ABSTRACT
Lung transplant recipients are among the patients most likely eventually to undergo diagnostic lung biopsy. Unfortunately, these patients are at particularly high risk for experiencing intra- and periprocedural complications. Percutaneous transthoracic needle biopsy (TNB) has over time emerged as an increasingly safe and reliable method of obtaining lung tissue for diagnosis. This article gives an overview of TNB including its indications, the imaging modalities currently used for guidance, and the special techniques utilized in performing the procedure and minimizing complications with an emphasis placed upon the special case of TNB performed in lung transplant recipients.
Keywords: Transthoracic needle biopsy, lung transplant, interventional radiology
Percutaneous transthoracic needle biopsy (TNB) has emerged over the past three decades as an invasive procedure of choice for the diagnosis of pulmonary nodules. Improvements in imaging technology, needle design, and cytopathologic techniques have rendered TNB safe, inexpensive, reliable, and widely used.1,2,3 Lesions previously inaccessible can now be biopsied using cross-sectional imaging, and tissue samples sufficient for histologic diagnosis can now be safely obtained with the small gauge cutting needles currently available.1
Among patients more likely to need a diagnostic lung biopsy at some point in time are lung transplant recipients, as these patients are at increased risk for developing conditions and complications that necessitate tissue sampling for diagnosis. For instance, because the most common indications for lung transplantation are emphysema and pulmonary fibrosis, both of which are risk factors for lung cancer, lung transplant recipients are at increased risk for developing primary lung malignancies within their native lung.4 In addition, the immunosuppressive regimens required to prevent allograft rejection place lung transplant recipients at increased risk for both infection and neoplasm.5 Unfortunately, TNB is considerably more dangerous when performed in patients with only one functional lung, as is the case in single lung transplant recipients. In fact, prior pneumonectomy and other instances of single lung have been cited as contraindications to TNB.1,2,3 However, with improvements in imaging technology, needle design, biopsy technique, and strategies for preventing and managing potential complications, TNB may now be a viable option for lung transplant recipients, single or double.
INDICATIONS AND CONTRAINDICATIONS
Indications
The most common indication for TNB is an indeterminate solitary pulmonary nodule or mass.3 Although some physicians still perform TNB on all patients with undiagnosed pulmonary lesions even when the likelihood of avoiding thoracotomy is relatively low, an increasing number of operators now perform TNB only when tissue diagnosis is likely to affect patient management.1,2,6 TNB is most likely to be of value in cases where the nodule in question is almost certainly malignant but unsuitable for resection or of an equivocal or likely benign nature. In such cases, the diagnosis provided by TNB may determine appropriate therapy while avoiding thoracotomy. As previously mentioned, lung transplant recipients are at increased risk for developing both primary lung cancer (as a result of concomitant emphysema or pulmonary fibrosis) and secondary lung malignancies (resulting from systemic immunosuppression). Because patients who have undergone single lung transplantation often have insufficient lung reserve to withstand thoracotomy, TNB may play a crucial role in planning their future therapy.
An increasingly common indication for TNB, particularly in the population of immunosuppressed patients, is a focal area of consolidation or suspected lung abscess in which bronchoscopy or sputum cytology and culture has not yielded a diagnosis.7,8 This is another reason for which lung transplant recipients may undergo TNB, as they require life-long immunosuppression to prevent allograft rejection. Other indications for TNB include confirmation of metastatic disease (multiple nodules), diagnosis of a mediastinal mass, diagnosis of focal or diffuse pleural thickening, and staging of lung cancer or extrathoracic malignancy (mediastinal or hilar lymph node biopsy).3 In all cases, the potential benefits of TNB should outweigh the risks, and the biopsy results should be considered likely to affect the patient's management.
The position of the lesion and appearance on computed tomography (CT) determine whether bronchoscopic biopsy or TNB is the more appropriate technique for establishing a diagnosis. In general, central lesions with an endobronchial component are likely to be diagnosed accurately with flexible bronchoscopy, whereas peripheral tumors, especially those smaller than 3 cm in diameter, without a bronchus seen entering the proximal portion of the lesion are best suited for TNB.1,3 More recently, new technologies have rendered TNB an accurate method of diagnosing smaller lesions—even nodules less than 1 cm in diameter.9
Contraindications
Although some authors claim that any case of single lung is an absolute contraindication to TNB,2 others insist that there are no absolute contraindications to the procedure.3 Most authors agree that several preexisting conditions significantly increase the complication rate of TNB or render any complication more significant and therefore constitute relative contraindications to TNB. These conditions include contralateral pneumonectomy, bulla or severe emphysema, relatively prominent airways or vasculature within the anticipated biopsy path, pulmonary hypertension, cardiac insufficiency, inability of the patient to follow instructions (i.e., breath hold), and abnormal clotting function (international normalized ratio > 1.3 or platelets < 50,000).1,3 Above all, TNB should be limited to cases that are truly indicated, technically feasible, and for which the anticipated benefits outweigh the risks. Again, with regard to single lung transplant recipients, although TNB carries an increased risk of potentially serious complications, advances in imaging technology, needle design, and special techniques used in performing TNB and managing complications have rendered TNB a viable option for these patients.
