ABSTRACT
Up to 85% of patients who present with colonic obstruction have a colorectal cancer. Between 7% and 29% of these patients present with total or partial intestinal obstruction. Only 20% of these patients presenting with acute colonic obstruction due to malignancy survive 5 years. Emergent surgical intervention in patients with colonic obstruction is associated with significant morbidity and mortality rates. Only 40% of patients with obstructive carcinoma of the left colon can be treated with surgical resection without the need for a colostomy. The use of a temporary or permanent colostomy has a significant impact on quality of life. The decompressive effect seen with colonic stenting is a durable, simple, and effective palliative treatment of patients with advanced disease. Stent deployment provides an effective solution to acute colonic obstruction and allows surgical treatment of the patient in an elective and more favorable condition. In addition, colonic stenting reduces costs and avoids the need for a colostomy.
Keywords: Colon cancer, intestinal obstruction, colonic stenting
Colorectal cancer is an important health problem: there are nearly one million new cases of colorectal cancer diagnosed worldwide each year and half a million deaths.1 Between 10% and 30% of these patients have complete or partial obstruction of the colon at the time of presentation.2 Traditionally, the treatment of intestinal obstruction due to colon cancer has been surgery.3 However, acute obstruction of the left side of the colon poses an important medical problem.4 Emergency surgery in a patient with an unprepared colon is associated with high morbidity and mortality.5 Colon cancer patients who present with colonic obstruction have a 5-year survival rate of less than 20%, a far poorer prognosis than patients who present without obstruction.6 Additionally, mortality decreases from 15 to 20% to 0.9 to 6% when patients with colon cancer undergo elective surgery.7 Only 40% of left-sided colonic obstructions secondary to carcinoma can be treated with intraoperative lavage and subtotal colectomy.8 The remaining patients need a temporary or permanent colostomy, which has a significant impact on quality of life.9
Colonic stents were first used by Dohmoto10 in 1990. Metallic endoprostheses are used as a palliative treatment to manage the acute phase of intestinal obstruction in patients with colonic malignancy.11,12,13 Tejero and colleagues in 1994 described the use of stents as a “bridge to surgery.” 13 Other nonsurgical treatments were used with limited effectiveness such as balloon dilatation, endoscopic laser ablation, and decompression tubes.14
Metallic stent placement is an adequate alternative to emergency surgery. Colonic stenting can provide an effective, nonsurgical decompression of the obstructed left colon avoiding an emergency colostomy.13
INDICATIONS AND CONTRAINDICATIONS
The main indications for endoluminal metallic stent placement in the colon and rectum are: (1) for temporary colonic decompression in patients with acute malignant obstruction as a “bridge” to elective surgery; (2) for long-term colonic decompression in patients with obstruction due to an unresectable colonic carcinoma; (3) for long-term colonic decompression in patients with benign colonic strictures because of fibrosis associated with surgery or radiotherapy; (4) for temporary colonic decompression in patients with diverticulitis to permit elective surgical resection; (5) as a palliative treatment, for closure of ileocolic, colovesical, or colocutaneous fistulae.
The principal absolute contraindication to this procedure is clinical and/or radiological evidence of acute perforation of the colon. Relative contraindications or limitations are the presence of a long segment colonic tumor, lesions that are too proximal or too distal in the colon, and lesions in tortuous portions of the colon.15,16 These latter situations are associated with an increase in technical difficulty during stent placement and can be responsible for technical failure.
STENT DESIGN
Many metallic stents have been used for the management of colonic obstruction. Despite the commercial availability of a variety of designs, the current generation of stents continues to be modified and improved. The ideal device should include the following features: (1) high expansion ratio; (2) high flexibility; (3) large diameter (> 25 mm); (4) mechanical stability; (5) adequate radial expandable force (dumb-bell shape); (6) prevention of restenosis due to tumor ingrowth; (7) prevention of restenosis due to hyperplasia; (8) small delivery system; (9) biodegradable or readily removable stent for benign strictures; and (10) lack of interference during imaging and tumor staging.
The majority of the current stents in use are made of Nitinol or stainless steel. They are self-expandable, cylindrical in shape, with diameters of 20 to 24 cm, and a length that varies between 40 mm and 100 mm.
The different types of metallic stents include (1) Wallstent Uni Endoprosthesis (Meditech-Boston Scientific; La Garenne Colombes Cedex, France) (Fig. 1); (2) Colonic Z-Stent (William Cook Europe; Bjaeverskov, Denmark) (Fig. 2) (3) Memotherm colorectal stent (CR Bard Inc; Billerica, MA, USA); (4) Precision stent (Microvasive-Boston Scientific, La Garenne Colombes Cedex, France) (Fig. 3); (5) Stent Choostent (Life Europe; Bagnolet, France) (Fig. 4); (6) Colonic Stent (Tecnostent, Medellin, Colombia).17,18,19,20,21
Figure 1.
