ABSTRACT
Gastrostomy allows enteral nutrition to continue in patients who are unable to meet their caloric requirements orally. Though the indications for gastrostomy placement are varied, dysphagia secondary to a neurological condition is the most common. These catheters were initially placed surgically, but percutaneous endoscopic placement is now the routine in most centers. Interventional radiologists have been performing this procedure under fluoroscopic guidance for several years with encouraging results. Percutaneous radiological gastrostomy is reported to have a success rate comparable to that of the endoscopic method, with lower morbidity and mortality rates. A further benefit is that it may be performed in patients for whom the endoscopic method would be difficult or dangerous, such as those with head and neck malignancies. One of the main factors currently limiting the use of this procedure is the shortage of interventional radiology facilities and specialists.
This article describes a technique for routine percutaneous radiological gastrostomy catheter placement and procedural variations for difficult cases. Indications and contraindications will be discussed, as will complication rates and how these compare with the traditional methods of gastrostomy tube placement.
Keywords: Gastrostomy, gastrojejunostomy, interventional radiology
Gastrostomy provides access for enteral nutrition in patients for whom oral intake is either impossible or unsafe, and for others who are unable to achieve an adequate caloric intake orally. It can also be used for palliative decompression of proximal small bowel or gastric outlet obstruction. The ideal procedure should provide an effective and aesthetically acceptable gastrostomy, at low cost, with minimal risk of both short- and long-term complications.
Surgical gastrostomy was initially described by Egeberg in 1837, but it was Verneuil who performed the first procedure in 1876,1 and Stamm who standardized the technique in 1894. The Stamm procedure, which is still used today for placement of some temporary gastrostomies, requires a general anesthetic and uses a midline or left sagittal incision in the upper abdomen with direct cut-down to the stomach, followed by the passage of a Foley catheter into the fundus of the stomach. The catheter is maintained in position by an intraluminal inflated balloon and a double row of purse-string sutures in the gastric wall. Other surgical techniques were described in the early twentieth century, though these did not translate into improved patient outcomes.2
Percutaneous endoscopic gastrostomy (PEG) was first performed in 1979 by Gauderer and Ponsky.3 This method, requiring only sedation and local anesthetic, was a major advance, resulting in successful placement in most patients, with less morbidity and mortality. The traditional “pull” technique involves apposition and transillumination of the anterior gastric wall with an endoscope. A small skin incision is made and the stomach punctured with an 18-gauge needle through which a long silk suture is passed. The proximal end of this thread is snared, pulled out via the mouth and is then used to pull the tube, which has a proximal phalange, into position. It is secured with a disk at the skin surface. The much simpler “push” technique begins in the same way, though it requires a slightly larger skin incision. After gastric puncture with the needle, a guide wire is passed through, the needle withdrawn, and a trochar with a peel-away catheter is advanced with pressure and rotation. The trochar is withdrawn and replaced with the feeding tube, which has either a proximal balloon or “mushroom,” and the catheter is peeled away. It is similarly fixed at the skin surface.
In 1981, a Canadian surgeon named Preshaw used fluoroscopic guidance to perform the first percutaneous radiological gastrostomy (PRG).4 Using fluoroscopy to avoid bowel and solid organs and to locate the stomach eliminates the need for upper endoscopy. As such, this procedure is both less invasive and available to a wider patient population, including those with contraindications to endoscopy. The technique, which will be described in detail below, is similar to that of a “push” PEG. It is a minimally invasive and effective procedure with low associated morbidity and mortality and, in addition to the PEG method, has now largely replaced open surgical gastrostomy.
Potential complications with PRG, as for all gastrotomy procedures, include: peritoneal catheterization leading to peritonitis; early tube dislodgment requiring repeated gastric puncture; entry site excoriation and/or infection; hemorrhage; ileus; aspiration of feed; and tube occlusion.5,6,7,8,9,10,11,12 In addition, the psychological challenge associated with the poor cosmetic appearance of traditional tubes must not be underestimated. These problems are being addressed with new catheter designs, which have higher long-term patency rates and more acceptable low-profile external appearances. Aspiration in patients with gastroesophageal reflux disease or neurological disorders can largely be avoided by the preferential insertion of a gastrojejunostomy, whereby the catheter tip is advanced through the pylorus to lie within the proximal jejunum.6
This article describes current techniques in percutaneous radiological gastrostomy and gastrojejunostomy, and compares success and complication rates with those of the traditional surgical and percutaneous endoscopic methods. Indications, contraindications, and current controversies related to this procedure are also addressed.
INDICATIONS
The most common indication for gastrostomy is dysphagia secondary to neurological disorder.5,13,14,15,16 Many neurological diseases cause swallowing difficulties, but patients with cerebrovascular disease, degenerative central nervous system disease, hypoxic brain injury, or traumatic brain injury form the majority of referrals for gastrostomy. Another group of patients that benefit from this procedure are those with head and neck or esophageal malignancies causing dysphagia due to local neurological involvement or physical obstruction with tumor.5,7,16,17,18,19,20,21,22,23,24,25 These patients, more so than any other group, should be referred for PRG in preference to PEG, as several studies have documented the infrequent but important complication of stomal or gastric tumor seeding when the endoscopic pull method is used.26,27,28,29,30,31
In pediatric populations, gastrostomy is more commonly indicated for supplemental nutrition in patients with chronic illnesses such as cystic fibrosis, hydrocephalus, or severe congenital heart disease.5,32,33,34 These patients are often unable to achieve their high caloric requirements orally, and nocturnal gastrostomy feeding provides additional nutrition with minimal disruption to their daytime diet. Adults with impaired intestinal absorption due to inflammatory bowel disease, radiation enteritis, or scleroderma can also occasionally benefit from supplemental gastrostomy feeding.17
With improved medical management of malignant gastric outlet and proximal small bowel obstruction, using agents such as octreotide, palliative gastrostomy is now an uncommon procedure.
