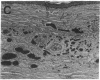
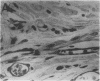
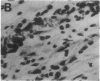
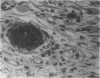
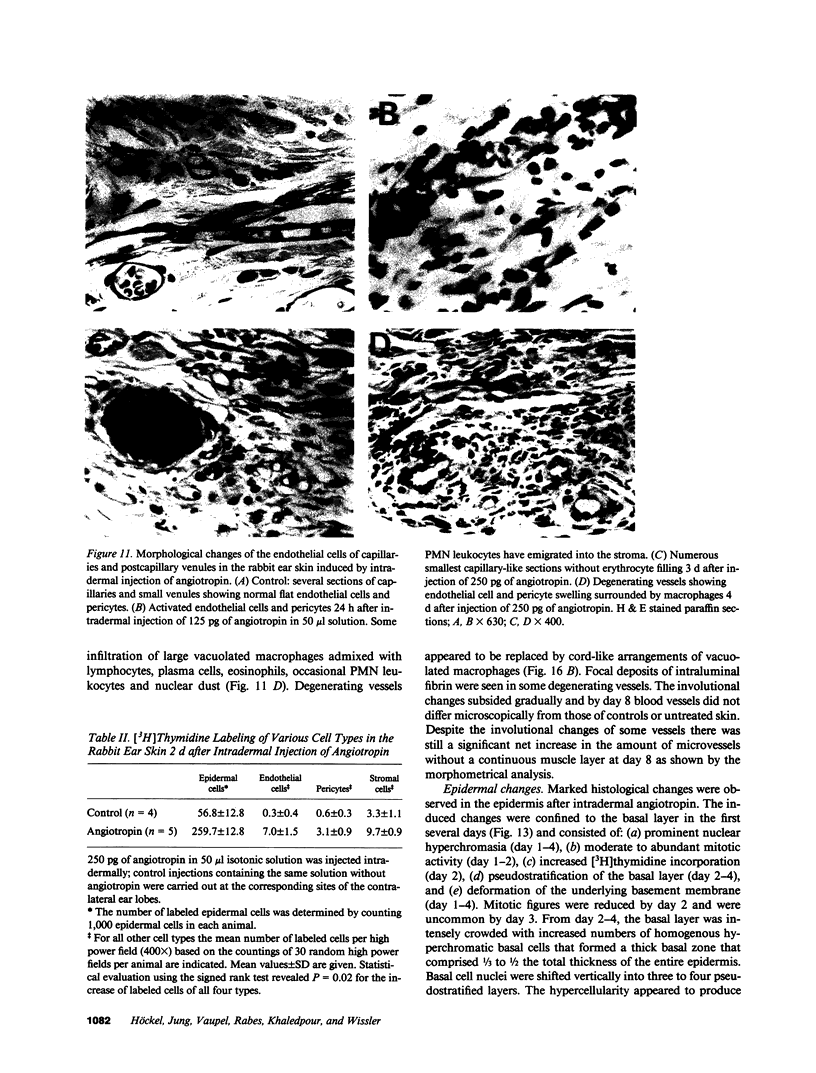
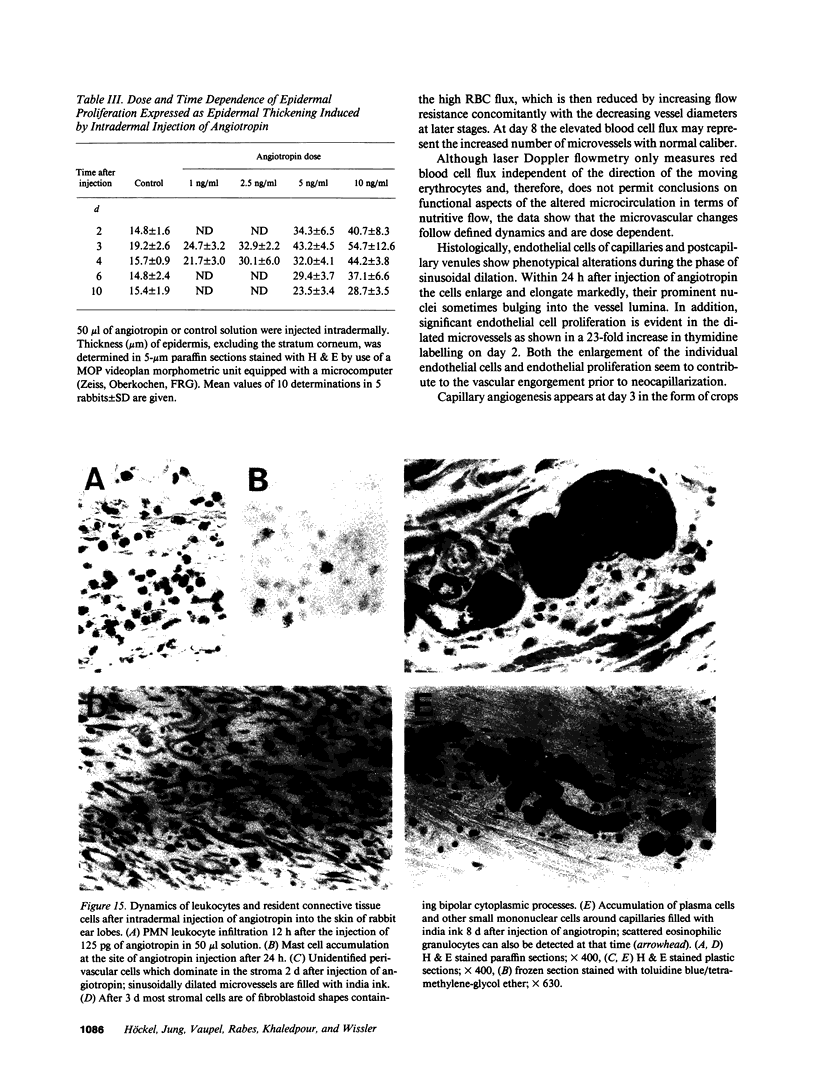
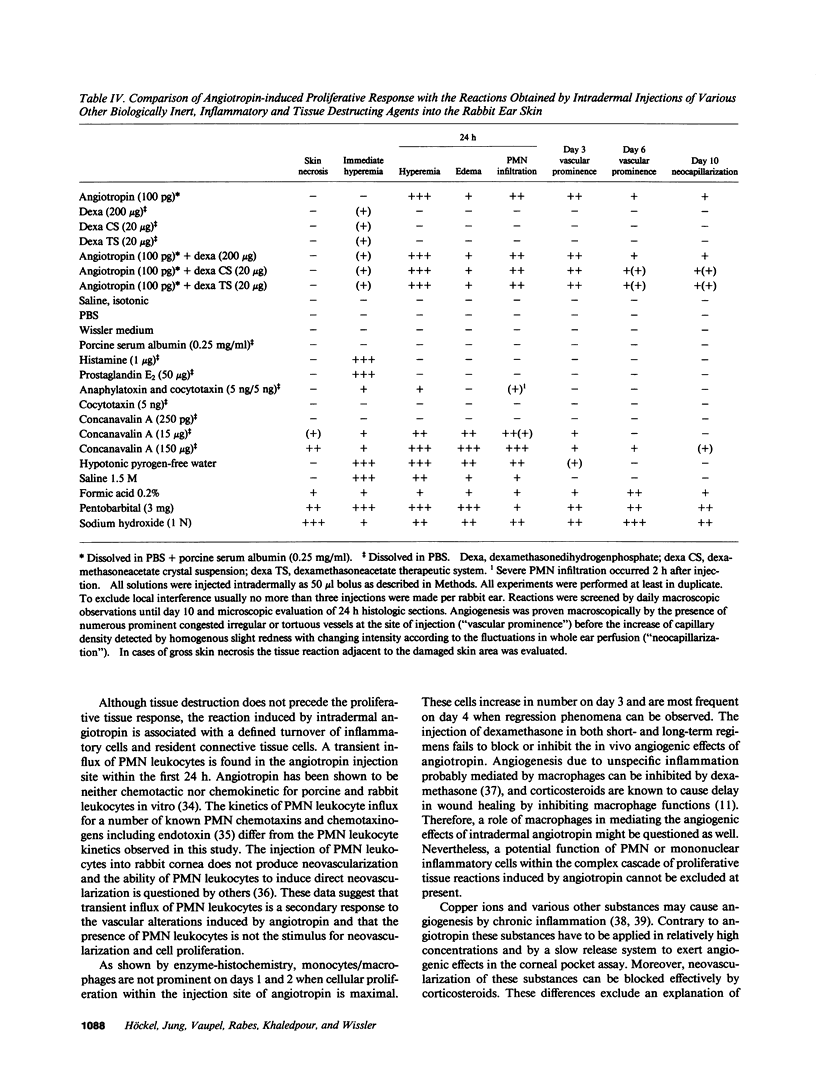
Abstract
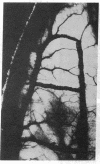
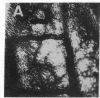
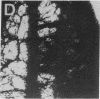
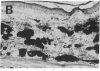
Angiotropin is a differentiation factor for microvascular endothelial cells isolated from serum-free cultures of lectin-activated, porcine monocytes. We used an ear lobe model in rabbits, single intradermal injection of angiotropin to induce phenotypical changes of the endothelial cells in capillaries and postcapillary venules, vascular engorgement, and subsequent angiogenesis in dose-dependent manner. The vascular changes are associated with epidermal and stromal cell proliferation. Angiogenesis and tissue proliferation occur in the absence of tissue necrosis and do not lead to scar formation. Angiotropin-induced angiogenesis is not inhibited by local dexamethasone although it involves a defined turnover of inflammatory cells. Proliferation is transient and regressive events follow. The overall tissue reaction resembles changes found in the undamaged skin margin of a primary healing wound during the inflammatory/proliferative phase. From these observations we conclude that angiotropin is an important secretory product of activated peripheral macrophages that triggers inflammatory and proliferative reactions in wound healing by activating microvascular endothelial cells.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M. Localized formation of new tissue in an adult mammal. Symp Soc Exp Biol. 1957;11:235–254. [PubMed] [Google Scholar]
- Ausprunk D. H., Falterman K., Folkman J. The sequence of events in the regression of corneal capillaries. Lab Invest. 1978 Mar;38(3):284–294. [PubMed] [Google Scholar]
