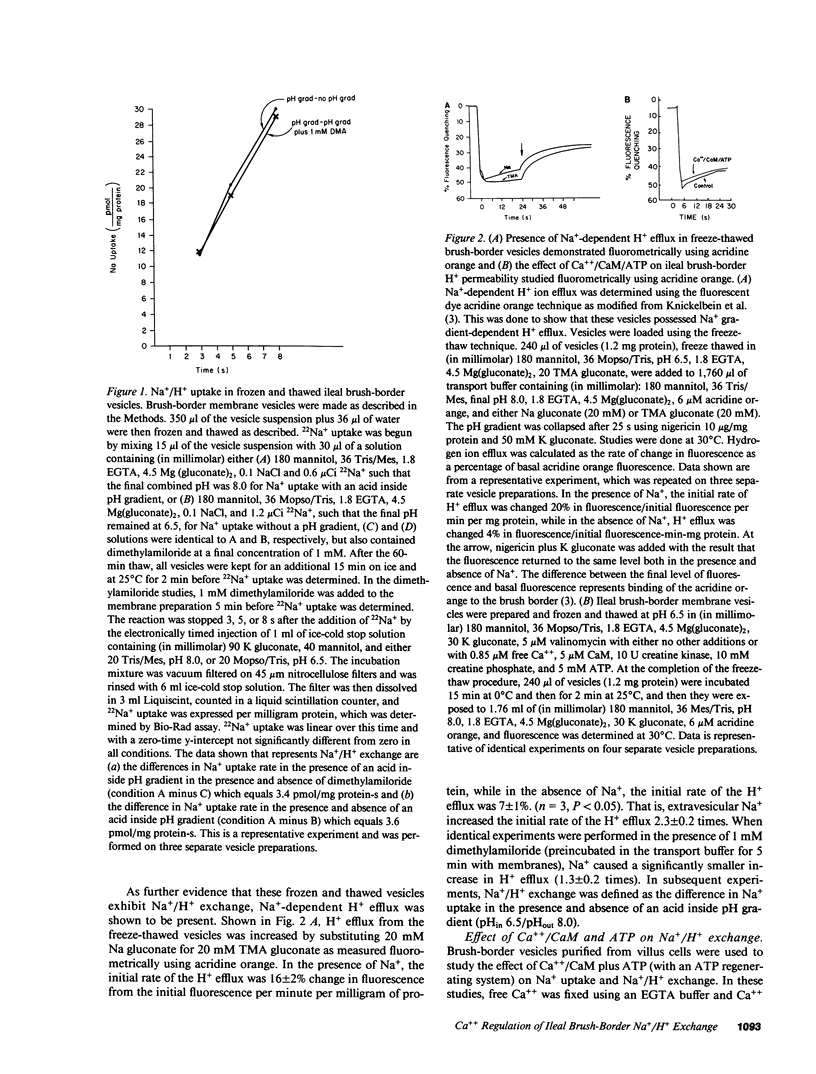
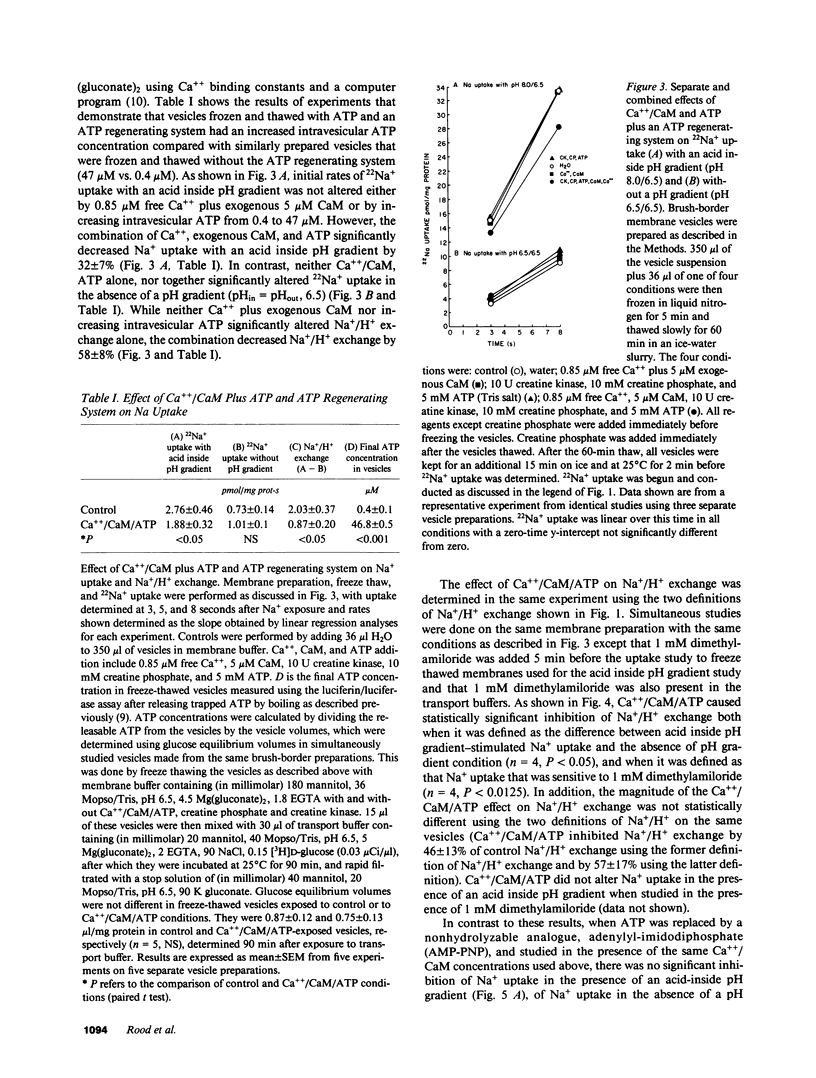
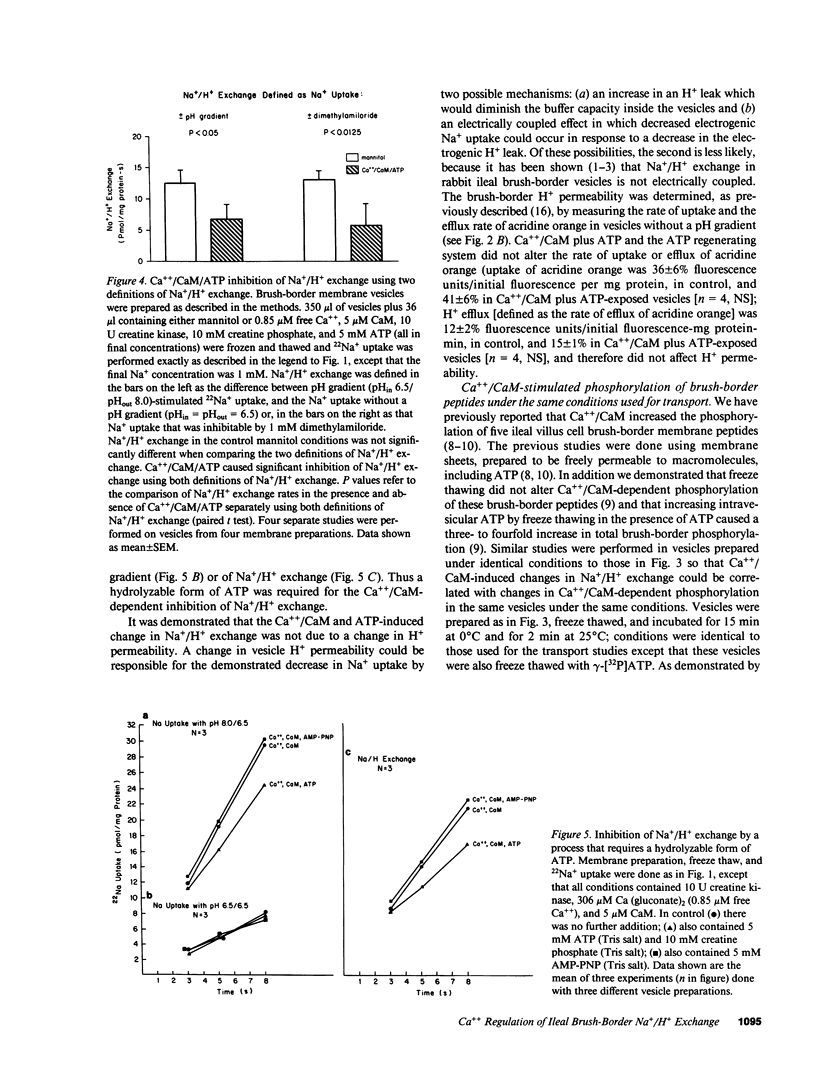
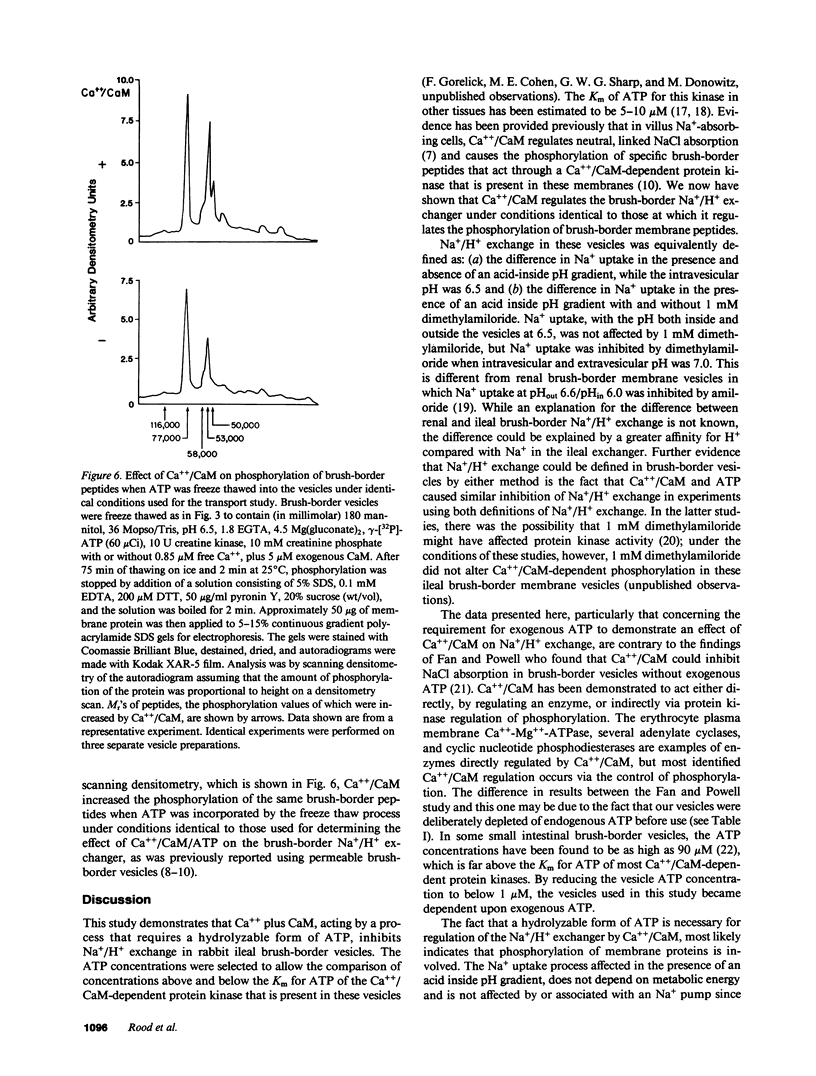
Abstract
Brush-border vesicles purified from rabbit ileal villus cells were used to evaluate how Ca++/calmodulin (CaM) regulates the neutral linked NaCl absorptive process, part of which is a Na+/H+ exchanger. After freezing and thawing to allow incorporation of macromolecules into the vesicles, the effect of Ca++/CaM on brush-border Na+ uptake with an acid inside pH gradient, and on Na+/H+ exchange was determined. Freezing and thawing vesicles with 0.85 microM free Ca++ plus 5 microM exogenous CaM failed to alter Na+/H+ exchange as did the addition of exogenous ATP plus an ATP regenerating system, which was sufficient to elevate intravesicular ATP to 47 microM from a basal level of 0.4 microM. However, the combination of Ca++/CaM plus ATP inhibited Na+ uptake in the presence of an acid inside pH gradient and inhibited Na+/H+ exchange, while Na+ uptake in the absence of a pH gradient was not altered. This effect required a hydrolyzable form of ATP, and did not occur when the nonhydrolyzable ATP analogue, AMP-PNP, replaced ATP. Under the identical intravesicular conditions used for the transport studies, Ca++ (0.85 microM) plus exogenous CaM (5 microM), in the presence of magnesium plus ATP, increased phosphorylation of five brush-border peptides. These data are consistent with Ca++/CaM acting via phosphorylation to regulate the ileal brush-border Na+/H+ exchanger.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S., Suhm M. A., Nee J. Interaction of external H+ with the Na+-H+ exchanger in renal microvillus membrane vesicles. J Biol Chem. 1983 Jun 10;258(11):6767–6771. [PubMed] [Google Scholar]