CONTRAINDICATIONS
Several contraindications to percutaneous gastrostomy once considered absolute are now only relative. However, uncorrected coagulopathy remains an absolute contraindication due to the possibility of uncontrollable internal hemorrhage. Patients proposed for this procedure should have a coagulation screen performed and any coagulopathy corrected prior to the procedure. Suggested acceptable parameters include an International Normalized Ratio (INR) of 1.3 or less and a platelet count of at least 80 × 109/L.6 If possible, this procedure should also be avoided in patients with portal hypertension and varices due to the potential for massive hemorrhage.
Many patients requiring gastrostomy placement are immunosuppressed, either due to their underlying illness or to the use of medications such as steroids. Immunosuppression has been associated with higher rates of pericatheter leakage, and as such, should be kept in mind when considering patients for this procedure.18
Interposition of either colon or liver between the stomach and anterior abdominal wall, previous major gastric surgery, and ascites can all pose technical difficulties for the interventional radiologist. Various procedural modifications, described in the following section, have been made to address these difficulties.
TECHNIQUES
Each radiology department will have its own variations on the method used for percutaneous gastrostomy placement. Because it is impossible to detail all variations here, we will describe the key features of a “routine” placement, followed by some important adaptations and recent technical advances.
Preparation
Each patient has a preprocedural evaluation including chart review and coagulation screen. Informed consent is obtained by the treating radiologist. A nasogastric tube is passed to allow gastric air insufflation prior to puncture. In some departments, this tube is also used to administer 200 mL of dilute barium 12 hours before the procedure to aid in fluoroscopic identification of the colon. Difficulty in passing a nasogastric tube may be overcome by using a guide wire under fluoroscopic control, and in cases when it cannot be passed, oral effervescent sodium bicarbonate can be used to produce gastric ventilation.35 Alternately, if an air bubble can be seen fluoroscopically in the stomach, a 22-gauge Chiba needle can be aimed at that, and the stomach inflated directly via this needle.7 If all else fails, direct gastric puncture can be made with CT or ultrasound guidance.17,18,35,36
Though this procedure can be performed with local anesthesia alone,7,8,37 conscious sedation and analgesia are usually performed with midazolam hydrochloride and fentanyl citrate. It is suggested that, in conjunction with sedation, children undergoing this procedure have restraint bands applied.17,32,33,34 All patients are monitored for cardiac rate and rhythm, respiratory rate, blood pressure, and oxygen saturation. The proposed puncture site, which should be at the junction of the upper two thirds and lower third of the stomach and halfway between the greater and lesser curvatures, is prepped, draped, and anesthetized with lignocaine.7 Some institutions use 0.5 to 1.0 mg of intravenous glucagon prior to gastric distension to inhibit gastric motility and emptying. Antibiotic cover is not routinely used for this procedure.
Gastropexy
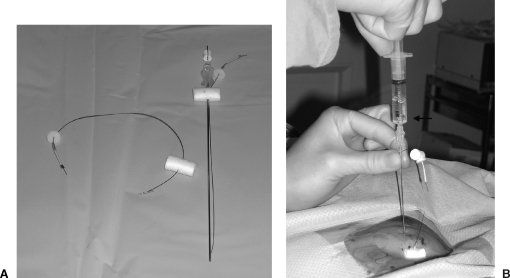
There is currently controversy as to whether gastropexy with T-fasteners (Boston Scientific, Natick, MA) should be a routine part of the procedure. Gastropexy involves the use of between two and four T-fasteners, which are deployed into the distended stomach via a slotted 18-gauge needle (Fig. 1A). After an intragastric position is confirmed by aspirating air (Fig. 1B), a stylet is advanced into each needle, pushing the preloaded T-fasteners into the stomach. After the needles and stylets are removed, gentle traction is applied to the T-fastener sutures to approximate the stomach to the abdominal wall (Fig. 2). A metal tube is then crimped around each suture to maintain the desired position. T-fasteners are removed anywhere from 2 to 20 days after the procedure, by cutting the suture between the skin and crimped metal and allowing the T-fastener to fall back into the stomach.
Figure 1.
(A) Gastropexy needle and T-fastener. (B) Intragastric confirmation of needle position through air aspiration (arrow).
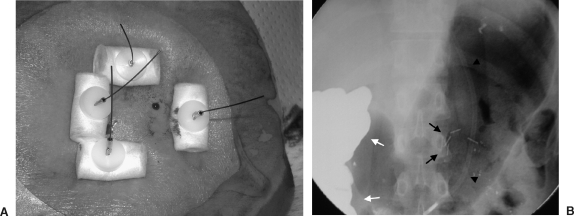
Figure 2.
(A) External appearance of gastropexy; sutures are cut at skin level at 2 to 20 days. (B) Fluoroscopic appearance in the same patient demonstrating T-fasteners (arrows), nasogastric tube (arrowheads), and colon containing barium (white arrows).
