ABSTRACT
Achalasia is an esophageal motor disorder characterized by increased lower esophageal sphincter (LES) pressure, diminished-to-absent peristalsis in the distal portion of the esophagus composed of smooth muscle, and lack of a coordinated LES relaxation in response to swallowing. These abnormalities are recognized radiographically by aperistalsis, esophageal dilatation, and decreased opening of the LES, with a characteristic “bird-beak” appearance. The principal symptom of this disorder is dysphagia and pneumatic dilatation is designed to alleviate or cure this symptom.
Keywords: Achalasia, balloon dilatation, fluoroscopy, dysphagia
Achalasia of the cardia is a disorder of esophageal motility characterized by loss of peristalsis and failure of relaxation of the lower esophageal sphincter (LES) on swallowing.1
Sir Thomas Willis first described achalasia in 1674. Willis successfully treated a patient by dilating the LES with a cork-tipped whalebone. Not until 1929 did Hurt and Rake2 first realize that the primary pathophysiology resulting in achalasia was a failure in LES relaxation.
Primary achalasia is the most common subtype and is associated with loss of ganglion cells in the esophageal myenteric plexus. These important inhibitory neurons induce LES relaxation and coordinate proximal-to-distal peristaltic contraction of the esophagus. LES pressure and relaxation are regulated by excitatory (e.g., acetylcholine, substance P) and inhibitory (e.g., nitric oxide, vasoactive intestinal peptide) neurotransmitters. Persons with achalasia lack nonadrenergic, noncholinergic, inhibitory ganglion cells, causing an imbalance in excitatory and inhibitory neurotransmission. The result is a hypertensive nonrelaxed esophageal sphincter.
Secondary achalasia is relatively uncommon. This condition exists when a process other than intrinsic disease of the esophageal myenteric plexus is the etiology. Examples of conditions causing secondary achalasia include certain malignancies, diabetes mellitus, and Chagas' disease.
The exact etiology of achalasia is not known. The most widely accepted current theories implicate autoimmune disorders, infectious diseases, or both.2
Epidemiology
Achalasia is a relatively uncommon disease with an incidence of 1 per 100,000 people per year in the United States. The disease prevalence is ∼8 cases per million population.3,4 Rates may be slightly higher in South America because of the prevalence of Chagas' disease there.
No racial predilection for achalasia has been described. The male-to-female ratio is 1:1. Achalasia typically occurs in adults aged 25 to 60 years. The disease has been diagnosed in infants and in patients well into their 80s.5
CLINICAL CHARACTERISTICS
The evaluation of dysphagia begins with a careful history, which points to the underlying cause in up to 80% of cases.6
The following symptoms and signs characterize achalasia:
Dysphagia: This is the most common presenting symptom in patients with achalasia. The ingestion of either solids or liquids can result in dysphagia, though dysphagia for solids is more common.
Regurgitation: Many patients with achalasia experience spontaneous regurgitation of undigested food from the esophagus during the course of the disease.
Chest pain: Approximately 25 to 50% of patients with dysphagia report episodes of retrosternal chest pain, which are frequently induced by eating.
Weight loss: This is usually a sign of advanced disease.
As the disease progresses, the likelihood that aspiration will occur increases. As a result, some patients may present with signs or symptoms of pneumonia. Lung abscesses, bronchiectasis, and hemoptysis are some of the more severe pulmonary consequences of achalasia-associated aspiration. Patients with achalasia are at increased risk for esophageal cancer. When esophageal cancer occurs, it is usually found in patients with a long history of achalasia.
DIAGNOSIS
We consider three tests to be important to confirm the diagnosis and eliminate other disorders:
barium esophagogram;
esophageal manometry and pH studies;
endoscopy to rule out a tumor at the gastroesophageal junction (pseudoachalasia).
Characteristic changes on the barium esophagogram include dilatation of the esophagus and absence of peristaltic activity. A smooth narrowing in the distal esophagus (“bird's beak” appearance) is characteristic but is not always seen.
