external massage (using Betadine solution as the skin lubricant) can be performed to force small thrombi out of the dilated vein segments. However, if any significant amounts of thrombus are being dislodged to the pulmonary vasculature, the use of thrombolytic agents, in addition to adequate heparinization, is recommended.
Angioplasty of the Entire Venous Portion of the Fistula
Angioplasty will depend on the fistula diameter. In general, if the stenosis is 90% or less we use angioplasty to 7 to 9 mm, depending on the size of the adjacent veins. If the stenosis is greater than 90%, we will perform angioplasty in a staged procedure. The initial angioplasty is performed to 6 to 7 mm and the patient returns to radiology in 1 to 3 weeks for follow-up angioplasty to re-evaluate the outflow and repeat angioplasty with a slightly larger balloon (7 to 9 mm).
Angioplasty of the Arterial Anastomosis
This is the crucial step in creating adequate flow for dialysis, as well as preventing repeat thrombosis. Fistulae, because they are created from native tissue, are inherently nonthrombogenic. As a result, a single venous outflow stenosis will generally not cause a fistula to thrombose. Typically, both a venous outflow stenosis and an arterial inflow stenosis are required to clot this form of access. Thus, to restore good flow and ensure adequate long-term function, the arterial inflow needs to be crossed, dilated, and thoroughly evaluated.
Catheterization of the arterial inflow is initially done with an angled glide wire (Boston Scientific) and angled glide catheter (Boston Scientific) using the 7-French sheath directed toward the arteriovenous anastomosis. If the angled glide catheter is unsuccessful, other more sharply angulated catheters might be helpful. After the angled glide catheter is placed in the radial artery inflow and advanced to at least the mid-forearm, the angled glide wire is exchanged for a Rosen guide wire (Cook). Angioplasty of the inflow artery to a minimum of 4 mm is performed (occasionally to 3 mm in immature fistulae). Angioplasty needs to be done with the balloon directed toward the heart and not toward the hand (Fig. 2). Stealing flow from the arterial arch of the hand will not provide adequate flow for a fistula. If the wire will not pass toward the inflow, external manipulation should be attempted. If manipulation does not work, forming a V-18 guidewire into a small Simmons or loop shape can be helpful. The curve is then advanced across the anastomosis and toward the arch of the hand (Fig. 3). The wire is then pulled back and hooked into the radial artery inflow. Then with external manipulation and support, the wire and balloon can often pass into the radial artery inflow.
Figure 2.
(A) Incorrect balloon placement for angioplasty. (B) Correct balloon placement for angioplasty.
Figure 3.
(A) Simmons loop is created with tip of wire. Loop is either formed before advancing the wire into the fistula or within the fistula. If the loop is to be made in the fistula a small curve is created at the tip of the guide wire to facilitate forming the Simmons loop. (B) Wire is pulled out slightly and tip of the wire will engage the arteriovenous anastomosis.
In the most difficult cases, a transbrachial puncture might be required.10,19,20 The brachial artery is visualized with ultrasound and a single wall puncture is made with a 21-gauge needle from a Micropuncture kit. Palpation can be used if the artery can be identified, but again, a careful single wall puncture is required since the patient is anti-coagulated. The radial artery is accessed with an 0.018-in. wire from the kit, the needle withdrawn and a 3-French dilator (inner dilator from a Micropuncture kit) is advanced into the artery. This puncture cannot be closed with purse-string sutures and the size of the hole needs to be kept to a minimum. The 0.018-in. wire used for initial access is exchanged for a V-18 guide wire. The wire is then advanced toward the radiocephalic anastomosis, into the fistula, and finally into the vascular sheath facing the arterial inflow. Occasionally, the wire might need to be snared and pulled into the sheath and out of the patient to secure through-and-through access. The wire is only pulled out for ∼10 to 20 cm—just enough to allow a 4-mm Symmetry balloon (Boston Scientific) to be advanced over the tip of the wire, into the fistula, and across the radial artery anastomosis. The entire wire is not pulled through because the V-18 is a very stiff wire and can potentially traumatize the arterial inflow in a “rope-saw” fashion. The V-18 wire is simply used as a reverse guide for advancing the balloon from the fistula. Some forward tension on the wire is required because the balloon is advanced into the fistula and across the arteriovenous anastomosis. The balloon is never advanced onto the wire entering the brachial artery puncture site (Figs. 4 and 5).