Supporters of routine gastropexy argue that it reduces the risk of initial peritoneal catheterization, of pericatheter gastric leakage, and of later intraperitoneal tube migration.6,9,15,38,39,40 Replacement of dislodged tubes is easier, leading to lower rates of repeat gastrostomy,7,8 as the formation of adhesions maintains alignment and allows the mucocutaneous tract to mature earlier. Animal studies have shown that when gastropexy is used, adhesion between the stomach and abdominal wall occurs as early as 24 hours after the procedure.38,41 It may also make the primary placement of larger tubes, which are believed to have lower occlusion rates, easier, and by acting as a tamponade, may decrease the risk of early gastric hemorrhage.42
Thornton and colleagues performed a prospective randomized trial comparing gastropexy with nongastropexy PRG in 90 patients.9 In 48 patients receiving T-fastener gastropexy, no major complications occurred. Minor complications attributable to gastropexy were pain in 5 patients and skin excoriation at the T-fastener site in 6 patients. These complications all resolved on removal of the T-fasteners. Of the 42 patients in whom gastropexy was not used, 4 had technically difficult insertions, resulting in misplacement of the tube within the peritoneal cavity in 2 of these patients (5%). Three of these patients had the procedure completed radiologically, but one was referred for PEG. This would support the use of routine gastropexy.
While few would argue against the use of gastropexy in certain clinical situations, such as the presence of ascites,10,17,18,43 some believe the routine use of gastropexy is not justified. The traditional belief that the use of smaller catheters, which have higher occlusion rates, is safer in nongastropexy cases has not been confirmed in the literature. In one study using canine stomachs, Moote and associates found no significant occurrence of pericatheter leakage when using gastrostomy tubes of various calibres (8 to 18 F) without routine gastropexy.41 Furthermore, Deutsch and colleagues found that gastropexy was not required to safely place large-calibre catheters (16 F) at the time of initial gastrostomy.44 They also claim the additional gastric punctures required for gastropexy may be associated with a higher risk of hemorrhage. In addition, there is the potential for T-fasteners to cause skin ischemia and pressure necrosis, leading to enlargement of the tract and an increased rate of gastric leakage.45
Some also argue that gastropexy adds complexity and time, and as a result, cost, to the procedure. However, others feel the additional few minutes required for gastropexy is more than justified when the time saved in difficult cases and in repeated procedures after early dislodgment is taken into account. Dewald and associates performed a cost evaluation of gastropexy, but they were unable to support or refute its routine use on a purely financial basis.8
Gastrostomy Placement
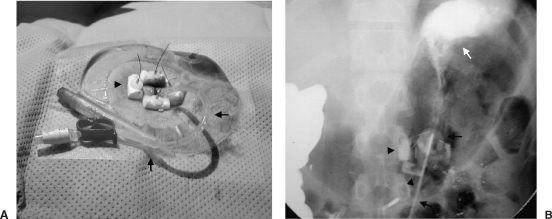
There are many catheters available for percutaneous gastrostomy, and the choice of catheter depends on the patient's clinical situation and the operator's personal preference. A skin incision is made and the subcutaneous tissues carefully dissected. When gastropexy is performed, the incision is positioned between the T-fastener sites. The stomach is punctured with an 18-gauge needle, through which a stiff guide wire is passed. The needle is removed and the tract dilated to allow passage of the catheter. The tract must be larger than the catheter, with some authors suggesting the tract be dilated to two French sizes greater than the chosen catheter.6 The catheter is then advanced over the guide wire and is maintained in position with a pigtail or balloon, depending on the catheter. If the Foley balloon type of catheter is used, it must be inserted through a peel-away sheath. An intragastric position should be confirmed by injecting contrast material at the end of the procedure (Fig. 3).
Figure 3.
Balloon-retained (A) gastrostomy tube (arrows) and (B) gastropexy (arrowheads) are shown, while intragastric position is confirmed with contrast (white arrow).
Some departments have described the use of per-oral image-guided gastrostomy (PIG).31,46,47 This involves gastric location and puncture as for routine radiologic gastrostomy, then retrograde catheterization of the esophagus, passage of a guide wire up the catheter and out the patient's mouth, and attachment of a pull-type gastrostomy tube. The tube is pulled down into the stomach and fixed as for PEG. Laasch and coworkers compared results of 250 PEG, 50 PRG, and 60 PIG procedures, and found this technique to be successful with similar complication rates to other methods.46 Of interest, they found that prophylactic antibiotics reduced the rate of stomal infection in all per-oral placements, and that no significant pathology other than clinically silent peptic disease (21%) was detected with endoscopy. This pull technique should not be used in patients with head and neck tumors due to the risk of stomal or gastric seeding.26,27,28,29,30,31
The patient must usually fast for 12 hours postprocedure, after which time enteral feeding via the catheter is commenced. It is imperative that the catheter be flushed well after each use to avoid tube occlusion. If blockage does occur, it can often be cleared by flushing with warm or carbonated water. If unsuccessful, an attempt can be made to clear the tube using a guide wire, but if this too fails, the tube must be replaced. Cases of gastrostomy-related complication should be referred to the treating radiologist, both for optimal management of the complication and for internal auditing purposes.