- BEUTLER E., BALUDA M. C. SIMPLIFIED DETERMINATION OF BLOOD ADENOSINE TRIPHOSPHATE USING THE FIREFLY SYSTEM. Blood. 1964 May;23:688–698. [PubMed] [Google Scholar]
- Besterman J. M., May W. S., Jr, LeVine H., 3rd, Cragoe E. J., Jr, Cuatrecasas P. Amiloride inhibits phorbol ester-stimulated Na+/H+ exchange and protein kinase C. An amiloride analog selectively inhibits Na+/H+ exchange. J Biol Chem. 1985 Jan 25;260(2):1155–1159. [PubMed] [Google Scholar]
- Cassano G., Stieger B., Murer H. Na/H- and Cl/OH-exchange in rat jejunal and rat proximal tubular brush border membrane vesicles. Studies with acridine orange. Pflugers Arch. 1984 Mar;400(3):309–317. doi: 10.1007/BF00581565. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Cohen M. E., Gudewich R., Taylor L., Sharp G. W. Ca2+-calmodulin-, cyclic AMP- and cyclic GMP-induced phosphorylation of proteins in purified microvillus membranes of rabbit ileum. Biochem J. 1984 Apr 15;219(2):573–581. doi: 10.1042/bj2190573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Emmer E., McCullen J., Reinlib L., Cohen M. E., Rood R. P., Madara J., Sharp G. W., Murer H., Malmstrom K. Freeze-thaw and high-voltage discharge allow macromolecule uptake into ileal brush-border vesicles. Am J Physiol. 1987 Jun;252(6 Pt 1):G723–G735. doi: 10.1152/ajpgi.1987.252.6.G723. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Welsh M. J. Ca2+ and cyclic AMP in regulation of intestinal Na, K, and Cl transport. Annu Rev Physiol. 1986;48:135–150. doi: 10.1146/annurev.ph.48.030186.001031. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Wicks J., Madara J. L., Sharp G. W. Studies on role of calmodulin in Ca2+ regulation of rabbit ileal Na and Cl transport. Am J Physiol. 1985 Jun;248(6 Pt 1):G726–G740. doi: 10.1152/ajpgi.1985.248.6.G726. [DOI] [PubMed] [Google Scholar]
- Fan C. C., Powell D. W. Calcium/calmodulin inhibition of coupled NaCl transport in membrane vesicles from rabbit ileal brush border. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5248–5252. doi: 10.1073/pnas.80.17.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Knickelbein R., Aronson P. S., Atherton W., Dobbins J. W. Sodium and chloride transport across rabbit ileal brush border. I. Evidence for Na-H exchange. Am J Physiol. 1983 Oct;245(4):G504–G510. doi: 10.1152/ajpgi.1983.245.4.G504. [DOI] [PubMed] [Google Scholar]
- Knickelbein R., Aronson P. S., Schron C. M., Seifter J., Dobbins J. W. Sodium and chloride transport across rabbit ileal brush border. II. Evidence for Cl-HCO3 exchange and mechanism of coupling. Am J Physiol. 1985 Aug;249(2 Pt 1):G236–G245. doi: 10.1152/ajpgi.1985.249.2.G236. [DOI] [PubMed] [Google Scholar]
- Lai Y., Nairn A. C., Greengard P. Autophosphorylation reversibly regulates the Ca2+/calmodulin-dependence of Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4253–4257. doi: 10.1073/pnas.83.12.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Liedtke C. M., Hopfer U. Mechanism of Cl- translocation across small intestinal brush-border membrane. I. Absence of Na+-Cl- cotransport. Am J Physiol. 1982 Mar;242(3):G263–G271. doi: 10.1152/ajpgi.1982.242.3.G263. [DOI] [PubMed] [Google Scholar]