Figure 4.
(A) Wire is advanced into the radial artery from a transbrachial approach, manipulated across the arteriovenous anastomosis, into the fistula and finally out the opposing sheath for a short length of 10 to 20 cm outside the skin. (B) Balloon is advanced onto wire and into the fistula in the direction of arrow to perform angioplasty of the arteriovenous anastomosis.
Figure 5.
Contrast study of fistula. (A) Initial contrast injection into the fistula with reflux into the arterial arch of the hand and ulnar artery. Radial artery is not visualized. (B) Magnified view of a transbrachial injection visualizing the radial artery inflow. (C) Contrast injection of balloon positioned in the radial artery prior to angioplasty. Stenosis of arteriovenous anastomosis was severely underestimated by initial transbrachial injection in Figure 5B. Therefore, it is important to catheterize the radial artery inflow selectively in all cases. (D) Angiogram after initial angioplasty to 4 mm.
Closure with Purse-String Sutures
Once the entire length of the fistula has been treated with angioplasty and flow has been restored to an acceptable level (contrast clears from the fistula within 3 to 4 seconds), the puncture sites are closed with purse-string sutures and a miniature tourniquet.18 Suture material is 2-0 nylon and the tourniquet is made of any dilator or plastic tube that is available. In the past, we have used 3-0 nylon. However, it did not always provide the tension required for adequate hemostasis. The suture is left in place for at least 24 hours because we have had delayed bleeding complications if the suture is taken out sooner. In patients that are anticoagulated, we prefer the suture to be left in place for at least 48 hours, given that these patients are at even higher risk for rebleeding. Safety should not be traded for cosmetics, as some authors have suggested.21
NONMATURING FISTULA
Brescia Cimino fistulae have a higher failure rate (i.e., the fistula does not mature or enlarge sufficiently to be useable) after placement than synthetic loop grafts.22 In cases in which a fistula is not maturing adequately for dialysis, the patients need to be referred to radiology for evaluation—ideally, within 2 months of placement.23
Every a poorly functioning fistula should be evaluated with venography or arteriography that includes evaluation of the arterial inflow.24 An aggressive approach is needed in these instances because patient care is improved by having as many native fistulae as possible vs. grafts and central lines. Typically, the fistula is poorly palpated because of its immature state. If a venous puncture cannot be performed, a transbrachial approach should be used for access and an arteriogram should be performed. Turmel-Rodrigues et al24 reported that a stenosis existed in every case of fistula malfunction. It is incumbent upon the interventional radiologist to find this stenosis and treat it. Often, the radiologist can improve inflow sufficiently to allow the fistula to mature. However, even if the fistula cannot be salvaged, venous mapping and evaluation of the arterial inflow will help guide surgical revision. The advantages of having a functioning fistula are so great in comparison with all other access alternatives that it would be a great disservice to hemodialysis patients to not try and salvage each fistula.
MAINTENANCE OF NATIVE FISTULAE
Venogram on a Dysfunctional Native Fistula
If a follow-up venogram is performed for continued poor function (i.e., high venous resistance or high recirculation) or repeat dilation of a venous stenosis, the entire length of the fistula needs to be re-examined. We use pressure measurements in the venous segment of fistulae if the stenosis is not angiographically obvious. Pressure gradients at the arteriovenous anastomosis are expected. At this location, blood flow changes direction rapidly and a gradient of 30 to 40 mm Hg or more can remain, even if the fistula is widely patent. Equivocal venous outflow stenoses are treated with angioplasty to at least 7 mm and, preferably, 8 mm. Following angioplasty, pressure measurements across the venous outflow can be helpful to evaluate areas requiring repeat angioplasty with an even larger balloon or simply tortuous vessels appearing to have a stenosis without a pressure gradient.