TECHNIQUE
Imaging Modalities
Percutaneous TNB can be performed under fluoroscopy, CT, or ultrasonography; the choice of image guidance modality is dependent upon the size and location of the lesion, the availability of imaging systems, and operator expertise. Localization of the lesion by chest radiography and CT is necessary to determine the method of biopsy. The depth of the lesion and its relations to the major airways and vessels, fissures, mediastinum, and ribs are used to plan the biopsy route. Traditionally, percutaneous TNB was performed under fluoroscopic guidance. This technique has the advantages of real-time visualization, short procedure duration, and low cost. However, because of its limited ability to guide access to central lesions and to avoid bullae and vascular structures in the needle track, fluoroscopy is now reserved for large, peripheral lesions.1,2
In many centers, CT is now the standard imaging modality for TNB. CT enables selection of a biopsy route with the shortest distance between pleura and lesion and allows access to central lesions, while avoiding airways and vessels, fissures, bullae, and pronounced emphysema. In addition, CT aids in distinguishing necrotic from solid portions of the lesions and allows unequivocal documentation of the needle tip within the lesion (paramount in the interpretation of absence of malignant cells).10 Procedure duration, one of the most commonly cited drawbacks of CT guidance, has significantly decreased with the advent of CT fluoroscopy.11 This is of particular importance in less cooperative patients. In addition, CT fluoroscopy offers the advantage of real-time visualization of needle placement. Real-time imaging is of particular importance within the lung, where extensive respiratory movement is present, causing small target lesions to shift and even disappear from the scan plane during needle advancement. Lack of real-time imaging has been cited as one of the most considerable reasons why CT-guided biopsies are unsuccessful or need to be repeated.12 CT fluoroscopy has been shown to facilitate biopsy of smaller lesions as well as lesions situated in less favorable locations such as those in the costophrenic recess or close to the mediastinum. One of the major concerns regarding CT fluoroscopy remains the increased operator-related radiation exposure (although patient-related radiation exposure is essentially unchanged).11
In some instances, TNB can be performed under ultrasound guidance. Major advantages of ultrasound include real-time visualization of needle placement, portability, ability to target nonvascular, non-necrotic portions of a mass for sampling, and lack of exposure to ionizing radiation. Like CT, ultrasound also increases the likelihood of a positive biopsy in cavitating lesions. Ultrasound-guided TNB is limited to regions that provide an adequate acoustic window to the lesion and is best suited for peripheral pulmonary or mediastinal masses that abut the pleura.1,3
Needles
A variety of needle sizes, tip designs, and sampling mechanisms are used in TNB. Ideally, a biopsy needle should maximize specimen yield while minimizing bleeding complications. Bleeding complications are avoided today by using needles smaller than 18 gauge. Both single- and multiple-pass coaxial needles have been used in percutaneous TNB. The coaxial technique, which involves inserting a thin inner needle through a larger outer needle positioned at the edge of or within the lesion, has several advantages over the single-needle technique, including limitation of the number of pleural punctures, easy repositioning of the needle, and the possible use of techniques to prevent leakage of air.13
The biopsy needles currently used in TNB can be divided into two broad categories: aspirating and cutting needles.3 Commonly used aspirating needles include the Chiba, Spinal, Westcott, and Greene needles, most ranging in size from 20 to 22 gauge.2 Aspirating needles, when accurately placed within the lesion of interest, can provide high-quality cellular material for the diagnosis of malignancy.3 When TNB is used to isolate causative organisms in pneumonia or lung abscess, a 22-gauge Chiba or Spinal needle usually provides an adequate specimen.3 Cutting or core needles are generally larger than aspirating needles (usually 18–20 gauge) and are designed to provide histologic material using a circumferential cutting tip (e.g., Greene or Turner needles), a receptacle slot proximal to the tip (e.g., Westcott needle), an automated spring-loaded end cut or side-notched device (e.g., ASAP or Temno needles), or a drill bit stylet contained within a guiding cannula.3 Core biopsy is best suited for large (>3 cm) peripheral lesions that abut the