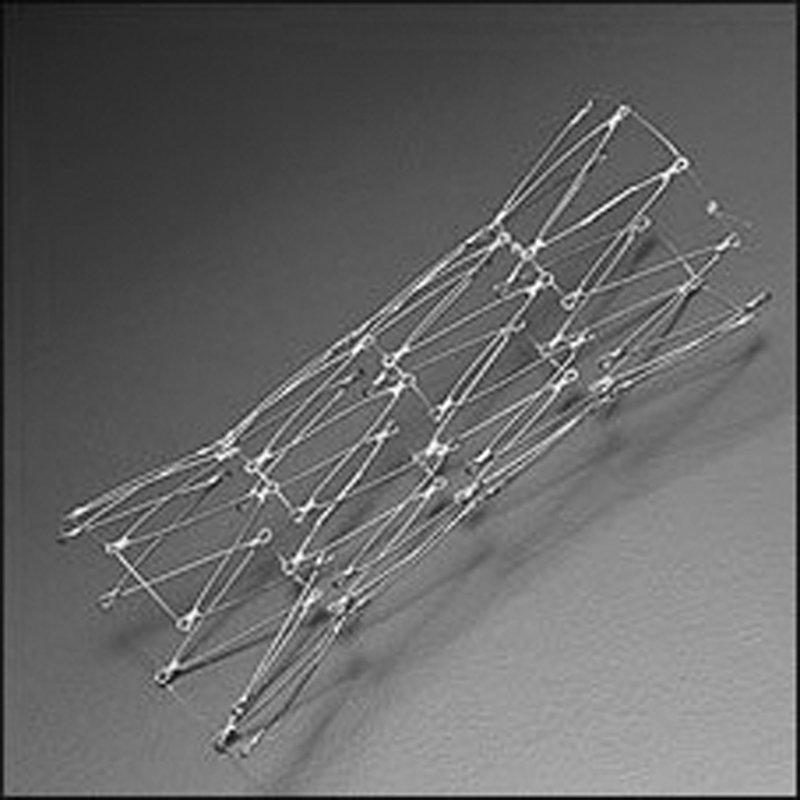
Photograph of an enteral Wallstent. This enteral endoprosthesis is a self-expandable metallic stent designed for colonic use. It consists of a monofilament wire. It has a diameter of 18 to 22 mm and is available in lengths of 6 and 9 cm (courtesy of Meditech-Boston Scientific).
Figure 2.
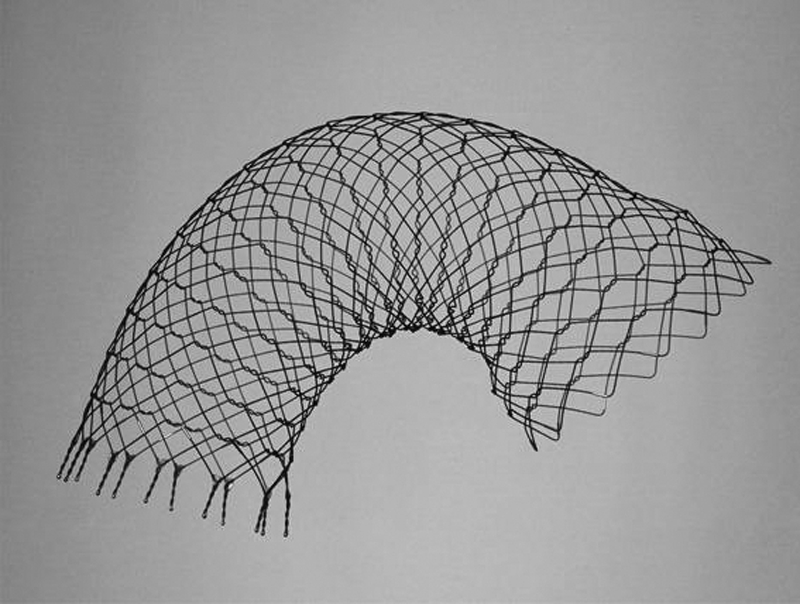
Photograph of colonic Z-stent. The colonic endoprosthesis Z-stent is a self-expandable metallic stent. It has a flared end diameter of 35 mm and a shaft diameter of 25 mm. It is available in 4- and 12-cm lengths (courtesy of Cook Europe).
Figure 3.
Photograph of a Precision Wallstent. The Precision endoprosthesis is a self-expandable metallic stent. It has a flared end diameter of 30 mm and a shaft diameter of 25 mm. It is available in 6- and 12-cm lengths. The Precision delivery system is a 16 F sheath (courtesy of Microvasive, Boston Scientific).
Figure 4.

Photograph of a covered colonic Choostent. The Choostent is a stainless cylindrical zigzag stent. It is available as a covered and uncovered stent (courtesy of Life Europe).
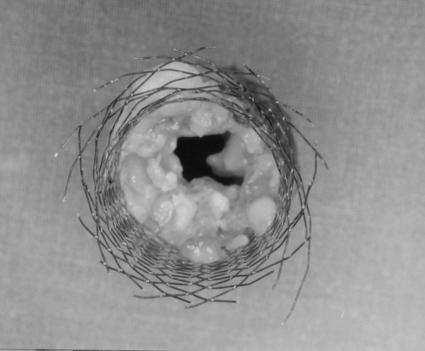
The Wallstent is a self-expandable stent made with a nonferromagnetic alloy. It is the most commonly used device. Advantages of this stent include the small delivery system and adequate flexibility. A disadvantage of this stent is the termination of the stent with the presence of free filaments at the ends of the stent.22 See Figure 5. Some authors have used a flexible, not completely covered stent with PTFE. The principal disadvantage of this design is the high rate of migration.20,21,22,23
Figure 5.
Surgical specimen from colectomy. Wallstent visualized within the tumor. Free wire filaments are appreciated in the distal portion of the stent.
TECHNICAL CONSIDERATIONS
Stent deployment in the colon can be achieved under fluoroscopic guidance alone, under colonoscopic guidance alone, and by using a combined approach (fluoroscopy and colonoscopy). In the distal sigmoid colon and rectum, stent deployment can be performed under fluoroscopic or endoscopic guidance alone. Due to the redundant course of more proximal portions of the colon, especially when associated with a tortuous sigmoid segment, some authors14,15 prefer guidance with fluoroscopy and endoscopy for stent placement in the descending colon. Endoscopy is frequently used to cross colonic strictures and place stents, although it is not strictly necessary. Fluoroscopy, however, is considered by the authors of this article to be an absolute requirement for stent placement.16 We prefer to use fluoroscopic guidance alone.
Distal bowel cleansing may be desirable to facilitate endoscopic stenting, although it is not always possible to perform completely. Moreover, oral bowel preparation is contraindicated because of the risk of perforation.