The venogram performed on a dysfunctional fistula also needs to include a close evaluation of the arteriovenous anastomosis, which includes the crossing of the anastomosis (Fig. 5C). Dilation of this region is required until flow is rapid or the native fistula has an acceptable thrill. A 4- to 5-mm balloon is placed across the arteriovenous anastomosis and into the radial artery inflow. Dilation is routinely performed during follow-up venograms on all patients and this prevents a subtle lesion from being overlooked. Typically, we do not anticoagulate patients prior to repeat angioplasty and thus we routinely use balloons that are 1 to 2 mm larger than those used during initial declotting.
Treating Collateral Veins
Some authors advise tying off collateral vessels with relative frequency.25 In our experience, tying off collaterals should only be done after complete angioplasty of the main segment of the fistula has been performed and no measurable outflow gradient is identified. Turmel-Rodrigues23 reports there is never a reason to tie off collateral vessels and that collateral vessels occur due to proximal stenoses. Practically, we agree, but there are rare instances where tying off a small collateral of the forearm is helpful to maintain flow in the main channel of the fistula or to prevent flow down the forearm, as in the case of upper arm fistulae (Fig. 6). In these instances, tying off should be done with a single 2-0 nylon stitch and a large dilator or gauze to create pressure on the collateral vein (Fig. 7). Occasionally, a second stitch in series is required, as some authors have described,26 but these authors did not describe use of dilator segments gauze to create direct compression on the vein. In general, the tying off of segments is helpful, but occasionally the vein will recanalize and the collateral flow will return, albeit the flow is not as rapid.
Figure 6.
Contrast study of fistula. (A) Initial injection is only within veins. Arterial inflow is not visualized. (B) Transbrachial injection was required. Collateral vein stealing flow down into the patient's forearm was tied off (a loop of wire was placed in the collateral vein and used to help identify it).
Figure 7.
(A) Suture is placed around the vein (shaded). (B) If suture is simply tied around vein, flow in a reduced diameter vein can persist. (C) Suture is tied around an external segment of large dilator, which helps to oppose the walls of the vein and to better occlude flow.
The tying off of collateral vessels that are at the mid-forearm or more central, as well as banding of the medial cubital vein, should not be performed on radiocephalic fistulae. Recent published reports suggest that high flow contributes to maturation of both the vein27 and artery,28 rather than high venous pressure. Thus, banding of outflow veins is self-defeating because it will decrease flow in both regions.
Finally, sclerosing agents and coils are the least useful. Coils are very tricky to use in the venous system because, in contrast to the arterial system, the terminal vessels of the venous system become larger and the coils have a tendency to migrate centrally. With regard to sclerosing agents, we have had little success occluding collateral veins with ethanol. Despite the use of occlusion balloons to decrease flow in the collateral vein being treated (thus increasing dwell time of the ethanol for sometimes longer than a minute), the veins invariably remained patent. High flow and perhaps low venous thrombogenicity prevented us from ever successfully sclerosing collateral veins.
Follow-Up
Patency after declotting is excellent. Checking function every year or 18 months is likely prudent. Any recurring stenoses can be addressed at these times and adequate dialysis can be ensured. Unfortunately, many patients will not return unless the fistula is completely clotted. Others require multiple repeat studies.
FAILURES OF PERCUTANEOUS DECLOTTING
Operator inexperience is the most common cause for failure because there is a steep learning curve during the initial two to three cases, after which nearly all previously functioning native fistulae can have flow restored, given enough time and patience. This is especially true in fistulae that have been functioning previously. In contrast, immature fistulae, either thrombosed or low flow, have a slightly lower reported rate of successful declotting, restoration of flow, and long-term function.24,29 However, it is still important to perform angiography, either diagnostic or therapeutic, in these cases rather than simply giving up.
Ultrasound should not be used to exclude fistulae from diagnostic angiograms. Some authors have reported ultrasound is helpful in deciding when to evaluate fistulae and to help prevent unsuccessful angioplasties.30 Turmel-Rodrigues et al23 pointed out that this study did not correlate all findings with angiography. Thus, we currently see no reason to exclude a patient from the gold standard test of angiography and angioplasty based on yet-unproven ultrasound criteria. Venography and angioplasty are simple, safe, and relatively noninvasive techniques to maintain fistulae. Measurement of pressure gradients directly within the fistula will give more information regarding a stenosis than an indirect test such as ultrasound.