Esophageal manometry findings are characteristic and show aperistalsis in the smooth muscle portion of the esophageal body, with waves that are not true contractions and that are mirror images of each other (common-cavity phenomenon).
Pressure in the LES is elevated in about half of cases, but relaxation of the sphincter is almost always abnormal, with ~75% of patients demonstrating absent or incomplete relaxation and 20 to 30% showing relaxation that is very short (less than 6 seconds) but complete.
Endoscopy is essential to confirm the absence of structural abnormalities such as a stricture and, more importantly, to rule out a tumor in the region of the gastroesophageal junction, which can mimic pseudoachalasia but should not be treated with pneumatic dilatation. In some cases endoscopic ultrasound may be utilized to examine the lower esophagus and cardia to ensure the absence of tumor. This is particularly important in elderly patients with a short duration of symptoms and profound weight loss.
TREATMENT
Treatment is directed toward symptomatic relief of the disorder by disrupting the circular muscle fibers of the lower esophageal sphincter.7 This can be achieved by surgical cardiomyotomy or by balloon dilatation. The therapeutic results following either technique are broadly similar1 but balloon dilatation has several advantages: thoracic or abdominal surgery is avoided, the average hospital stay is shorter, and there is a lower incidence of subsequent esophageal reflux.8
Minimally invasive surgery using laparoscopic or thoracoscopic myotomy has shortened hospitalization and decreased morbidity compared with traditional myotomy, without increasing complications.9 However, no long-term results are available yet.
Medical treatment with anticholinergic agents or calcium antagonists is disappointing and bougienage produces only transient relief with a reported 6% incidence of perforation of the esophagus.10 Injection of botulinum toxin is an attractive alternative because of its safety and low cost per treatment, but the response is short-lasting and there is a need for repeat injections, leading to increased overall cost compared with pneumatic dilatation.11 The medical treatment with drugs and injection of botulinum toxin can be reserved for elderly patients who have contraindications to pneumatic dilatation or surgery.
Pneumatic dilatation remains the first choice in the treatment of esophageal achalasia in many institutions.11,12,13,14 Many different types of balloon dilators were used in the past, but the Rigiflex balloon (Boston Scientific, Watertown, MA) is currently the most popular.14
PROCEDURE
In our institution all esophageal dilatations for achalasia patients are performed in the interventional suite under fluoroscopic guidance.15
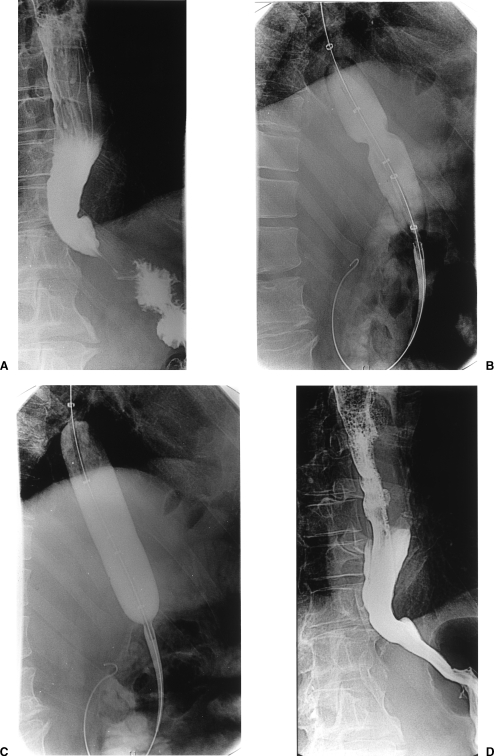
The majority of procedures are performed under intravenous sedation and analgesia using Midazolam (Hypnovel, Roche Products Ltd, England) and Fentanyl (Sublimaze, Janssen-Cilag Ltd, England). After an initial esophagogram with water-soluble nonionic contrast medium to identify the site and length of the narrow segment (Fig. 1A), the patient is placed on the fluoroscopic table in the left lateral position, the oropharynx is sprayed with xylocaine, and a mouth guard is inserted. A 6.5-F biliary manipulation catheter (William Cook, Bloomington, IN) is advanced over a Bentson guide wire (William Cook, Europe) to the level of the gastroesophageal junction. Injection of contrast medium is used to outline the narrow segment and enable safe manipulation of the catheter and guide wire into the stomach; if the catheter can be easily advanced into the duodenum, this is done to allow easier catheter exchange. If the esophagus is grossly dilated with