Gastrojejunostomy
Aspiration is a cause of considerable morbidity and mortality in patients receiving enteral nutrition via a gastrostomy tube. Controversy exists regarding the routine use of gastrojejunostomy to decrease the incidence of aspiration. While the use of primary gastrojejunostomy in high-risk patients, including those with neurological diseases, previous aspiration, history of reflux, or known hiatus herniae, is recommended,6 a standard gastrostomy tube should be adequate in most cases. In a small number of patients, such as those with gastric outlet obstruction, the use of a dual lumen tube with a gastric opening for suctioning and a jejunal opening for feeding may be indicated.18
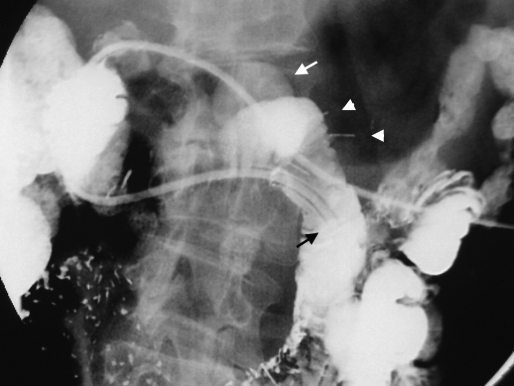
The technique for gastrojejunostomy placement begins as for gastrostomy, except that gastric puncture is made with the needle angled toward the pylorus. A torquable catheter is advanced through the pylorus into the duodenum and a guide wire passed beyond the ligament of Trietz under fluoroscopic guidance. The tract is dilated and a 14- to 18-F catheter is passed over the guide wire and positioned to lie in the proximal small bowel. Contrast is injected to confirm an intraluminal position (Fig. 4).
Figure 4.
Immediate postprocedure fluoroscopic image of a percutaneous radiological gastrojejunostomy. The catheter tip is sited in the proximal jejunum (arrow). Note the gastropexy (white arrowheads) and retaining balloon (white arrow).
Conversion of Gastrostomy to Gastrojejunostomy
There are many patients deemed low-risk for aspiration who then demonstrate signs of reflux and aspiration after gastrostomy placement. Lee and Kiely found that 46% of their patients had scintigraphic evidence of gastroesophageal reflux after gastrostomy tube insertion.48 Patients who exhibit reflux of feed should be considered for conversion to a gastrojejunostomy to decrease the risk of subsequent aspiration.
As the initial puncture for gastrostomy alone is usually aimed toward the fundus rather than the antrum of the stomach, a more acute angle lies between the entry point and the pylorus, making negotiation of the catheter into the pylorus more difficult. Rigid sheaths or cannulae can often be used to broaden this angle and allow conversion without the need for a new tract. One series also described the successful use of a 10-F curve-tipped vascular dilator to aid in directing the guide wire to the pylorus.18 When converting a surgical gastrostomy, the existing tube is removed over a guide wire and replaced with a gastrojejunostomy, whereas when an endoscopic gastrostomy is converted, the PEG tube is simply cut and the tract redirected as for a PRG.48 If these techniques are unsuccessful, an entirely new tract may need to be formed.
Modifications for Difficult Cases
Interposition of colon or liver was once considered an absolute contraindication to PRG. Some operators routinely identify the position of the colon using dilute barium given orally the night prior to the procedure or via enema on the same day as the procedure. Others, including Hicks and colleagues, feel that as the colon is usually visible with fluoroscopy, transcolonic puncture should be avoidable without routine contrast administration.49 Either way, if the colon is found to lie between the anterior abdominal wall and the stomach, a problem arises. In these cases, an infracolic approach, as described by Mirich and Gray, may be used.50 Importantly, gastric fixation is not recommended with this method due to the ensuing risk of mechanical bowel obstruction.51 Preprocedural CT or ultrasound has been used to overcome the difficulty associated with an interposed liver.17,18,33,35,48
For the interventional radiologist, patients with ascites pose the challenges of a higher degree of technical difficulty and an increased risk of pericatheter leakage.10,17 This can cause skin excoriation at the stomal site and, more importantly, can lead to potentially fatal peritonitis. The risk is greatest for patients with gross ascites, and, as such, it is recommended that paracentesis be performed prior to procedure and that gastropexy be a routine for this group, to decrease the risk of intraperitoneal tube placement and of early tube dislodgment. Patients receiving peritoneal dialysis should be treated in a similar way, having all dialysate drained out of the peritoneal cavity prior to procedure. Successful placement with few complications has been reported in patients with minimal ascites.22
Neurological disorders such as amyotrophic lateral sclerosis may result in a superiorly displaced stomach due to diaphragmatic denervation. Thornton and associates have described an intercostal route or angled subcostal route with no additional morbidity for PRG. They recommend using the lowest anterior intercostal space, where the pleural reflection is less likely to be traversed, if intercostal puncture is necessary.52
While previous gastric surgery, such as partial gastrectomy, Bilroth II gastroenterostomy, and gastric pull-up procedures, can make the percutaneous approach to gastrostomy more difficult, it is now uncommon for this to render the procedure impossible. Numerous modifications have been described to improve access to the surgically altered stomach, including balloon distension or CT guidance to better identify the stomach, longer needles and peel-away sheaths to access higher or deeper stomachs, and even a transhepatic approach can be undertaken in certain cases.15,17,48,53,54,55 Despite these advances, it is impossible in some instances to safely achieve percutaneous access to the stomach, and at such times, the patient should be considered for an open surgical approach.
RESULTS
Success rates for PRG insertion have been high, with most series reporting technical success in 98 to 100% of patients.25,40,47,52,56,57 Wollman and coworkers performed a meta-analysis of 5752 patients, comparing surgical gastrostomy, PEG, and PRG. Not surprisingly, surgical gastrostomy had a 100% success rate. PRG also had a high success rate at 99.2%, and this was significantly better than for PEG (95.7%, p < 0.001).58 Surgical placement was associated with the highest procedure-related mortality at 2.5%, significantly higher than for PRG (0.3%) or PEG (0.53%) [p < 0.001]. Significantly higher total and major complication rates occurred in the surgical group (29% and 19.9%, respectively) when compared with PEG (15.4% and 9.4%) and PRG (13.3% and 5.9%) [p < 0.001]. Surgery also had the highest minor complications rate (9%), followed by PRG (7.8%) and PEG (5.9%), though this did not reach statistical significance.