In difficult cases or repeat occlusions, changing of operators can be helpful because interventionalists have slightly different approaches to declotting. In addition, given that declotting fistulae is time consuming and strenuous, one's first fistula declot should not be scheduled for the late afternoon, but rather for the next morning when one is fresh and has plenty of time to perform an adequate procedure. During longer procedures, patient compliance can become a problem, which often is a reflection of inadequate sedation. If the patient needs emergent dialysis, that is all the more reason to place a temporary line and declot the next day. Declotting a patient in fluid overload makes a difficult procedure all that much more strenuous and will decrease the chances of success.
COMPLICATIONS
Potential Small Emboli to the Lungs and Hand
Many fistula declots may have small emboli that cannot be removed percutaneously, regardless of the method. Authors have reported varying amount of residual thrombus following percutaneous declotting.9 If adequate amounts of heparin are used, these small fragments will lyse. If visible amounts of thrombus remain, use of a small amount of 2- to 5-mg rt-PA or 250,000 U urokinase (Abbott Laboratories) might be indicated to facilitate thrombolysis. In addition, an attempt to clear the fistula by intentionally macerating and embolizing small fragments is acceptable, especially if adequate heparinization and thrombolytics are used. Embolization of large clots should not be done and there has been at least one reported death.31
Arterial emboli to the hand will occasionally occur. However, these rarely are symptomatic and generally can be left alone. Injection of a lytic agent into the radial artery can be performed (2 to 5 mg rt-PA or 250,000 to 500,000 U urokinase) if the patient has discomfort or the interventionalist is uncertain. In addition, for symptomatic thrombi, a back-bleeding technique can be performed with occlusion of the feeding artery, which then induces reverse flow in the more distal radial or brachial artery.32 In our experience, asymptomatic clots will generally clear either with or without the use of lytic agents or additional intervention. However, close follow-up might be warranted.
Venous Rupture and Placing Metallic Stents
Venous rupture will occur more commonly if declotting is done more aggressively. Venous rupture and extravasation should be controlled with prolonged balloon inflation. In more difficult cases, reversal of heparin with protamine can be performed. Care should be taken when giving protamine to diabetics using neutral protamine Hagedorn (NPH) insulin because cross-reactions can occur.33
If venous rupture is not controlled with prolonged balloon inflation up to 30 minutes, a metallic stent can be placed. However, never use a Wallstent. The interstices are spaced too closely together and these stents will distort with repeated puncture.34 In addition, Wallstents need to foreshorten axially to expand radially. This makes repeat angioplasty much more difficult once the stent has become endothelialized in the vein. Other self-expanding stents can be used and oversizing by at least 30 to 40% is recommended to allow future redilations. In general, placing a 10- to 12-mm-diameter stent in the axillary vein is recommended. Similarly, the brachial vein can be stented to 9 to 10 mm even if the measured lumen is 7 to 8 mm. Oversized stents are also much less likely to migrate centrally. Complications of migration occur when stents are inadvertently undersized or closely approximate the true lumen of the vein. Uncovered stents are adequate and the use of covered stents is usually unnecessary in cases of venous rupture. Finally, in the rare instance where an uncovered stent is inadequate and covered stents are unavailable, occlusion of the fistula itself can be performed if the extravasation is severe. Depending on the location of the rupture, occlusion of the fistula or radial artery itself could be performed. Fortunately, this is an extremely rare occurrence.
SPECIAL CONSIDERATIONS IN BRACHIOCEPAHLIC FISTULAE
Brachiocephalic or upper arm fistulae occlude more frequently with lower long-term patencies than radiocephalic or wrist fistulae.35 In addition, stenoses of the origin of the cephalic vein are more common, and often are resistant to angioplasty.36 In our experience, the use of extremely high pressure balloons (Conquest Balloon; Bard, Inc., Covington, GA) has been useful in all of our difficult cases. A 7- to 8-mm balloon is hand-inflated with a 3-cm3 syringe to an estimated pressure of 40 to 50 atm (typical pressure syringes do not go this high). We have not been able to burst a 7- to 8-mm balloon using a small 3-cm3 syringe and consider this a safe high-pressure inflation device. If we were unable to resolve a stenosis with a high-pressure balloon, the use of a “cutting” balloon would be an option, especially when larger sizes become commonly available. Finally, percutaneous needle puncture of the vein has been described as a tenderizing procedure prior to venous angioplasty and can help in difficult cases.