food/liquid residue, a large-bore nasogastric tube is inserted and the contents aspirated. In most cases a Bentson guide wire is used to cross the stricture, but in tortuous or very tight strictures a hydrophilic wire may be necessary. The guide wire is then changed for a stiff exchange wire (Amplatz Superstiff, Boston Scientific, Inc., Watertown, MA) over which the balloon catheter is advanced to the cardia. The balloon is partially inflated until a waist appears (Fig. 1B), indicating the position of the muscular ring of the lower esophageal sphincter. The balloon is inflated under continuous fluoroscopic guidance and its position readjusted if it starts to slip. The balloon is inflated using hand pressure and dilute contrast medium of Ultravist 300 (concentration 300 mg I/mL, Schering) mixed with equal volume sterile water). Elimination of the waist is used as an indicator of successful dilatation (Fig. 1C). Balloon inflation is maintained for 1 minute to allow time for disruption of the muscular ring to take place. If the waist of the balloon is not eliminated completely during the first inflation, a second or third inflation is performed.
Figure 1.
(A) Contrast esophagogram showing the smooth, tapered beak-like appearance at the level of the esophageal hiatus, characteristic of achalasia. (B) Partially inflated Rigiflex balloon shows a waist at the level of the cardia. (C) Further inflation of the balloon shows complete obliteration of the waist. (D) Barium esophagogram following dilatation shows successful dilation and no evidence of leak.
In patients having balloon dilatation for the first time, a 20-mm balloon is used initially. If there is no blood on the balloon, or severe pain (pain intolerable despite adequate sedation and analgesia) occurs during the dilatation, 30-mm and 35-mm diameter balloons are used. If blood is seen on the balloon after dilatation, or the patient experiences severe pain, the procedure is terminated. Balloons larger than 35 mm are not used for patients having their first dilatation, even if no blood is seen on the balloon. However, in patients having their second or subsequent dilatation a 40-mm diameter balloon is used if no blood is present on the 35-mm balloon.
Immediately after the dilatation, a catheter esophagogram is performed using water-soluble contrast medium to exclude perforation. After 4 to 6 hours, when the patient has recovered from the sedation, a barium esophagogram is performed to look for a mucosal tear or perforation (Fig. 1D). Barium can demonstrate small perforations not shown when water-soluble media are used. If no perforation is seen, the patient is allowed to drink normally. Persistent chest pain and fever should suggest the possibility of perforation. Surgery is usually required following full thickness perforation and it is essential to explain this to the patient prior to balloon dilatation. Most patients are discharged the same evening.16
RESULTS AND DISCUSSION
The success rate of balloon dilatation reported in several series is high (70 to 80%), with low complication rates.11,12,15 More than one dilatation may be required in as many as 50% of patients.17 Dilatation is successful in decreasing LES pressure in 60 to 80% of patients; however, this change does not always translate into the relief or improvement of symptoms. Approximately 50% of patients experience recurrent symptoms within 5 years. The decision whether to proceed to repeated dilatation is based on recurrence of the presenting symptoms. A second procedure is performed if symptoms recur. In most cases of recurrence, the disease responds well to repeated dilatation therapy. However, recurrence in under 3 months after the second or subsequent dilatation is considered an indication for surgical myotomy.