pleura; however, its use in the diagnosis of smaller (<3 cm) lesions has been reported.14 Coaxial models with small diameters have become available, allowing multiple core specimens to be obtained with a single pleural puncture.15 Debate exists in the literature as to whether cutting needles offer any advantage over fine-needle aspiration in the diagnosis of malignancy16,17; however, cutting needles are felt to be more accurate in the specific diagnosis of benign lesions, lymphoma, and in settings where expert cytopathology is unavailable.15,18,19 The two techniques are often used in complementary fashion, with cutting needles proposed when the diagnosis of malignancy is not made with fine-needle aspiration.20 The initial choice between fine-needle aspiration and core needle biopsy depends on several factors, including operator expertise, the risk of complication, and the availability of an on-site cytopathologist. Several institutions recommend the initial use of core biopsy in the absence of an on-site pathologist when the diagnosis of a benign lesion or lymphoma is suspected; however, when a lesion is strongly suspicious for malignancy and an experienced cytopathologist is available, fine-needle aspiration is attempted first.21
Biopsy Process
The goal in performing TNB is to obtain sufficient material for diagnosis while minimizing complications for the patient. With this in mind, special techniques may be utilized in TNB to maximize diagnostic yield and to avoid possible complications. These techniques are of utmost importance when performing TNB in single lung transplant recipients and other high-risk patients as the potential impact of having to repeat a false-negative biopsy or experiencing a complication in these patients is much greater. The following section focuses on several important aspects of the biopsy process and, for each, outlines the various techniques that have been described to be helpful as a means of facilitating them. These include positioning of the patient, advancement and accurate placement of the biopsy needle within the target lesion, and procurement and evaluation of the specimen obtained.
Proper positioning of the patient on the biopsy table is crucial for the success of TNB. In general, the patient should be positioned so that the skin entry site allowing the shortest, most vertical path to the lesion that avoids fissures, bullae, and major airways and vessels is placed upright or nondependent. Some have suggested that TNB is best performed with the patient in prone position whenever possible (i.e., when the difference in distance to the lesion from a supine approach versus a prone approach is not very large).22 Because there is less motion of the posterior aspect of the ribs relative to their anterior aspect, there is less motion of the needle during the patient's breathing when using a posterior approach. In addition, because it is preferable for the patient to lie with the biopsy site dependent after completion of the procedure and it is generally much easier for patients to lie on their backs for extended periods of time as compared with on their stomachs, a prone position may facilitate the patient's comfort after the procedure. Further, with the patient in prone position, there is less need to rely upon patients' breathing techniques.22
As a corollary to positioning of the patient, the approach used to guide the needle must be carefully planned. This often involves maneuvering around obstacles, including the bone structures (most often), thoracic blood vessels, and visceral pleura. Whenever possible, a needle path through abnormal lung and/or pleura should be chosen to minimize potential complications23 (Figs. 1 and 2). In the prone position, the scapula is often the most difficult bone structure around which to navigate. The scapula can be rotated out of the way by the following mechanism first described by Yankelevitz; the patient is positioned so that the arm on the ipsilateral side of the nodule is by his or her side and is then externally rotated. This usually rotates the scapula out of the way; however, if the scapula remains an obstacle, the patient is rotated so that the side with the nodule is in a less dependent position. A pillow may be placed under the side with the nodule, allowing more space for the scapula to move laterally when the arm is externally rotated.22 The ribs may also block direct access to the lesion. One approach used to avoid overlying ribs involves angling the CT gantry24 (Fig. 3). A plane can then be selected that starts above or below the rib and passes through the intercostal space into the lesion without the rib directly in the plane of scanning.
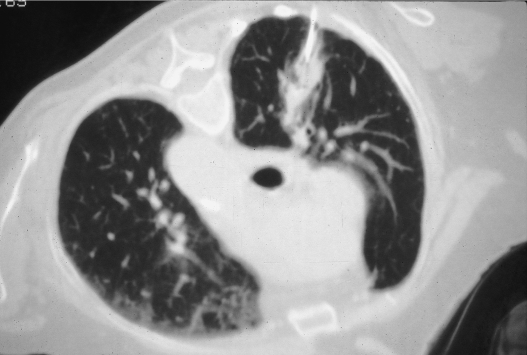
Figure 1.