Upper arm fistulae are occasionally difficult to palpate because their outflow might be directed into the deep brachial vein or medial basilic vein. Often, these need to be “superficialized” by surgery. Other upper arm fistulae will dilate in a tortuous manner, creating a “kink.” This may be related to the vein being too long during fistula creation and then elongating even further during fistula maturation.22 These cases should generally be referred to a surgeon. Stent placement will likely create additional future problems than surgical shortening of the vein.
CONCLUSION
The initial success rate of fistula declotting is low. However, the interventionalist should not become discouraged. The learning curve is steep and, after only a few cases (albeit long, time-consuming cases), the success rate will approach 100%. At this point, one's confidence will increase dramatically. However, not every case will be angiographically perfect. In cases in which the flow remains suboptimal despite angioplasty of the entire fistula and arteriovenous anastomosis, close follow-up is recommended. Close follow-up prevents recurrent thrombosis and is much preferred to re-declotting the fistula or abandoning the access site. If there are any concerns at dialysis regarding fistula flow, angiographic follow-up is required. As the number of patients with native fistulae increases, declotting fistulae will become the most important service that an interventionalist can provide to hemodialysis patients.
REFERENCES
- Brescia M J, Cimino J E, Appel K, Hurwich B J. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med. 1966;275:1089–1092. doi: 10.1056/NEJM196611172752002. [DOI] [PubMed] [Google Scholar]
- Chazan J A, London M R, Pono L M. Long-term survival of vascular accesses in a large chronic hemodialysis population. Nephron. 1995;69:228–233. doi: 10.1159/000188461. [DOI] [PubMed] [Google Scholar]
- Stevenson K B, Hannah E L, Lowder C A, et al. Epidemiology of hemodialysis vascular access infections from longitudinal infection surveillance data: predicting the impact of NKF-DOQI clinical practice guidelines for vascular access. Am J Kidney Dis. 2002;39:549–555. doi: 10.1053/ajkd.2002.31405. [DOI] [PubMed] [Google Scholar]
- Aruny J E. In: Kandarpa K, Aruny JE, editor. Handbook of Interventional Radiology Procedures. Boston: Little, Brown; 1996. Dialysis access shunt and fistula recanalization. pp. 122–124.
- Vorwerk D, Schurmann K, Muller-Leisse C, et al. Hydrodynamic thrombectomy of haemodialysis grafts and fistulae: results of 51 procedures. Nephrol Dial Transplant. 1996;11:1058–1064. [PubMed] [Google Scholar]
- Overbosch E H, Pattynama P M, Aarts H J, et al. Occluded hemodialysis shunts: Dutch multicenter experience with the hydrolyser catheter. Radiology. 1996;201:485–488. doi: 10.1148/radiology.201.2.8888246. [DOI] [PubMed] [Google Scholar]
- Turmel-Rodrigues L, Sapoval M, Pengloan J, et al. Manual thromboaspiration and dilation of thrombosed dialysis access: mid-term results of a simple concept. J Vasc Interv Radiol. 1997;8:813–824. doi: 10.1016/s1051-0443(97)70666-3. [DOI] [PubMed] [Google Scholar]
- Zaleski G X, Funaki B, Kenney S, et al. Angioplasty and bolus urokinase infusion for the restoration of function in thrombosed Brescia-Cimino dialysis fistulas. J Vasc Interv Radiol. 1999;10:129–136. doi: 10.1016/s1051-0443(99)70454-9. [DOI] [PubMed] [Google Scholar]