- BULLOUGH W. S., LAURENCE E. B. The control of epidermal mitotic activity in the mouse. Proc R Soc Lond B Biol Sci. 1960 Mar 1;151:517–536. doi: 10.1098/rspb.1960.0014. [DOI] [PubMed] [Google Scholar]
- Baird A., Mormède P., Böhlen P. Immunoreactive fibroblast growth factor in cells of peritoneal exudate suggests its identity with macrophage-derived growth factor. Biochem Biophys Res Commun. 1985 Jan 16;126(1):358–364. doi: 10.1016/0006-291x(85)90614-x. [DOI] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J., Tippins J. R., Morris H. R., MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985 Jan 3;313(5997):54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Stone R. D., Leung D. Y., Silver I., Hohn D. C., Hunt T. K. Role of macrophages in would healing. Surg Forum. 1976;27(62):16–18. [PubMed] [Google Scholar]
- Colditz I. G., Movat H. Z. Kinetics of neutrophil accumulation in acute inflammatory lesions induced by chemotaxins and chemotaxinigens. J Immunol. 1984 Oct;133(4):2169–2173. [PubMed] [Google Scholar]
- Ehrlich H. P., Griswold T. R., Rajaratnam J. ATP-induced cell contraction with epidermolysis bullosa dystrophica recessive and normal dermal fibroblasts. J Invest Dermatol. 1986 Feb;86(2):96–100. doi: 10.1111/1523-1747.ep12284026. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Garvey W. Modified elastic tissue-Masson trichrome stain. Stain Technol. 1984 Jul;59(4):213–216. doi: 10.3109/10520298409113858. [DOI] [PubMed] [Google Scholar]
- Glenn K. C., Ross R. Human monocyte-derived growth factor(s) for mesenchymal cells: activation of secretion by endotoxin and concanavalin A. Cell. 1981 Sep;25(3):603–615. doi: 10.1016/0092-8674(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Höckel M., Beck T., Wissler J. H. Neomorphogenesis of blood vessels in rabbit skin induced by a highly purified monocyte-derived polypeptide (monocyto-angiotropin) and associated tissue reactions. Int J Tissue React. 1984;6(4):323–331. [PubMed] [Google Scholar]