Approach considerations. When possible, the needle path should traverse abnormal or atelectatic lung. Following right upper lobectomy for non–small cell lung carcinoma, a new right upper lung mass is evident, the chosen needle path exploiting the presence of the associated parenchymal bands extending to the posterolateral pleural surface.
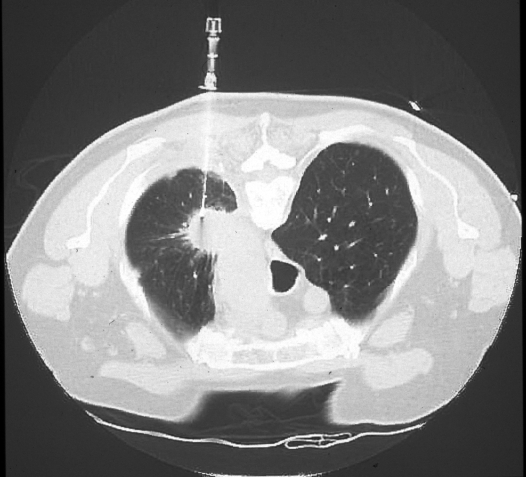
Figure 2.
Approach considerations. Crossing abnormal or thickened pleura can minimize the rate of pneumothorax. Although both anterior and posterior needle approaches are feasible to reach the spiculated left upper lobe mass, a posterior access was chosen, to pass via the thickened pleural rind.
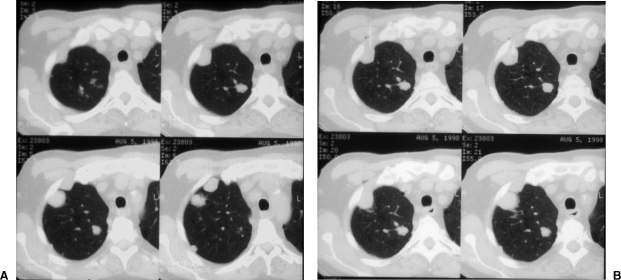
Figure 3.
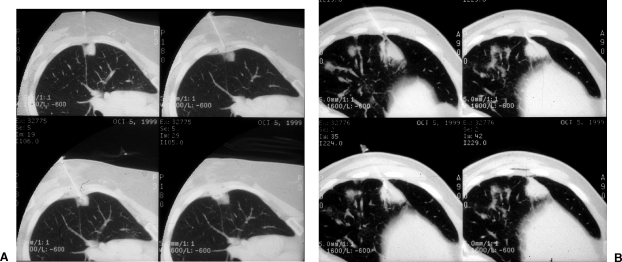
Scanning techniques. Cranial or caudal gantry tilt can expose a path for needle entry not readily apparent on diagnostic imaging. Comparative levels between images without and with gantry tilt demonstrate the relative differences in the appearances of the target lesion and intended needle path. (A) Images without gantry tilt. (B) Images with gantry tilt demonstrate a larger intercostal window into the target lesion and eventual needle placement.
Once the plane has been selected, the needle is inserted parallel to this new plane, allowing direct visualization of the needle as it avoids the rib, crosses the intercostal space and pleura, and enters the lesion. As an alternative method, the needle may be angled using basic geometric principles. In this direct approach, the needle is placed above or below the obstructing rib and inferiorly or superiorly angled, respectively, toward the nodule. Using several contiguous axial images, the craniocaudal needle angle can be estimated and the needle appropriately advanced. Using these techniques, TNB can be performed with relative ease, even for small nodules close to an obstructing rib.25 On rare occasion, TNB has been performed via a transsternal approach; however, this approach is usually unnecessary. In addition to the bone structures, it is sometimes necessary to maneuver around the thoracic blood vessels. Positioning the patient's arm either up or down can alter vascular anatomy, allowing vessels to be avoided. For instance, raising the patient's arms moves the axillary artery and vein superiorly, facilitating biopsy of a left upper lobe lesion.26
Various techniques can be employed to avoid transgression of the visceral pleura. For example, anterior mediastinal masses can be biopsied by a parasternal approach, taking care to avoid the internal mammary artery and vein (Fig. 4). In addition, a saline and anesthetic solution can be injected into the paravertebral soft tissues, thus elevating the pleura off the spine and providing a route through which posterior mediastinal masses can be biopsied without traversing the pleura (Fig. 5). Extrapleural infusion of normal saline has also been described as a technique for improving the safety of large-bore biopsies of subpleural pulmonary lesions27 (Fig. 6). Other techniques for avoiding pleural transgression include a pleural space approach through a preexisting or iatrogenically induced pleural effusion (Fig. 7), induced controlled pneumothorax, and lateral decubitus positioning.26
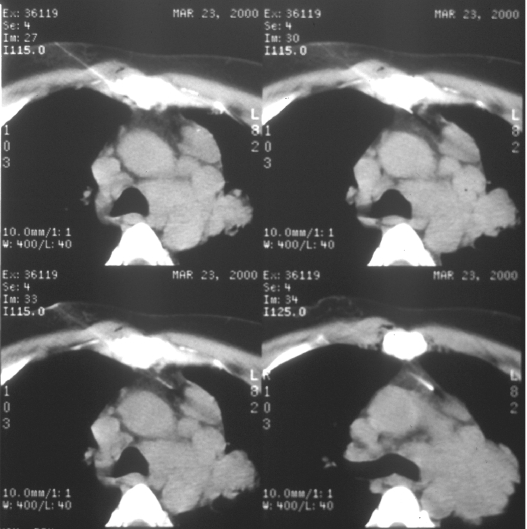
Figure 4.