- Haage P, Vorwerk D, Wildberger J E, et al. Percutaneous treatment of thrombosed primary arteriovenous hemodialysis access fistulae. Kidney Int. 2000;57:1169–1175. doi: 10.1046/j.1523-1755.2000.00944.x. [DOI] [PubMed] [Google Scholar]
- Turmel-Rodrigues L, Pengloan J, Rodrigue H, et al. Treatment of failed native arteriovenous fistulae for hemodialysis by interventional radiology. Kidney Int. 2000;57:1124–1140. doi: 10.1046/j.1523-1755.2000.00940.x. [DOI] [PubMed] [Google Scholar]
- Rocek M, Peregrin J H, Lasovickova J, et al. Mechanical thrombolysis of thrombosed hemodialysis native fistulas with use of the Arrow-Trerotola percutaneous thrombolytic device: our preliminary experience. J Vasc Interv Radiol. 2000;11:1153–1158. doi: 10.1016/s1051-0443(07)61356-6. [DOI] [PubMed] [Google Scholar]
- Schmitz-Rode T, Wildberger J E, Hubner D, et al. Recanalization of thrombosed dialysis access with use of a rotating mini-pigtail catheter: follow-up study. J Vasc Interv Radiol. 2000;11:721–727. doi: 10.1016/s1051-0443(07)61630-3. [DOI] [PubMed] [Google Scholar]
- Liang H L, Pan H B, Chung H M, et al. Restoration of thrombosed Brescia-Cimino dialysis fistulas by using percutaneous transluminal angioplasty. Radiology. 2002;223:339–344. doi: 10.1148/radiol.2232010821. [DOI] [PubMed] [Google Scholar]
- Rajan D K, Clark T W, Simons M E, et al. Procedural success and patency after percutaneous treatment of thrombosed autogenous arteriovenous dialysis fistulas. J Vasc Interv Radiol. 2002;13:1211–1218. doi: 10.1016/s1051-0443(07)61967-8. [DOI] [PubMed] [Google Scholar]
- Schon D, Mishler R. Pharmacomechanical thrombolysis of natural vein fistulas: reduced dose of TPA and long-term follow-up. Semin Dial. 2003;16:272–275. doi: 10.1046/j.1525-139x.2003.16052.x. [DOI] [PubMed] [Google Scholar]
- Berman S S, Gentile A T. Impact of secondary procedures in autogenous arteriovenous fistula maturation and maintenance. J Vasc Surg. 2001;34:866–871. doi: 10.1067/mva.2001.118086. [DOI] [PubMed] [Google Scholar]
- Turmel-Rodrigues L. Regarding “impact of secondary procedures in autogenous fistula maturation and maintenance. J Vasc Surg. 2002;36:867–868. doi: 10.1067/mva.2002.127963. [DOI] [PubMed] [Google Scholar]
- Zaleski G X, Funaki B, Gentile L, Garofalo R S. Purse-string sutures and miniature tourniquet to achieve immediate hemostasis of percutaneous grafts and fistulas: a simple trick with a twist. AJR Am J Roentgenol. 2000;175:1643–1645. doi: 10.2214/ajr.175.6.1751643. [DOI] [PubMed] [Google Scholar]
- Manninen H I, Kaukanen E T, Ikaheimo R, et al. Brachial arterial access: endovascular treatment of failing Brescia-Cimino hemodialysis fistulas—initial success and long-term results. Radiology. 2001;218:711–718. doi: 10.1148/radiology.218.3.r01mr38711. [DOI] [PubMed] [Google Scholar]
- Haage P, Vorwerk D, Wildberger J, et al. Percutaneous treatment of thrombosed primary arteriovenous hemodialysis access fistulae. Kidney Int. 2000;57:1169–1175. doi: 10.1046/j.1523-1755.2000.00944.x. [DOI] [PubMed] [Google Scholar]
- Simons M E, Rajan D K, Clark T W. The Woggle technique for suture closure of hemodialysis access catheterization sites. J Vasc Interv Radiol. 2003;14:485–488. doi: 10.1097/01.rvi.0000064851.87207.d0. [DOI] [PubMed] [Google Scholar]
- Konner K. The anastomosis of the arteriovenous fistula—common errors and their avoidance. Nephrol Dial Transplant. 2002;17:376–379. doi: 10.1093/ndt/17.3.376. [DOI] [PubMed] [Google Scholar]