- Höckel M., Ott S., Siemann U., Kissel T. Prevention of peritoneal adhesions in the rat with sustained intraperitoneal dexamethasone delivered by a novel therapeutic system. Ann Chir Gynaecol. 1987;76(6):306–313. [PubMed] [Google Scholar]
- Höckel M., Sasse J., Wissler J. H. Purified monocyte-derived angiogenic substance (angiotropin) stimulates migration, phenotypic changes, and "tube formation" but not proliferation of capillary endothelial cells in vitro. J Cell Physiol. 1987 Oct;133(1):1–13. doi: 10.1002/jcp.1041330102. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Hunt T. K., Scheuenstuhl H., Halliday B. J., Werb Z., Banda M. J. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983 Sep 23;221(4617):1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Silver I. A., Hunt T. K. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981 Aug;90(2):262–270. [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Unanue E. R., Cotran R. S. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981 Apr;126(4):1510–1515. [PubMed] [Google Scholar]
- McAuslan B. R., Reilly W. G., Hannan G. N., Gole G. A. Angiogenic factors and their assay: activity of formyl methionyl leucyl phenylalanine, adenosine diphosphate, heparin, copper, and bovine endothelium stimulating factor. Microvasc Res. 1983 Nov;26(3):323–338. doi: 10.1016/0026-2862(83)90080-8. [DOI] [PubMed] [Google Scholar]
- Moore J. W., 3rd, Sholley M. M. Comparison of the neovascular effects of stimulated macrophages and neutrophils in autologous rabbit corneas. Am J Pathol. 1985 Jul;120(1):87–98. [PMC free article] [PubMed] [Google Scholar]
- Myers M. B., Cherry G. Blood supply of healing wounds: functional and angiographic. Arch Surg. 1971 Jan;102(1):49–52. doi: 10.1001/archsurg.1971.01350010051013. [DOI] [PubMed] [Google Scholar]