Further predictors of outcome include manometry and barium studies. A 50% decrease in LES pressure or decline below 10 mm Hg is a predictor of symptomatic response.18 Timed barium esophagography is an objective test, which may be helpful in evaluating the success of achalasia treatment.19
Metman and colleagues20 found that previous Heller's myotomy was not a risk factor for complications following pneumatic dilatation. This accords with our own experience15 in 76 patients who underwent a total of 110 dilatations. No perforations were encountered, including the subgroup with previous esophageal surgery. Reported rates of perforation range from 0 to 18%.12,14,21,22,23,24 The maximum balloon diameter in two thirds (67%) of dilatations performed in our study was 30 mm (the balloon sizes used were similar to those in other series).12,14,24 Most of the patients had a good or excellent response and did not require further dilatations with a larger balloon. Progressive balloon dilatations yield excellent results, with balloons larger than 30 mm needing to be used in relatively few patients.11 Laplace's law, which states that “the tension applied on the wall for a given pressure increases rapidly with the square of the balloon radius,” suggests that use of larger balloons is likely to be associated with a substantially higher risk of perforation. However, in a retrospective study evaluating risk factors for esophageal perforation in 218 patients who had 270 pneumatic dilatations, Borotto and coworkers25 reported that perforation always occurred during the first dilatation.
Our policy of not progressing to a balloon of greater size when blood is detected on the balloon being used for the procedure is based on the assumption that tissue damage has occurred in such cases and that this is a useful marker of success. By terminating the procedure when blood staining on balloon is observed, we believe that we decrease the likelihood of unnecessary further injury to tissues and reduce the risk of perforation.
Accurate positioning of the balloon across the lower esophageal sphincter and maintenance of the position throughout inflation using fluoroscopic guidance is essential to success. We believe that the abolition of a balloon “waist” and the presence of blood on the balloon are indicators of a successful myotomy.
Some investigators11 do not consider that there is a need for the routine use of fluoroscopy for balloon dilatations. However, the American Society for Gastrointestinal Endoscopy guidelines state that fluoroscopy is mandatory for placement of a balloon dilator.26 We believe that fluoroscopic guidance facilitates the negotiation of tight strictures and enables less traumatic crossing of the cardia, thus helping to minimize the risk of perforation due to inappropriate advancement of instruments outside the esophageal lumen. Furthermore, it is not uncommon during inflation for the balloon to slide into the stomach or into the esophagus. Fluoroscopy enables immediate detection and correction of misplacement; this could be missed on endoscopy, leading to full inflation of a large balloon in the esophagus and consequent perforation. However, it is important for new patients to undergo endoscopy and manometry initially for confirmation of the diagnosis and exclusion of malignancy.
Symptomatic gastroesophageal reflux occurs frequently after cardiomyotomy.27,28 Antireflux surgical procedures are frequently performed at the same time as cardiomyotomy, especially when an abdominal route is used for the operation.29 In our study, symptomatic gastroesophageal reflux was seen in only 4% of patients following dilatation and resolved spontaneously in all cases. It appears that balloon myotomy is less likely to lead to permanent loss of competence of the lower esophageal sphincter than is a surgical incision. This may be related to retention of the phrenicoesophageal ligament.
Some patients experience chest pain following balloon dilatation.21,30,31,32,33 In the study by Eckardt and associates,32 chest pain occurred in 15% of patients between 1 and 10 hours following pneumatic dilatation. We encountered four cases of severe postdilatation chest pain in our study. Patients who had chest pain after the procedure responded as well or better than patients who did not experience pain after the dilatation.
Recurrent dysphagia occurs in 3 to 20% of patients and can be treated by repeated balloon dilatation.23 Some authors recommend surgery for patients in whom dysphagia recurs after three pneumatic dilatations, as multiple dilatations may lead to fibrosis and diminish the likelihood of successful surgery.34,35 However, Eckardt and colleagues18 found that surgery was equally effective in patients who had several dilatations as in those who chose to undergo surgery as the initial treatment. As surgery offers no clear benefits over balloon dilatation, we believe that balloon myotomy should be repeated, except in those patients in whom it is ineffective or leads to very short-lasting relief of dysphagia.