The procedure doesn't require general anesthesia. In uncooperative patients, those with high levels of anxiety, or those in whom colonoscopy is going to be used for stent deployment, sedation with midazolam and analgesia is used.
A water-soluble contrast enema should be performed prior to stent placement to identify the location, length, and caliber of the obstructing lesion. With the patient in either a supine or a lateral decubitus position, a high-torque angiographic catheter or guiding catheter is advanced over a 0.035-inch angled hydrophilic stiff guide wire (Radiofocus Terumo Europe; Leuven, Belgium) to traverse the obstructive segment under fluoroscopic guidance. If an excessively tortuous or redundant rectosigmoid region is encountered, the use of a 0.035-inch Lunderquist extra stiff guide wire (William Cook Europe; Bjaeverskov, Denmark) is recommended to facilitate the progression of the angiographic catheter to the level of the obstruction.
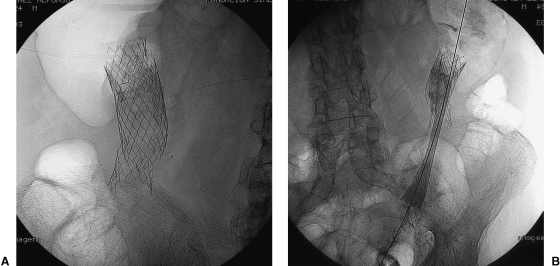
As soon as the catheter is positioned proximal to the obstruction, water-soluble contrast medium is injected through the catheter to document the characteristics and length of the obstruction and to rule out the possibility of perforation. This procedure makes fluoroscopy necessary. Radiopaque markers can be placed to identify the two ends of the obstruction. An appropriately sized stent and delivery system are chosen based on the information obtained from the enema examination. A 0.038-inch Amplatz super stiff (Boston Scientific Meditech, Lagarene Colombes Cedex, France) guide wire is introduced through the catheter into the proximal segment well beyond the obstruction. After the catheter is withdrawn, the delivery system with a loaded stent is advanced over the stiff wire and is positioned in the obstructed segment (Fig. 6). The deployed stent should be long enough not only to cover the entire obstructed segment, but also to extend beyond the proximal and distal margins of the lesion by at least 1 to 2 cm. If stent coverage is inadequate, an additional stent can be deployed to completely cover the lesion and its margins. See Figure 7.
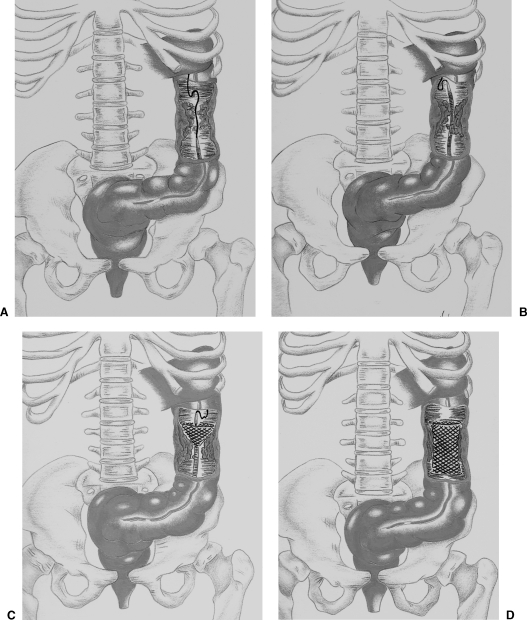
Figure 6.
Diagrams demonstrate colorectal stent deployment with fluoroscopic guidance. An obstructive carcinoma is present in the descendent colon. (A) The guide wire and the catheter are advanced through the area of obstruction. (B) The guide wire is replaced by an Amplatz stiff guide wire to straighten the tortuous colon, and the delivery system is introduced. (C) The stent is initially deployed at the proximal portion of the lesion. (D) The stent is fully expanded.
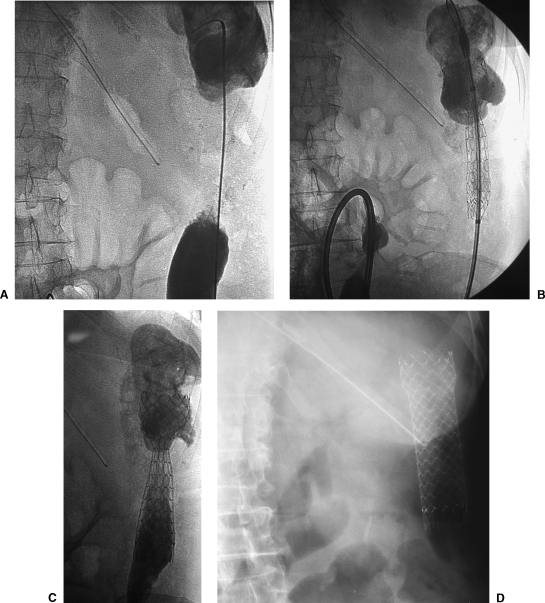
Figure 7.
Images obtained during fluoroscopic placement of a colonic stent showing the stent deployment technique. (A) Neoplasia within the descending colon. The guide wire has crossed the area of obstruction. (B) Precison stent partially deployed in the area of malignant obstruction. The delivery system can be seen. (C) Stent totally deployed and partially expanded. (D) Radiographic control with stent totally expanded.