- PADAWER J. A stain for mast cells and its application in various vertebrat43es and in a mastocytoma. J Histochem Cytochem. 1959 Sep;7:352–353. doi: 10.1177/7.5.352. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- SWANN M. M. The control of cell division: a review. II. Special mechanisms. Cancer Res. 1958 Nov;18(10):1118–1160. [PubMed] [Google Scholar]
- Thakral K. K., Goodson W. H., 3rd, Hunt T. K. Stimulation of wound blood vessel growth by wound macrophages. J Surg Res. 1979 Apr;26(4):430–436. doi: 10.1016/0022-4804(79)90031-3. [DOI] [PubMed] [Google Scholar]
- Thompson W. D., Campbell R., Evans T. Fibrin degradation and angiogenesis: quantitative analysis of the angiogenic response in the chick chorioallantoic membrane. J Pathol. 1985 Jan;145(1):27–37. doi: 10.1002/path.1711450103. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K., Sugino Y. Tumor angiogenesis activity in clonal cells transformed by bovine adenovirus type 3. Cancer Res. 1979 Apr;39(4):1305–1309. [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z. How the macrophage regulates its extracellular environment. Am J Anat. 1983 Mar;166(3):237–256. doi: 10.1002/aja.1001660302. [DOI] [PubMed] [Google Scholar]
- Wharton W., Gillespie G. Y., Russell S. W., Pledger W. J. Mitogenic activity elaborated by macrophage-like cell lines acts as competence factor(s) for BALB/c 3T3 cells. J Cell Physiol. 1982 Jan;110(1):93–100. doi: 10.1002/jcp.1041100115. [DOI] [PubMed] [Google Scholar]
- Wissler J. H. Chemistry and biology of the anaphylatoxin related serum peptide system. II. Purification, crystallization and properties of cocytotaxin, a basic peptide from rat serum. Eur J Immunol. 1972 Feb;2(1):84–89. doi: 10.1002/eji.1830020116. [DOI] [PubMed] [Google Scholar]
- Ziche M., Jones J., Gullino P. M. Role of prostaglandin E1 and copper in angiogenesis. J Natl Cancer Inst. 1982 Aug;69(2):475–482. [PubMed] [Google Scholar]