Fellows and coworkers7 report that symptomatic relief is more difficult to achieve with pneumatic dilatation in patients under 45 years of age and this view is shared by Vantrappen and associates.36 Eckardt and colleagues18 advocate surgery as the first line of treatment for patients under 18 with achalasia. Our experience15 supports this approach: out of the 76 patients treated, the 5 patients who required more than two dilatations in our series were all under 48 years, and the 2 who required five dilatations were 17 and 25 years old.
Furthermore, patients who have chest pain as the predominant symptom before treatment are usually young individuals with vigorous achalasia (distal esophageal amplitude > 37 mm Hg).37 Pneumatic dilatation is reported to be less effective for control of chest pain,38 and Perretta and coworkers37 recommend laparoscopic Heller's myotomy for this group of patients. They recently published their experience showing that chest pain resolved in 84% of patients and improved in 11% following laparoscopic Heller's myotomy.37
A randomized comparison of balloon dilatation and laparoscopic myotomy in the treatment of achalasia would provide valuable information. However, as the results of balloon dilatation are comparable to surgery, it is unlikely that informed consent can be obtained for random allocation to these two methods of treatment in the light of a high degree of patient satisfaction with dilatation.7
Two recent studies have reported the results of balloon dilatation in children.39,40 The first study documented the author's experience in three children with achalasia, including one treated successfully with balloon dilatation. The second study compares the results of balloon dilatation of strictures due to caustic ingestion, achalasia, esophagitis, congenital stenosis, and epidermolysis bullosa with those of primary repair of esophageal atresia. In both groups, dilatation is shown as a good method for alleviating symptoms. There were no complications and the authors noted that chronic diseases such as epidermolysis bullosa and esophageal atresia required repeated dilatations. They also concluded that radiation exposure from multiple procedures, over an extended period, is comparable to that from a single abdominal CT, and can be considered acceptable when complex surgery is the alternative treatment option, or when chronic incurable disease is the cause of the stricture.
Our experience in treating esophageal strictures other than achalasia in adults includes strictures caused by radiation, previous surgery, caustic ingestion, epidermolysis bullosa, and malignant strictures either prior to stenting or to allow endoscopic access for assessment and biopsy. We would advise caution when dilating this group of patients. They are more likely to have severe pain during the procedure and may be more prone to complications. We would limit the balloon size to 20 mm and progress to 30 mm in only very few cases.
CONCLUSION
Balloon dilatation under fluoroscopic control is a safe and successful method of treatment for esophageal achalasia, even after previous esophageal surgery. The dilatations should start with small-size balloons, progressing to larger sizes only when necessary, to minimize the risk of esophageal perforation.
REFERENCES
- Vantrappen G, Hellemans J. Treatment of achalasia and related motor disorders. Gastroenterology. 1980;79:144–154. [PubMed] [Google Scholar]
- Patti M. Achalasia. Emedicine. Available at: http://www.emedicine.com/med/topic16.htm. Accessed August 2003. Available at: http://www.emedicine.com/med/topic16.htm
- Mayberry J F, Rhodes J. Achalasia in the city of Cardiff from 1926 to 1977. Digestion. 1980;20:248–252. doi: 10.1159/000198446. [DOI] [PubMed] [Google Scholar]