There are several referred patients who do not go on to have a PRG due to anticipated technical difficulty, though the rates are decreasing as experience is gained with complex cases. Bell and associates had a 7.7% refusal rate in their series of 562 referred patients, citing reasons for refusal as overlying viscera, high position of stomach, or massive ascites.12 Dewald and colleagues were referred 643 patients, of whom 28 were deemed inappropriate for PRG (4.4%) due to reasons mentioned above as well as uncorrected coagulopathy and, early in the series, partial gastrectomy.8 It must be remembered that the patients currently referred for PRG are often a complex subset of an already ill population, with many patients having first failed, or having contraindications to, the more widely used PEG procedure.52
It is important to note that long-term follow-up can be difficult in these patients as rates of early mortality are high due to the severe nature of their underlying illnesses. Bell and associates found 30-day mortality in their series to be 17.1%, with 71 deaths in 416 treated patients, only 2 of which were procedure-related.12 Halkier and coworkers found a similar 30-day mortality of 14% in 248 patients, with 2 deaths related to tube placement,24 and Saini and colleagues reported 11% mortality at 30 days, with no deaths attributable to the procedure.15
COMPLICATIONS
Gastrostomy-related complications are commonly described as being either major or minor. Major complications include peritonitis, septicemia, significant stomal infection, aspiration, hemorrhage, gastrointestinal perforation, dislodgment of tube requiring repeat procedure, and any complication leading to permanent catheter removal, prolonged hospitalization, permanent adverse outcomes, or death. Complications considered minor result in no sequelae but may require nominal therapy. These include low-grade pericatheter leakage, superficial stomal infection, tube dislodgment not requiring repeat procedure, tube occlusion, and tube or balloon rupture. In the series by Dewald and coworkers, pneumonia and new-onset aspiration not requiring tube removal were considered minor rather than major complications.8
In the meta-analysis by Wollman and colleagues, the major complication rate was only 5.9% for PRG, which was significantly lower than that for PEG (9.4%) or surgical gastrostomy (19.9%).58 In more recent studies, the results for PRG have been even better. Both Lyon and associates40 and Funaki and coworkers59 reported no major complications in their studies of 53 and 80 PRG procedures, respectively. Of note, both of these studies used routine gastropexy.
The minor complication rate with PRG reported by Wollman and colleagues was slightly higher than for PEG, though this difference did not reach statistical significance.58 This may be related to a lower risk of tube dislodgment with phalange-retained pull-type PEG tubes compared with the traditional balloon-retained catheters used for PRG. One would expect the results for PRG to improve as new catheters designed to reduce these complications, such as button and mushroom-retained catheters, become more widely used. This has already been suggested by the lower minor complication rate of 3.6% reported by Funaki and coworkers with the use of the mushroom-retained devices.60
Tube dislodgment is one of the leading causes for repeat intervention. In its simplest form, this involves repositioning an inadvertently dislocated tube. At other times, such as after balloon rupture, a new tube must be inserted through an established tract, and in the worst-case scenario, an entirely new gastrostomy tract may have to be formed. Dewald and colleagues found that 46 of 83 (55%) repeat procedures were for tube dislodgment.8 Of these, all tubes dislodged in the first week were replaced with ease, and only 2 patients with tubes dislodged between 7 and 14 days required repeat gastric puncture (2.4%). This is a favorable result when compared with that of Bell and associates, who had to perform repeat gastric puncture in 28 of 41 procedures (68%).12 This difference may have been due to the routine use of gastropexy by Dewald and colleagues, while Bell and associates did not use gastropexy in their series.
Catheter occlusion requiring tube replacement is a common complication, particularly with the long, narrow Foley-type catheters. In comparing 10-F and 22-F catheters, Hoffer and associates found that the small-calibre 10-F tubes had a much higher 30-day occlusion rate (13.6%) than the wide-bore 22-F tubes (1.6%).57 Dewald and colleagues reported a 2.4% 30-day occlusion rate in their 643 patients with 14-F catheters.8 Using death and tube removal as endpoints, De Baere and coworkers reported a 7.3% occlusion rate in 496 patients with 16- to 18-F catheters.7 The newer mushroom and button catheters are proving to have more promising occlusion rates. Funaki and associates reported only one tube occlusion in 55 patients with mushroom-retained button catheters,59 and Lyon and coworkers had no tube occlusions at all, using balloon-retained button catheters in 53 patients.40 By using the shorter and wider-bore button catheters, the morbidity associated with tube occlusion can be decreased.
When it occurs, pericatheter leakage is an annoying and unpleasant problem for patients, causing skin excoriation, increasing the risk for peristomal infections, and at the extreme end of the spectrum, increasing the risk of peritonitis. It can be managed effectively by exchanging the catheter for a 2-F–larger size and using a cutaneous protection paste.7 In the instance of any complication leading to a catheter being replaced, the proceduralist should consider replacing the defective tube with a mushroom-retained button gastrostomy due to its lower rates of occlusion and dislodgment.
CONCLUSION
PRG and gastrojejunostomy are safe and effective procedures for the provision of enteral alimentation. The technique results in high success rates, including in patients who have failed or have contraindications to PEG, with lower complication rates than either PEG or surgical placement. There is now much evidence to support the preferential use of radiologically placed gastrostomy tubes in most patients; however, access to fluoroscopy facilities and interventional radiologists can be a limiting factor.