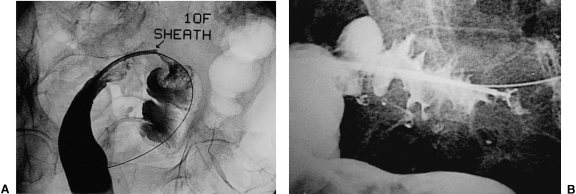
Several types of self-expanding metallic stents can be used. Covered stents are not indicated, because they migrate more easily. Choo and associates reported a 50% migration rate with the use of two covered stent types in 20 patients.24 Endoscopy can help in advancing the guide wire in patients with proximal colonic lesions (high descending colon and transverse colon), and in those patients who have marked colonic angulation (Fig. 8). In the latter type of patient we have successfully used a 10- to 12-F, 40-cm introducer sheath25 with double guide wires and a catheter: a hydrophilic guide wire with a catheter and Lunderquist super stiff guide wire (William Cook Europe; Bjaeverskov, Denmark) combination. The introducer sheath helps in getting to the lesion if the colon is tortuous. Additional advantages of the introducer sheath are that it (1) facilitates forceps biopsy of the lesion; (2) allows introduction of contrast medium to estimate the length of the lesion; and (3) facilitates stent deployment. See Figure 9.
Figure 8.
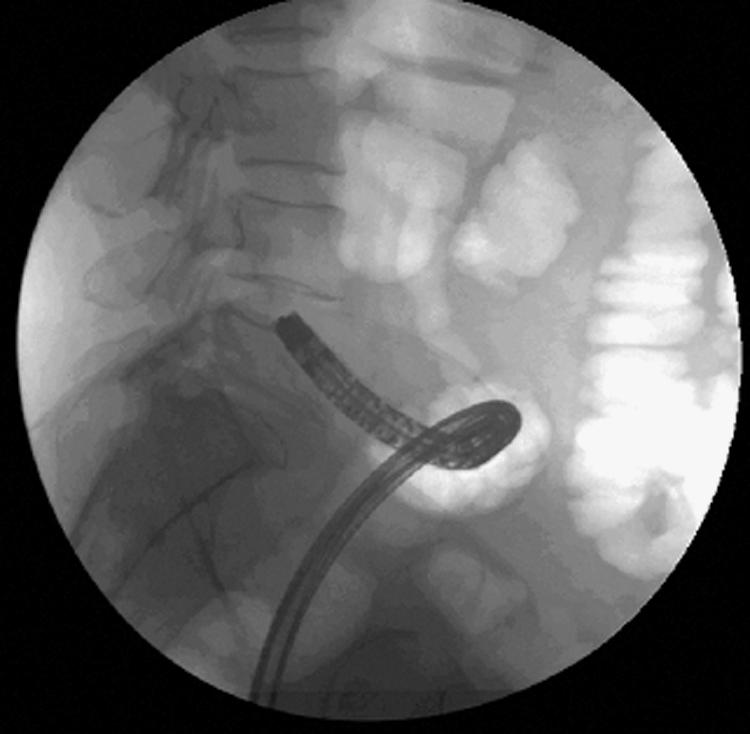
Lateral view of the abdomen. Colonoscope can be seen close to a lesion in the sigmoid colon.
Figure 9.
Utilization of a 10-F introducer sheath to facilitate the technique. (A) Introduction of contrast through the sheath to determine the morphology and the extension of the lesion keeping a guide wire distal to it. (B) Gathering a biopsy sample with a forceps through the sheath, guide wire distal to the lesion for safety purposes.
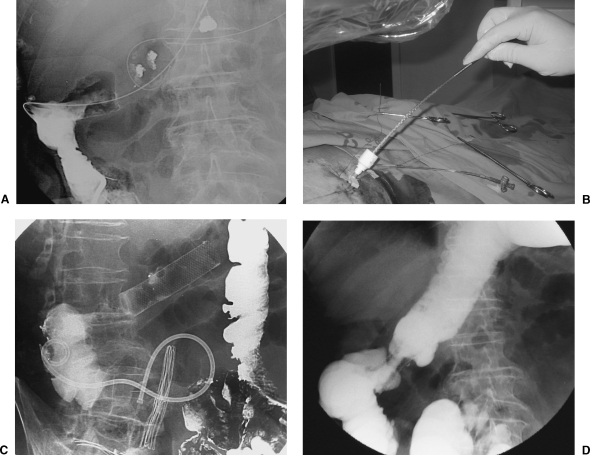
In palliative cases when it is not possible to access the colonic tumor from a retrograde approach, antegrade placement of colonic stents through a cecostomy or colostomy is a feasible alternative.26 In two nonsurgical patients with obstruction in the transverse colon and marked tortuosity of the colon, we deployed stents through a percutaneous colostomy and cecostomy, respectively27 (Fig. 10).
Figure 10.
Antegrade approach for the deployment of a colonic stent. (A) Guide wire crossing the area of malignant obstruction with access through a percutaneous cecostomy. (B) Delivery system introduction carrying the stent through a 10-F sheath placed in the cecostomy. (C) Radiographic control after stent placement. A 10-F pigtail catheter is present in the cecum. (D) Control performed 3 months after stent placement showing patency of the stent. Pigtail catheter was withdrawn without complications.
After stent deployment, additional balloon dilatation is not recommended because it is associated with a high risk of perforation. Due to the nature of the self-expansion, stents are allowed to slowly expand over time. The peristaltic movements of the colon after decompression may facilitate full expansion.
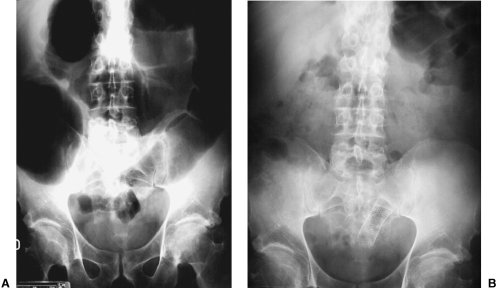
Immediately after stent placement, the enema examination can be repeated to document post-stenting patency and correct positioning, although this is not always needed. The ends of the stent should not be covered by colonic folds. If this happens, the position of the stent should be adjusted after deployment. Some authors suggest a low-residue diet and mineral oil to prevent stent occlusion by impacted fecal material. Twenty-four hours after stent placement, a plain radiograph of the abdomen is obtained to evaluate the position of the stent and to assess changes in the radiographic appearance of the obstruction (Fig. 11). After the procedure is completed the patient's vital signs are monitored and serial electrolyte measurements are obtained until they return to normal values. During the recovery period, the patient is staged and surgical risk is determined. Tumor staging is achieved by computed tomography or ultrasound of the abdomen. If the patient recovers satisfactorily the preoperative assessment can be performed on an outpatient basis.