- Mayberry J F, Atkinson M. Studies of incidence and prevalence of achalasia in the Nottingham area. Q J Med. 1985;56:451–456. [PubMed] [Google Scholar]
- Nihoul-Fekete C, Bawab F, Lortat-Jacob S, Arhan P. Achalasia of the esophagus in childhood. Surgical treatment in 35 cases, with special reference to familial cases and glucocorticoid deficiency association. Hepatogastroenterology. 1991;38:510–513. [PubMed] [Google Scholar]
- Lind C D. Dysphagia: evaluation and treatment. Gastroenterol Clin North Am. 2003;32:553–575. doi: 10.1016/s0889-8553(03)00024-4. [DOI] [PubMed] [Google Scholar]
- Fellows I W, Ogilvie A L, Atkinson M. Pneumatic dilatation in achalasia. Gut. 1983;24:1020–1023. doi: 10.1136/gut.24.11.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J R, Bargaza E, Hendrix T R, Siegel C I. Treatment of achalasia by pneumatic dilatation of the cardia. Gut. 1968;9:727. [PubMed] [Google Scholar]
- Patti M G, Pellegrini C A, Arcerito M, et al. Comparison of medical and minimally invasive surgical therapy for primary oesophageal motility disorders. Arch Surg. 1995;130:609–615. doi: 10.1001/archsurg.1995.01430060047009. [DOI] [PubMed] [Google Scholar]
- Yon J, Christensen J. An uncontrolled comparison of treatments for achalasia. Ann Surg. 1975;182:672–676. doi: 10.1097/00000658-197512000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P O, Gilbert J, Castell D O. Pneumatic dilatation is effective long-term treatment for achalasia. Dig Dis Sci. 1998;43:1973–1977. doi: 10.1023/a:1018886626144. [DOI] [PubMed] [Google Scholar]
- Kadakia S C, Wong R KH. Graded pneumatic dilation using Rigiflex achalasia dilators in patients with primary oesophageal achalasia. Am J Gastroenterol. 1993;88:34–38. [PubMed] [Google Scholar]
- Lambroza A, Schuman R W. Pneumatic dilation for achalasia without fluoroscopic guidance: safety and efficacy. Am J Gastroenterol. 1995;90:1226–1229. [PubMed] [Google Scholar]
- Cox J, Buckton G K, Bennett J R. Balloon dilatation in achalasia: a new dilator. Gut. 1986;27:986–989. doi: 10.1136/gut.27.8.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharwal T, Cowling M, Dussek J, et al. Balloon dilation for achalasia of the cardia: experience in 76 patients. Radiology. 2002;224:719–724. doi: 10.1148/radiol.2243011049. [DOI] [PubMed] [Google Scholar]
- Sabharwal T, Cowling M, Dussek J, et al. Balloon dilation for achalasia of the cardia: a day case procedure. Radiology. 2003;228:594. doi: 10.1148/radiol.2282030120. [DOI] [PubMed] [Google Scholar]
- Uthappa M C, Uberoi J, Phillip-Hughes J, Boardman P. Balloon dilation for achalasia of the cardia: a day case procedure. Radiology. 2003;228:594. doi: 10.1148/radiol.2282030120. [DOI] [PubMed] [Google Scholar]
- Eckardt V F, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732–1738. doi: 10.1016/0016-5085(92)91428-7. [DOI] [PubMed] [Google Scholar]
- Vaezi M F, Baker M E, Achkar E, et al. Timed barium oesophagram: better predictor of long-term success after pneumatic dilation in achalasia than symptom assessment. Gut. 2002;50:765–770. doi: 10.1136/gut.50.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metman E H, Lagasse J P, d'Alteroche L, Picon L, Scotto B, Barbieux J P. Risk factors for immediate complications after progressive pneumatic dilation for achalasia. Am J Gastroenterol. 1999;94:1180–1185. doi: 10.1111/j.1572-0241.1999.01062.x. [DOI] [PubMed] [Google Scholar]
- Barkin J S, Guelrud M, Reiner D K, et al. Forceful balloon dilatation: an outpatient procedure for achalasia. Gastrointest Endosc. 1990;36:123–126. doi: 10.1016/s0016-5107(90)70964-9. [DOI] [PubMed] [Google Scholar]
- Csendes A, Braghetto I, Henriquez A, et al. Late results of a prospective randomised study comparing forceful dilatation and esophagomyotomy in patients with achalasia. Gut. 1989;30:299–304. doi: 10.1136/gut.30.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried R L, Rosenberg S, Goyal R. Perforation rate in achalasia with polyethylene balloon dilators. Gastrointest Endosc. 1991;37:405. doi: 10.1016/s0016-5107(91)70753-0. [DOI] [PubMed] [Google Scholar]