- Turmel-Rodrigues L A, Bourquelot P, Pengloan J. Hemodialysis arteriovenous fistula maturity: US evaluation Radiology. 2003;227:906–907. doi: 10.1148/radiol.2273021730. [DOI] [PubMed] [Google Scholar]
- Turmel-Rodrigues L, Mouton A, Birmele B, et al. Salvage of immature forearm fistulas for haemodialysis by interventional radiology. Nephrol Dial Transplant. 2001;16:2365–2371. doi: 10.1093/ndt/16.12.2365. [DOI] [PubMed] [Google Scholar]
- Beathard G A, Settle S M, Shields M W. Salvage of the nonfunctioning arteriovenous fistula. Am J Kidney Dis. 1999;33:910–916. doi: 10.1016/s0272-6386(99)70425-7. [DOI] [PubMed] [Google Scholar]
- Faiyaz R, Abreo K, Zaman F, et al. Salvage of poorly developed arteriovenous fistulae with percutaneous ligation of accessory veins. Am J Kidney Dis. 2002;39:824–827. doi: 10.1053/ajkd.2002.32003. [DOI] [PubMed] [Google Scholar]
- Corpataux J M, Haesler E, Silacci P, et al. Low-pressure environment and remodelling of the forearm vein in Brescia-Cimino haemodialysis access. Nephrol Dial Transplant. 2002;17:1057–1062. doi: 10.1093/ndt/17.6.1057. [DOI] [PubMed] [Google Scholar]
- Ene-Iordache B, Mosconi L, Antiga L, et al. Radial artery remodeling in response to shear stress increase within arteriovenous fistula for hemodialysis access. Endothelium. 2003;10:95–102. doi: 10.1080/10623320303365. [DOI] [PubMed] [Google Scholar]
- Beathard G A, Arnold P, Jackson J, Litchfield T. Physician Operators Forum of RMS Lifeline. Aggressive treatment of early fistula failure. Kidney Int. 2003;64:1487–1494. doi: 10.1046/j.1523-1755.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- Robbin M L, Chamberlain N E, Lockhart M E, et al. Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology. 2002;225:59–64. doi: 10.1148/radiol.2251011367. [DOI] [PubMed] [Google Scholar]
- Trerotola S O, Vesely T M, Lund G B. Treatment of thrombosed hemodialysis access grafts: Arrow-Trerotola percutaneous thrombolytic device versus pulse-spray thrombolysis. Arrow-Trerotola Percutaneous Thrombolytic Device Clinical Trial. Radiology. 1998;206:403–414. doi: 10.1148/radiology.206.2.9457193. [DOI] [PubMed] [Google Scholar]
- Trerotola S O, Johnson M S, Shah H, Namyslowski J. Backbleeding technique for treatment of arterial emboli resulting from dialysis graft thrombolysis. J Vasc Interv Radiol. 1998;9:141–143. doi: 10.1016/s1051-0443(98)70496-8. [DOI] [PubMed] [Google Scholar]
- Funaki B, Szymski G X, Hackworth C A, Rosenblum J D. Vascular or interventional procedures in patients with diabetes. J Vasc Interv Radiol. 1997;8:1080. doi: 10.1016/s1051-0443(97)70715-2. [DOI] [PubMed] [Google Scholar]
- Zaleski G X, Funaki B, Rosenblum J, et al. Metallic stents deployed in synthetic arteriovenous hemodialysis grafts. AJR Am J Roentgenol. 2001;176:1515–1519. doi: 10.2214/ajr.176.6.1761515. [DOI] [PubMed] [Google Scholar]
- Turmel-Rodrigues L, Pengloan J, Rodrigue H, et al. Treatment of failed native arteriovenous fistulae for hemodialysis by interventional radiology. Kidney Int. 2000;57:1124–1140. doi: 10.1046/j.1523-1755.2000.00940.x. [DOI] [PubMed] [Google Scholar]
- Rajan D K, Clark T W, Patel N K, et al. Prevalence and treatment of cephalic arch stenosis in dysfunctional autogenous hemodialysis fistulas. J Vasc Interv Radiol. 2003;14:567–573. doi: 10.1097/01.rvi.0000071090.76348.bc. [DOI] [PubMed] [Google Scholar]