Figure 11.
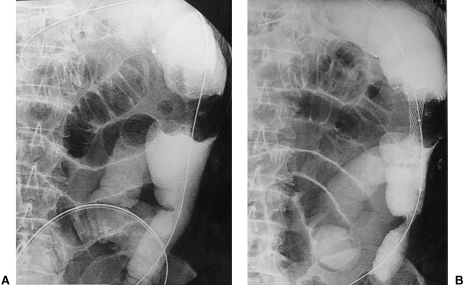
(A) Single view of the abdomen showing significant abdominal distention with dilatation of the colon proximal to the site of obstruction. (B) Radiographic control 24 hours postimplantation of the stent. Resolution of the obstructive pattern.
After discharge, the patient is instructed to contact the managing team if clinical deterioration occurs. Periodically the patient is seen in the clinic to ensure stability. Follow-up radiography should be taken when the patient develops obstructive symptoms or peritoneal signs. During physical examinations (digital rectal examination) and at surgery, special consideration should be given to the presence of a metallic stent so as to avoid injury to the examining physician.
FLUOROSCOPIC VERSUS ENDOSCOPIC GUIDANCE
The placement of colorectal stents has been performed with endoscopic guidance, fluoroscopic guidance, or by using a combined technique. Both methods have advantages and disadvantages. Stent placement in the right colon benefits from endoscopic assistance, whereas fluoroscopic guidance for lesions in the left colon is associated with tolerable radiation doses.28 In both situations the effectiveness and the technical success is similar in experienced hands. Endoscopic assistance reduces radiation dose but increases the cost and requires sedation in all patients. Nevertheless, the combination of both techniques appears to be the technique of choice in most cases.
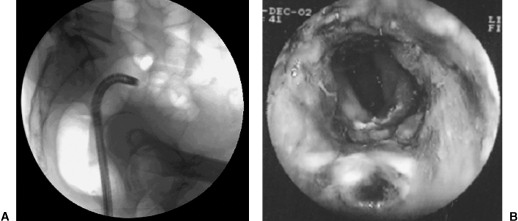
To compare both methods of guidance, from November 1999 to December 2002, our group performed a prospective, nonrandomized study analyzing the feasibility of the procedure, the technical success of stent implantation, procedure time, and radiation dose using fluoroscopy alone and fluoroscopy assisted by endoscopy.29 The endoscope was used by interventional radiologists without previous formal training. A total of 25 stents were implanted in 26 patients, 13 in each group (15 men and 11 women). See Figure 12. There was a technical failure using the combined technique in 1 patient with stenosis at the rectosigmoid level. The mean age was 58.7 (range 42 to 82 years). The lesions were located as follows: 2 in the rectum, 16 in the sigmoid colon, 6 in the proximal descending colon, and 2 in the proximal transverse colon.
Figure 12.
Implantation of a colonic stent with a combined guidance (fluoroscopy and colonoscopy). (A) Colonoscope is close to the area of obstruction within the sigmoid colon. (B) Endoscopic view of the area of obstruction.
The mean distance from the anus to the lesion was 23.69 cm (range = 11–35 cm) for the fluoroscopy group and 30.8 cm (range = 10–63 cm) in the combined technique group. The mean fluoroscopy time and radiation dose for the procedure were 19.76 mins (range = 12–45 mins) and 2763.6 dGy/cm2 (range = 1026–6789 dGy/cm2) for the fluoroscopy group and 15.0 mins (range = 15–35 mins) and 2250 dGyc/m2 (range = 1362–4523 dGy/cm2) for the combined technique group.
RESULTS
Technical success in deploying colonic stents with fluoroscopic, endoscopic, or combined guidance ranges from 88 to 100%.15 Clinical success (improvement of obstructive symptoms) has been reported in 80 to 92% of patients.15 In a systematic review of the published data (1990 to 2000) totaling 598 patients with colonic stents, the technical success was 92% and clinical success 88%. Palliation was achieved in 302 of 336 cases (90%). In 223 of 262 surgical candidates (85%), the stent was an effective “bridge” to surgery.30 There were three deaths (1%). Perforation occurred in 22 patients (4%). Stent migration was reported in 54 of 551 technically successful cases (10%). The rate of stent reobstruction was 10% (52 of 525), mainly in the palliative group. In the same study technical failure was reported in 47 of 598 patients (8%). The main causes of failure were (1) inability to cross the stenosis with a guide wire in 36 patients; (2) poor stent position in 4 patients; and (3) perforation in 2 patients.
Beltran compared two groups of patients with carcinoma of the left colon.31 The total number of patients included in this study was 100, equally distributed between the two groups. Group A was formed by patients with carcinoma of the left colon and obstructive symptoms who had a colonic stent implanted before surgery. Patients in group B had left-sided colon malignancy but no obstruction and underwent elective surgical intervention. In this study, no significant differences were observed between these groups in terms of morbidity (50.5% vs. 45%), mortality (3.5% vs. 2.8%), recurrence (3.5% vs. 8.4%), and survival at 3 years (76% vs. 66%).31
Martinez and colleagues,32 in a study of 72 patients, compared the use of self-expandable stents before elective surgery (study group) and conventional emergency surgery (control group) for the treatment of malignant left-sided colorectal obstruction. They concluded that placement of colonic stents prevented 94% of unnecessary surgical interventions and 84.6% of colostomies. Total hospitalization stay, intensive care unit stay, and complications were significantly lower in the study group than in the control group.