There is ongoing controversy concerning routine use of gastropexy and primary gastrojejunostomy placement. We believe that the use of T-fasteners results in a safer initial procedure and easier replacement of dislodged tubes. This is achieved with minimal additional effort and cost, and with few related complications. As such, we recommend the routine use of gastropexy. While primary gastrojejunostomy may be appropriate in patients at high risk of aspiration, such as those with neurological disorders and history of reflux, it does not need to be performed as a routine procedure.
There are several different catheters available for use. Traditional Foley-type catheters have been troubled by high occlusion and dislodgment rates. New catheter designs, as described in detail in another article in this issue, are proving to have lower complication rates.
REFERENCES
- Cunha F. Gastostomy: its inception and evolution. Am J Surg. 1946;72:610–634. [Google Scholar]
- Schwartz S I, Ellis H. Maingot's Abdominal Operations. 8th ed. Norwalk, CT: Appleton-Century-Crofts; 1985. p. 973.
- Gauderer M W, Ponsky J L, Izant R J., Jr Gastrostomy without laparoscopy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15:872–875. doi: 10.1016/s0022-3468(80)80296-x. [DOI] [PubMed] [Google Scholar]
- Preshaw R M. A percutaneous method for inserting a feeding gastrostomy tube. Surg Gynecol Obstet. 1981;152:658–660. [PubMed] [Google Scholar]
- McLoughlin R F, So C B, Gray R R. Fluoroscopically guided percutaneous gastrostomy: current status. Can Assoc Radiol J. 1996;47:10–15. [PubMed] [Google Scholar]
- Given M F, Lyon S M, Lee M J. The role of the interventional radiologist in enteral alimentation. Eur Radiol [serial online] May 8, 2003 doi: 10.1007/s00330-003-1911-y. Accessed. [DOI] [PubMed] [Google Scholar]
- De Baere T, Chapot R, Kuoch V, et al. Percutaneous gastrostomy with fluoroscopic guidance: single centre experience in 500 consecutive cancer patients. Radiology. 1999;210:651–654. doi: 10.1148/radiology.210.3.r99mr40651. [DOI] [PubMed] [Google Scholar]
- Dewald C L, Hiette P O, Sewall L E, Fredenberg P G, Palestrant A M. Percutaneous gastrostomy and gastrojejunostomy with gastropexy; experience in 701 procedures. Radiology. 1999;211:651–656. doi: 10.1148/radiology.211.3.r99ma04651. [DOI] [PubMed] [Google Scholar]
- Thornton F J, Fotheringham T, Haslam P J, et al. Percutaneous radiological gastrostomy with and without T-fastener gastropexy: a randomised comparison study. Cardiovasc Intervent Radiol. 2002;25:467–471. doi: 10.1007/s00270-001-0089-4. [DOI] [PubMed] [Google Scholar]
- McFarland E G, Lee M J, Boland G W, Mueller P R. Gastropexy breakdown and peritonitis after percutaneous gastrojejunostomy in a patient with ascites. AJR Am J Roentgenol. 1995;164:189–193. doi: 10.2214/ajr.164.1.7998537. [DOI] [PubMed] [Google Scholar]
- Gauderer M W, Stellato T A, Wade D C. Complications related to gastrostomy button placement. Gastrointest Endosc. 1993;39:467. doi: 10.1016/s0016-5107(93)70137-6. [DOI] [PubMed] [Google Scholar]
- Bell S D, Carmody E A, Yeung E Y, Thurston W A, Simmons M E, Ho C S. Percutaneous gastrostomy and gastrojejunostomy: additional experience in 519 patients. Radiology. 1995;194:817–820. doi: 10.1148/radiology.194.3.7862985. [DOI] [PubMed] [Google Scholar]
- Sonnenberg E Van, Wittich G R, Cabrera O A, et al. Percutaneous gastrostomy and gastrojejunostomy: clinical experience. AJR Am J Roentgenol. 1986;146:581–586. doi: 10.2214/ajr.146.3.581. [DOI] [PubMed] [Google Scholar]
- Gray R R, St Louis E L, Grosman H. Modified catheter for percutaneous gastrojejunostomy. Radiology. 1989;173:276–278. doi: 10.1148/radiology.173.1.2506611. [DOI] [PubMed] [Google Scholar]
- Saini S, Mueller P R, Gaa J, et al. Percutaneous gastrostomy with gastropexy: experience in 125 patients. AJR Am J Roentgenol. 1990;154:1003–1006. doi: 10.2214/ajr.154.5.2108533. [DOI] [PubMed] [Google Scholar]
- McLoughlin R F, Gibney R G. Fluoroscopically guided percutaneous gastrostomy: tube function and malfunction. Abdom Imaging. 1994;19:195–200. doi: 10.1007/BF00203505. [DOI] [PubMed] [Google Scholar]
- Ho C S, Yeung E. Percutaneous gastrostomy and transgastric jejunostomy. AJR Am J Roentgenol. 1992;158:251–257. doi: 10.2214/ajr.158.2.1729776. [DOI] [PubMed] [Google Scholar]
- Munk P L, Lee M J, Poon P Y, et al. Percutaneous gastrostomy in radiologic practice. Australas Radiol. 1997;41:342–350. [PubMed] [Google Scholar]