- Liedtke C. M., Hopfer U. Mechanism of Cl- translocation across small intestinal brush-border membrane. II. Demonstration of Cl--OH- exchange and Cl- conductance. Am J Physiol. 1982 Mar;242(3):G272–G280. doi: 10.1152/ajpgi.1982.242.3.G272. [DOI] [PubMed] [Google Scholar]
- Lou L. L., Lloyd S. J., Schulman H. Activation of the multifunctional Ca2+/calmodulin-dependent protein kinase by autophosphorylation: ATP modulates production of an autonomous enzyme. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9497–9501. doi: 10.1073/pnas.83.24.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco G., Iglesias C. F., Domínguez P., Barros F., Gascón S., Lazo P. S. Protein kinase C from small intestine epithelial cells. Biochem Biophys Res Commun. 1986 Sep 30;139(3):875–882. doi: 10.1016/s0006-291x(86)80259-5. [DOI] [PubMed] [Google Scholar]
- Whitehouse S., Feramisco J. R., Casnellie J. E., Krebs E. G., Walsh D. A. Studies on the kinetic mechanism of the catalytic subunit of the cAMP-dependent protein kinase. J Biol Chem. 1983 Mar 25;258(6):3693–3701. [PubMed] [Google Scholar]
- van Dommelen F. S., Hamer C. M., De Jonge H. R. Efficient entrapment of large and small compounds during vesiculation of intestinal microvilli. Biochem J. 1986 Jun 15;236(3):771–778. doi: 10.1042/bj2360771. [DOI] [PMC free article] [PubMed] [Google Scholar]