COMPLICATIONS
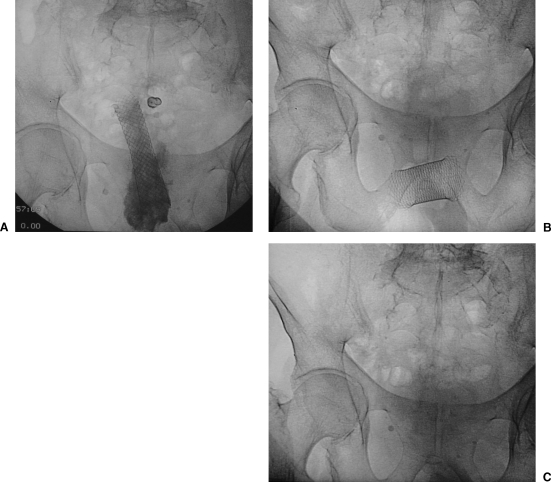
Minor complications related to colon stent placement such as mild to moderate rectal bleeding, transient anorectal pain, temporary incontinence, and fecal impaction are common in many reports.33 More severe life-threatening complications are also described, including procedure-related deaths. In a review by Khot and associates, 3 of 598 patients (1%) died from colon perforation and unsuccessful decompression.30 Perforation occurred in 22 of 598 patients (3.6%). There was a higher incidence of perforation in the prestent dilatation group (10%). Stent migration occurred in 54 of 551 patients (9.8%). See Figure 13. Stent migration usually occurred after an average of 3 days. Obstruction after successful initial stent decompression occurred in 52 of 525 patients (9.9%) (Fig. 14). This phenomenon was related to tumor ingrowth in 32 patients, stent migration in 7 patients, and fecal impaction in 13 patients. Mild to moderate low gastrointestinal bleeding occurred in 24 patients and major bleeding in 3 patients (4.5% total). Minor abdominal or rectal pain was described in 31 of 598 patients (5.1%). Another complication described with specific stent designs included stent fracture followed by obstruction and colonic perforation34 (Fig. 15).
Figure 13.
Stent migration. (B) Wallstent in posteroanterior (PA) view within the rectosigmoid lesion. Remains of barium can be seen. (B) Stent migration and change of position. (C) Radiographic control after stent expulsion.
Figure 14.
Surgical specimen from colectomy performed 24 days after stent implantation. Tumoral growth through the stent with narrowing of the lumen is noted. The patient remained asymptomatic.
Figure 15.
Rupture of a stent. A 76-year-old patient diagnosed with and treated for colon carcinoma 7 years previously. Placement of stent to treat a left colon lesion. Stent was implanted without complications (Memotherm 25 × 80 mm). Thirty months after procedure patient presented with new intestinal obstruction. Abdominal exam showed rupture of the stent. A new stent was implanted without incident (Wallstent 24 × 70 mm). (Images provided by Dr. J. Urbano.)
ECONOMIC IMPLICATIONS
Osman and coworkers estimated that the cost of palliative treatment with colon stenting was less than half that of surgical decompression, £1445 versus £3205, respectively.35 Those researchers also estimated a reduced cost of 12% in the preoperative stenting group versus those who have a two-stage surgical procedure, £5035 versus £5720, respectively. Binkert and associates performed a retrospective study comparing the total cost in two groups of patients: one group treated with preoperative stents (12 patients) versus the second group treated by surgery alone (11 patients).28 They reported savings of up to 20% for the first group. The decrease in cost was attributed mainly to the shorter hospital stay.
RADIATION DOSE
The radiation exposure to the patient and to the operator when the procedure is performed with fluoroscopic guidance is a very important issue. Our group measured the duration of the procedure and the fluoroscopy time and estimated the total radiation dose (dGy/cm2) in 12 patients.36 The average procedure time was 57.3 mins and fluoroscopy time was 20.9 mins. Mean total dose was 3593 dGy/cm2. Transanal placement of metallic stents performed in a combined fashion with endoscopic assistance may decrease fluoroscopy time.37
LIMITATIONS
Stents should not be inserted across distal rectal tumors because they can cause severe tenesmus or fecal incontinence.38 Although there are studies with large series of patients, there are no randomized prospective studies that compare preoperative stents with standard surgical treatment in patients with potentially resectable primary colorectal carcinoma and obstruction.39
OUR CLINICAL EXPERIENCE
Our experience16 includes 136 consecutive patients with large intestinal obstruction from colon carcinoma treated from January 1994 to September 1998. Barium enema located the lesions in these areas: the sigmoid colon (76 patients); descending colon (33 patients); rectosigmoid junction (11 patients); and transverse colon (6 patients). The stent most commonly used was the Wallstent (Boston Scientific; La Garenne Colombes Cedex, France) measuring 22 mm in diameter and with lengths ranging from 66 to 100 mm. The criteria for successful stent placement were relief of colonic obstruction allowing preoperative bowel preparation and successful palliation for those patients not eligible for surgery.
Colonic obstruction resolved in 115 patients (91%). See Figure 16. Bowel movements occurred within the first 24 hours in 98 patients (85%) and after 24 hours in 17 patients (15%). Bowel preparation was followed by colonic resection and primary anastomosis in 81 patients (70%). Palliative decompression was achieved in 34 patients (30%) who had a mean survival of 6.8 months (range = 1–12 months) after stent insertion. Colostomy was performed in 11 patients (9%) in whom colonic obstruction persisted because of inability to place the stent (9 patients) and in two patients despite appropriate stent placement. Complications occurred in 15 patients (12%): self-limited rectal bleeding (10 patients); stent migration (3 patients); and symptomatic colonic perforation (2 patients). In our experience, transanal metallic stent placement is an effective method for relief of malignant colonic obstruction. The procedure allows preoperative bowel preparation and a single-stage operation. Palliation of intestinal obstruction improves the quality of life of patients in whom resection is not indicated.