- O'Dwyer T, Gullane P, Ho C S. Percutaneous feeding gastrostomy in patients with head and neck tumours: a five year review. Laryngoscope. 1990;100:29–32. doi: 10.1288/00005537-199001000-00007. [DOI] [PubMed] [Google Scholar]
- Luetzow A M, Chafoo R A, Young H. Percutaneous gastrostomy: the Stanford experience. Laryngoscope. 1988;98:1035–1039. doi: 10.1288/00005537-198810000-00001. [DOI] [PubMed] [Google Scholar]
- Shike M, Berner Y N, Gerdes H, et al. Percutaneous endoscopic gastrostomy and jejunostomy for long term feeding in patients with cancer of the head and neck. Otolaryngol Head Neck Surg. 1989;101:549–554. doi: 10.1177/019459988910100506. [DOI] [PubMed] [Google Scholar]
- O'Keeffe F, Carrasco C H, Charnsangavej C, Richli W R, Wallace S, Freedman R S. Percutaneous drainage and feeding gastrostomies in 100 patients. Radiology. 1989;172:341–343. doi: 10.1148/radiology.172.2.2501821. [DOI] [PubMed] [Google Scholar]
- Wills J S, Oglesby J T. Percutaneous gastrostomy. Radiology. 1983;149:449–453. doi: 10.1148/radiology.149.2.6414043. [DOI] [PubMed] [Google Scholar]
- Halkier B K, Ho C S, Yee A C. Percutaneous feeding gastrostomy with the Seldinger technique, review of 252 patients. Radiology. 1989;171:359–362. doi: 10.1148/radiology.171.2.2495560. [DOI] [PubMed] [Google Scholar]
- Campos A CL, Butters M, Meguid M M. Home enteral nutrition via gastrostomy in advanced head and neck cancer patients. Head Neck. 1990;12:137–142. doi: 10.1002/hed.2880120208. [DOI] [PubMed] [Google Scholar]
- Thorburn D, Karim S N, Soutar D S, Mills P R. Tumour seeding following percutaneous endoscopic gastrostomy placement in head and neck cancer. Postgrad Med J. 1997;73:430–432. doi: 10.1136/pgmj.73.861.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strodel W E, Kenady D E. Stomal seeding of head and neck cancer by percutaneous endoscopic gastrostomy (PEG) tube. Ann Surg Oncol. 1995;2:462–463. doi: 10.1007/BF02306382. [DOI] [PubMed] [Google Scholar]
- Lee D S, Mhit-Tabatabai M A, Rush B F, Levine C. Stomal seeding of head and neck cancer by percutaneous endoscopic gastrostomy tube placement. Ann Surg Oncol. 1995;2:170–173. doi: 10.1007/BF02303634. [DOI] [PubMed] [Google Scholar]
- Schiano T D, Pfister D, Harrison L, Shike M. Neoplastic seeding as a complication of percutaneous endoscopic gastrostomy. Am J Gastroenterol. 1994;89:131–133. [PubMed] [Google Scholar]
- Huang D T, Thomas G, Wilson W R. Stomal seeding by percutaneous endoscopic gastrostomy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 1994;118:658–659. doi: 10.1001/archotol.1992.01880060108022. [DOI] [PubMed] [Google Scholar]
- Clark J A, Pugash R A, Pantalone R R. Radiologic peroral gastrostomy. J Vasc Interv Radiol. 1999;10:927–932. doi: 10.1016/s1051-0443(99)70140-5. [DOI] [PubMed] [Google Scholar]
- Keller M S, Lai S, Wagner D K. Percutaneous gastrostomy in a child. Radiology. 1986;160:261–262. doi: 10.1148/radiology.160.1.3715040. [DOI] [PubMed] [Google Scholar]
- Van Sonnenberg E, Wittich G R, Edwards D K, et al. Percutaneous diagnostic and therapeutic interventional radiologic procedures in children: experience in 100 patients. Radiology. 1987;162:601–605. doi: 10.1148/radiology.162.3.2949336. [DOI] [PubMed] [Google Scholar]
- Towbin R B, Ball W S, Bissett G S., III Percutaneous gastrostomy and percutaneous gastrojejunostomy in children: antegrade approach. Radiology. 1988;168:473–476. doi: 10.1148/radiology.168.2.3134669. [DOI] [PubMed] [Google Scholar]
- Kanazawa S, Naomoto Y, Hiraki Y, Yasui K, Matsuno T. Percutaneous feeding gastrostomy in patients with partial gastrectomy: transhepatic approach with CT guidance. Abdom Imaging. 1995;20:302–306. doi: 10.1007/BF00203358. [DOI] [PubMed] [Google Scholar]
- Sanchez R B, Sonnenberg E Van, D'Agostino H B, Goodacre B W, Moyers P, Casola G. CT guidance for percutaneous gastrostomy and gastroenterostomy. Radiology. 1992;184:201–205. doi: 10.1148/radiology.184.1.1609080. [DOI] [PubMed] [Google Scholar]
- Wollman B, D'Agostino H B. Percutaneous radiologic and endoscopic gastrostomy: a 3-year institutional analysis of procedure performance. AJR Am J Roentgenol. 1997;169:1551–1553. doi: 10.2214/ajr.169.6.9393163. [DOI] [PubMed] [Google Scholar]
- Brown A S, Mueller P R, Ferrucci J T. Controlled percutaneous gastrostomy: nylon T-fastener for fixation of the anterior gastric wall. Radiology. 1986;158:543–545. doi: 10.1148/radiology.158.2.2934763. [DOI] [PubMed] [Google Scholar]
- Gray R R, Rooney M, Grosman H. Use of T-fasteners for primary jejunostomy. Cardiovasc Intervent Radiol. 1990;13:93–94. doi: 10.1007/BF02577359. [DOI] [PubMed] [Google Scholar]