- Stark G, Castell D O, Richter J E, et al. Prospective randomized comparison of Brown-McHardy and Microvasive balloon dilators in treatment of achalasia. Am J Gastroenterol. 1990;85:1322–1325. [PubMed] [Google Scholar]
- Borotto E, Gaudric M, Danel B, et al. Risk factors for oesophageal perforation during pneumatic dilatation for achalasia. Gut. 1996;39:9–12. doi: 10.1136/gut.39.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esophageal dilation. Guidelines for clinical application. Gastrointest Endosc. 1991;37:122–124. [No authors listed.] [PubMed] [Google Scholar]
- Lobello R, Edwards D AW, Gummer J WP, et al. The antireflux mechanism after cardiomyotomy. Thorax. 1978;33:569–573. doi: 10.1136/thx.33.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce M, Ortiz V, Juan M, et al. Gastroesophageal reflux, quality of life, and satisfaction in patients with achalasia treated with open cardiomyotomy and partial fundoplication. Am J Surg. 2003;185:560–564. doi: 10.1016/s0002-9610(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Black J, Vorbach A N, Collis J L. Results of Heller's operation for achalasia of the oesophagus. The importance of hiatal repair. Br J Surg. 1976;63:949–953. doi: 10.1002/bjs.1800631215. [DOI] [PubMed] [Google Scholar]
- Nair L A, Reynolds J C, Parkman H P, et al. Complications during pneumatic dilation for achalasia or diffuse esophageal spasm. Analysis of risk factors, early clinical characteristics, and outcome. Dig Dis Sci. 1993;38:1893–1904. doi: 10.1007/BF01296115. [DOI] [PubMed] [Google Scholar]
- Heimlich H J, O'Connor T W, Flores D C. Cases for pneumatic dilatation in achalasia. Ann Otol Rhino Laryngol. 1978;87:519–522. doi: 10.1177/000348947808700410. [DOI] [PubMed] [Google Scholar]
- Eckardt V F, Kanzler G, Westermeler T. Complications and their impact after pneumatic dilation for achalasia: prospective long-term follow-up study. Gastrointest Endosc. 1997;45:349–353. doi: 10.1016/s0016-5107(97)70142-1. [DOI] [PubMed] [Google Scholar]
- Wehrmann T, Jacobi V, Jung M, et al. Pneumatic dilation in achalasia with a low-compliance balloon: results of a 5-year prospective evaluation. Gastrointest Endosc. 1995;42:31–36. doi: 10.1016/s0016-5107(95)70239-3. [DOI] [PubMed] [Google Scholar]
- Nanson E M. Treatment of achalasia of the cardia. Gastroenterology. 1966;51:236–241. [PubMed] [Google Scholar]
- Barnett J L, Eisenmann R, Nostrant T T, et al. Witzel pneumatic dilation for achalasia: safety and long-term efficacy. Gastrointest Endosc. 1990;36:482–485. doi: 10.1016/s0016-5107(90)71120-0. [DOI] [PubMed] [Google Scholar]
- Vantrappen G, Hellemans J, Deloof W, et al. Treatment of achalasia with pneumatic dilatation. Gut. 1971;12:268–275. doi: 10.1136/gut.12.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretta S, Fisichella P M, Galvani C, et al. Achalasia and chest pain: effect of laparascopic Heller myotomy. J Gastrointest Surg. 2003;7:692–700. doi: 10.1016/s1091-255x(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Eckardt V R, Stauf B, Bernhard G. Chest pain in achalasia: patient characteristics and clinical course. Gastroenterology. 1999;116:1300–1304. doi: 10.1016/s0016-5085(99)70493-2. [DOI] [PubMed] [Google Scholar]
- Ali M E. Achalasia of the cardia in children. Saudi Med J. 2003;24:S40. [Google Scholar]
- Fasulakis S, Andronikou S. Balloon dilatation in children for oesophageal strictures other than those due to primary repair of oesophageal atresia, interposition or restrictive fundoplication. Pediatr Radiol. 2003;33:682–687. doi: 10.1007/s00247-003-1011-9. E-pub 2003 Aug 6. [DOI] [PubMed] [Google Scholar]