Figure 16.
Intestinal obstruction with lesion in the splenic angle. (A) Apple-core lesion seen with guide wire crossing it. (B) Image after deployment.
CONCLUSIONS
There is agreement that management of the obstructed colon by means of stent placement is a safe and effective technique before definitive surgical treatment of the tumor. Colonic stent placement is also useful as a palliative treatment for the patient with obstructive carcinoma of the left colon who is not a surgical candidate.40 Nevertheless, there is a need for prospective randomized studies to confirm the results in published series. It is also necessary to continue designing new devices with better characteristics that will improve results and decrease the complexity of the procedure.
REFERENCES
- Boyle P, Leon M. Epidemiology of colorectal cancer. Br Med Bull. 2002;64:1–25. doi: 10.1093/bmb/64.1.1. [DOI] [PubMed] [Google Scholar]
- Deans G T, Krukowski Z H, Irwin S T. Malignant obstruction of the left colon. Br J Surg. 1994;81:1270–1276. doi: 10.1002/bjs.1800810905. [DOI] [PubMed] [Google Scholar]
- Duxbury M, Brodribb A, Oppong F, Hosie K. Management of colorectal cancer: variations in practice in one hospital. Eur J Surg Oncol. 2003;29:400–402. doi: 10.1053/ejso.2002.1426. [DOI] [PubMed] [Google Scholar]
- Griffith R. Prospective medical obstacles to surgery. Cancer. 1992;70:1333–1341. doi: 10.1002/1097-0142(19920901)70:3+<1333::aid-cncr2820701521>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Smothers L, Hynan L, Fleming J, Turnage R, Simmang C, Anthony T. Emergency surgery for colon carcinoma. Dis Colon Rectum. 2003;46:24–30. doi: 10.1007/s10350-004-6492-6. [DOI] [PubMed] [Google Scholar]
- Ohman U. Prognosis in patients with obstructing colorectal carcinoma. Am J Surg. 1982;143:742–747. doi: 10.1016/0002-9610(82)90050-2. [DOI] [PubMed] [Google Scholar]
- Witzig J A, Morel P, Erne M, Egeli R, Borst F, Rohner A. Chirugie des cancers digestifs des patients de plus de 80 ans. Helv Chir Acta. 1993;59:767–769. [PubMed] [Google Scholar]
- Barillari P, Aurello P, de Angelis R, et al. Management and survival of patients affected with obstructive colorectal cancer. Int Surg. 1992;77:251–255. [PubMed] [Google Scholar]
- Neugent K, Daniels P, Stewart B, Patankar R, Johnson C D. Quality of life in stoma patients. Dis Colon Rectum. 1999;42:1569–1574. doi: 10.1007/BF02236209. [DOI] [PubMed] [Google Scholar]
- Dohmoto M, Rupp K D, Hohlbach G. Endoscopically-implanted prosthesis in rectal carcinoma. Dtsch Med Wochenschr. 1990;115:915. [PubMed] [Google Scholar]
- Cwikiel W, Andren-Sandberg A. Malignant stricture with colovesical fistula: stent insertion in the colon. Radiology. 1993;186:563–564. doi: 10.1148/radiology.186.2.8421765. [DOI] [PubMed] [Google Scholar]
- Keen R, Orsay C P. Rectosigmoid stent for obstructing colonic neoplasms. Dis Colon Rectum. 1992;35:912–913. doi: 10.1007/BF02047883. [DOI] [PubMed] [Google Scholar]
- Tejero E, Mainar A, Fernandez L, Tobio R, de Gregorio M A. New procedure for the treatment of colorectal neoplastic obstructions. Dis Colon Rectum. 1994;37:1158–1159. doi: 10.1007/BF02049822. [DOI] [PubMed] [Google Scholar]
- Zollikofer C, Jost R, Schonh E, Decurtins M. Gastrointestinal stenting. Eur Radiol. 2000;10:1158–1159. doi: 10.1007/s003300050053. [DOI] [PubMed] [Google Scholar]
- Mauro M, Koehler R, Baron T. Advances in gastrointestinal intervention: the treatment of gastroduodenal and colorectal obstructions with metallic stents. Radiology. 2000;215:659–669. doi: 10.1148/radiology.215.3.r00jn30659. [DOI] [PubMed] [Google Scholar]
- de Gregorio M A, Mainar A, D'Agostino H, Herrera M, Tejero E, Medrano J. Trasanal metallic stents for malignant colonic obstructions in 126 patients. J Vasc Interv Radiol. 1999;10(suppl):219. [Google Scholar]
- Mainar A, de Gregorio M A, Tejero E, et al. Acute colorectal obstruction: treatment with self-expandable metallic stents before scheduled surgery—results of a multicenter study. Radiology. 1999;210:65–69. doi: 10.1148/radiology.210.1.r99ja0665. [DOI] [PubMed] [Google Scholar]
- Mischima R, Sawada S, Tanigawa N, Okuda Y, Kobayashi M, Koyama T. Expandable metallic stent treatment for malignant colorectal strictures. Cardiovasc Interv Radiol. 1999;22:155–158. doi: 10.1007/s002709900355. [DOI] [PubMed] [Google Scholar]
- Akle C A. Endoprostheses for colonic strictures. Br J Surg. 1998;85:310–314. doi: 10.1046/j.1365-2168.1998.00713.x. [DOI] [PubMed] [Google Scholar]
- Song H Y. Malignant gastric outlet obstruction: treatment by means of coaxial placement of uncovered and covered expandable Nitinol stents. J Vasc Interv Radiol. 2002;13:275–283. doi: 10.1016/s1051-0443(07)61720-5. [DOI] [PubMed] [Google Scholar]