- Lyon S M, Haslam P J, Duke D M, McGrath F P, Lee M J. De novo placement of button gastrostomies in an adult population: experience in 53 patients. J Vasc Interv Radiol. 2003;14:1283–1289. doi: 10.1097/01.rvi.0000092901.73329.eb. [DOI] [PubMed] [Google Scholar]
- Moote D J, Ho C S, Felice V. Fluoroscopically guided percutaneous gastrostomy: is gastric fixation necessary? Can Assoc Radiol J. 1991;42:113–118. [PubMed] [Google Scholar]
- Rose D B, Wolman S L, Ho C S. Gastric haemorrhage complicating percutaneous transgastric jejunostomy. Radiology. 1986;161:835–836. doi: 10.1148/radiology.161.3.3491377. [DOI] [PubMed] [Google Scholar]
- Lee M J, Saini S, Brink J A, et al. Malignant small bowel obstruction and ascites: not a contraindication to percutaneous gastrostomy. Clin Radiol. 1991;44:332–334. doi: 10.1016/s0009-9260(05)81270-x. [DOI] [PubMed] [Google Scholar]
- Deutsch L S, Kannegieter L, Vanson D T, Miller D P, Brandon J C. Simplified percutaneous gastrostomy. Radiology. 1992;184:181–183. doi: 10.1148/radiology.184.1.1609078. [DOI] [PubMed] [Google Scholar]
- Chung R, Schertzer M. Pathogenesis of complications of percutaneous endoscopic gastrostomy: a lesson in surgical principles. Am Surg. 1990;56:134–137. [PubMed] [Google Scholar]
- Laasch H U, Wilbraham L, Bullen K, et al. Gastrostomy insertion: comparing the options—PEG, RIG or PIG? Clin Radiol. 2003;58:398–405. doi: 10.1016/s0009-9260(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Szymski G X, Albazzaz A N, Funaki B, et al. Radiologically guided placement of pull-type gastrostomy tubes. Radiology. 1997;205:669–673. doi: 10.1148/radiology.205.3.9393519. [DOI] [PubMed] [Google Scholar]
- Lee M J, Kiely P. Percutaneous radiological gastrostomy and gastrojejunostomy. J Ir Coll Physicians Surg. 1998;27:13–16. [Google Scholar]
- Hicks M E, Surratt R S, Picus D, Marx M V, Lang E V. Fluoroscopically guided percutaneous gastrostomy and gastrojejunostomy: analysis of 158 consecutive cases. AJR Am J Roentgenol. 1990;154:725–728. doi: 10.2214/ajr.154.4.2107665. [DOI] [PubMed] [Google Scholar]
- Mirich D R, Gray R R. Infracolic percutaneous gastrojejunostomy: technical note. Cardiovasc Intervent Radiol. 1989;12:340–341. doi: 10.1007/BF02575435. [DOI] [PubMed] [Google Scholar]
- Hennigan T W, Forbes A. Colonic obstruction caused by endoscopic percutaneous gastrostomy. Eur J Surg. 1992;158:435. [PubMed] [Google Scholar]
- Thornton F J, Varghese J L, Haslam P J, McGrath F P, Keeling F, Lee M J. Percutaneous gastrostomy in patients who fail or are unsuitable for endoscopic gastrostomy. Cardiovasc Intervent Radiol. 2000;23:279–284. doi: 10.1007/s002700010069. [DOI] [PubMed] [Google Scholar]
- Varney R A, Sonnenberg E Van, Giovanna C, Sukthankar R. Balloon techniques for percutaneous gastrostomy in a patient with partial gastrectomy. Radiology. 1988;167:69–70. doi: 10.1148/radiology.167.1.3347748. [DOI] [PubMed] [Google Scholar]
- Sonnenberg E Van, Cubberly D A, Brown L K, et al. Percutaneous gastrostomy: use of intragastric balloon support. Radiology. 1984;152:531–532. doi: 10.1148/radiology.152.2.6739827. [DOI] [PubMed] [Google Scholar]
- Stevens S D, Picus D, Hicks M, et al. Percutaneous gastrostomy and gastrojejunostomy after gastric surgery. J Vasc Interv Radiol. 1992;3:679–683. doi: 10.1016/s1051-0443(92)72923-6. [DOI] [PubMed] [Google Scholar]
- Lee M J. PEG—is the E necessary? A comparison of percutaneous and endoscopic gastrostomy. Clin Radiol. 1997;52:77. doi: 10.1016/s0009-9260(97)80315-7. [DOI] [PubMed] [Google Scholar]
- Hoffer E K, Cosgrove J M, Levin D Q, Herskowitz M M, Sclafani S J. Radiologic gastrojejunostomy and percutaneous endoscopic gastrostomy: a prospective randomised comparison. J Vasc Interv Radiol. 1999;10:413–420. doi: 10.1016/s1051-0443(99)70058-8. [DOI] [PubMed] [Google Scholar]
- Wollman B, D'Agostino H B, Walus-Wigle J R, Easter D W, Beale A. Radiologic, endoscopic and surgical gastrostomy: an institutional evaluation and meta-analysis of the literature. Radiology. 1995;197:699–704. doi: 10.1148/radiology.197.3.7480742. [DOI] [PubMed] [Google Scholar]
- Funaki B, Zaleski G X, Lorenz J, et al. Radiologic gastrostomy placement: pigtail versus mushroom-retained catheters. AJR Am J Roentgenol. 2000;175:375–379. doi: 10.2214/ajr.175.2.1750375. [DOI] [PubMed] [Google Scholar]
- Funaki B, Pierce R, Lorenz J, et al. Comparison of balloon and mushroom-retained large-bore gastrostomy catheters. AJR Am J Roentgenol. 2001;177:359–362. doi: 10.2214/ajr.177.2.1770359. [DOI] [PubMed] [Google Scholar]