- Canon C L, Baron T H, Morgan D E, Dean P A, Koeler R E. Treatment of colonic obstruction with expandable metal stents: radiologic features. AJR Am J Roentgenol. 1997;168:199–205. doi: 10.2214/ajr.168.1.8976946. [DOI] [PubMed] [Google Scholar]
- Lopera J E, Ferral H, Wholey M, Maynar M, Castaneda-Zuniga W R. Treatment of colonic obstructions with metallic stents: indications, technique and complications. AJR Am J Roentgenol. 1997;169:1285–1290. doi: 10.2214/ajr.169.5.9353443. [DOI] [PubMed] [Google Scholar]
- Adamsen S, Holm J, Meisner S, Moller P, Naver L P, Wille-Jorgensen P A. Endoscopic treatment of colorectal obstruction with self-expandable metal endoprosthesis. Ugeskr Laeger. 2000;162:1560–1563. [PubMed] [Google Scholar]
- Choo I, Do Y, Suh S, et al. Malignant colorectal obstruction: treatment with a flexible covered stent. Radiology. 1998;206:415–421. doi: 10.1148/radiology.206.2.9457194. [DOI] [PubMed] [Google Scholar]
- de Gregorio M A, Mainar A, Tejero E, Alfonso E R, Gimeno M J, Herrera M. Use of an introducer sheath for colonic stent placement. Eur Radiol. 2002;12:2250–2252. doi: 10.1007/s00330-001-1290-1. [DOI] [PubMed] [Google Scholar]
- Velling T E, Hall L D, Brennan F J. Colonic stent placement facilitated by percutaneous cecostomy and antegrade enema. AJR Am J Roentgenol. 2000;175:119–120. doi: 10.2214/ajr.175.1.1750119. [DOI] [PubMed] [Google Scholar]
- Gimeno M J, Alfonso E R, Herrera M, Tobio R, Medrano J, de Gregorio M A. Palliative treatment of malignant stenoses of transverse colon by autoexpandable metallic stent through percutaneous cecostomy or colostomy. Cardiovasc Intervent Radiol. 2002;25(suppl):208. [Google Scholar]
- Binkert C A, Ledermann H, Jost R, Saurenmann P, Decurtins M, Zollikofer C. Acute colonic obstruction: clinical aspects and cost-effectiveness of preoperative and palliative treatment with self-expanding metallic stents—a preliminary report. Radiology. 1998;206:199–204. doi: 10.1148/radiology.206.1.9423673. [DOI] [PubMed] [Google Scholar]
- de Gregorio M A, D'Agostino H, Gimeno M J, et al. Trasanal colonic stent implantation under fluoroscopy alone and under fluoroscopy and endoscopy. A comparative study. Interventionismo. 2003;5:103–111. [Google Scholar]
- Khot U, Lang A, Murali K, Parker M. Systematic review of the efficacy and safety of colorectal stents. Br J Surg. 2002;89:1096–1102. doi: 10.1046/j.1365-2168.2002.02148.x. [DOI] [PubMed] [Google Scholar]
- Beltran J M. Left obstructive colonic carcinoma. Comparative study of short and middle-term results after a new therapeutic procedure based in self-expanding metallic stents placement [doctoral thesis] Zaragoza, Spain: Universidad de Zaragoza; 2003.
- Martinez C, Lobato R, Fradejas J M, Pinto I, Ortega P, Moreno M. Self-expandable stent before elective surgery vs emergency surgery for the treatment of malignant colorectal obstructions: comparison of primary anastomosis and morbidity rates. Dis Colon Rectum. 2002;45:401–406. doi: 10.1007/s10350-004-6190-4. [DOI] [PubMed] [Google Scholar]
- Harris G, Senagore A, Lavery I, Fazio V. The management of neoplastic colorectal obstruction with colonic endolumenal stenting devices. Am J Surg. 2001;181:499–506. doi: 10.1016/s0002-9610(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Odurny A. Colonic anastomotic stenoses and Memotherm stent fracture: a report of three cases. Cardiovasc Intervent Radiol. 2001;24:336–339. doi: 10.1007/s00270-001-0033-7. [DOI] [PubMed] [Google Scholar]
- Osman H, Rashid H, Sathananthan N, Parker M. The cost-effectiveness of self- expanding metal stents in the management of malignant left-sided bowel obstruction. Colorectal Dis. 2000;2:233–237. doi: 10.1046/j.1463-1318.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- de Gregorio M A, Mainar A, Gimeno M J, et al. Radiation exposure during metallic stent placement for the treatment of malignant colonic obstruction: experience with 12 patients. Cardiovasc Intervent Radiol. 2001;24:S189. [Google Scholar]
- Gimeno M J, Medrano J, Alfonso E R, de Gregorio M A. Utility of fluoroscopy combined with endoscopy in the treatment of malignant colorectal stenosis with metallic stents. Cardiovasc Intervent Radiol. 2002;25:S203. [Google Scholar]
- Turegano F, Echenagusia A, Simo A, et al. Transanal self-expanding metal stents as an alternative to palliative colostomy in selected patients with malignant obstruction of the left colon. Br J Surg. 1998;85:232–235. doi: 10.1046/j.1365-2168.1998.00565.x. [DOI] [PubMed] [Google Scholar]
- Baron T H. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med. 2001;344:1681–1687. doi: 10.1056/NEJM200105313442206. [DOI] [PubMed] [Google Scholar]
- Maynar M, Qian Z. Current status of the expandable metallic stent for the treatment of colorectal obstruction. Cardiovasc Intervent Radiol. 2001;21(suppl):114–116. [Google Scholar]