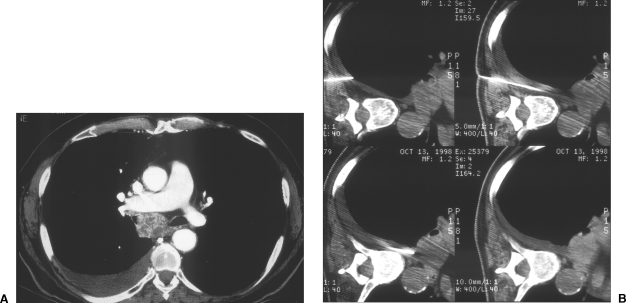
Approach considerations. Anterior mediastinal masses can be biopsied via a parasternal approach, using the anterior mediastinal fat as a window for needle placement.
Figure 5.
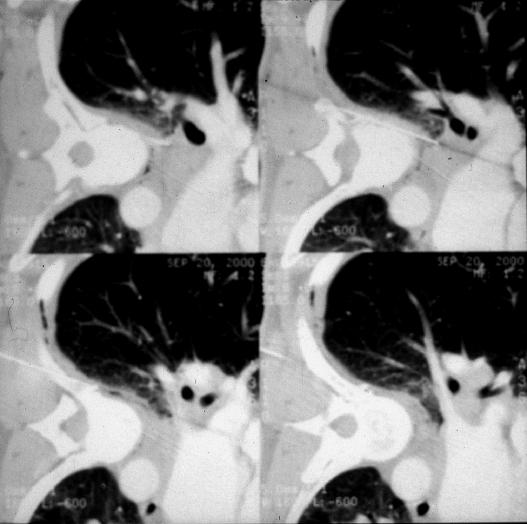
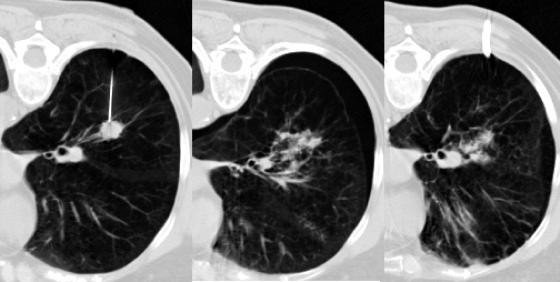
Artificial window creation. Normal saline and/or local anesthetic can be injected or infused into the extrapleural space to create an artificial and larger window for needle access. Series of intraprocedural images demonstrate paravertebral access to an enlarged subcarinal lymph node by saline injection into the paravertebral extrapleural space.
Figure 6.
(A,B) Artificial window creation. To help minimize traversal of normal aerated lung, liberal local anesthesia with or without added normal saline can be administered into the extrapleural space to displace insinuating lung.
Figure 7.
(A,B) Approach considerations. Preexistent or iatrogenic pleural effusions can create passage for needle placement to reach mediastinal lesions. Access to this subcarinal mass was achieved via right pleural effusion, some of which was artificially created with normal saline.
Once the patient is properly positioned and an appropriate route has been selected, needle advancement must be planned. Because even slight misalignment can cause the needle tip to miss its target, careful planning is crucial. To compensate for potential needle misalignment, the use of bevel steering has been described.28,29 Bevel steering relies on the principle that the needle deviates in the direction opposite the bevel. If the needle is found to be off course, it is partially withdrawn, rotated so that the bevel faces in a direction opposite to the intended direction change, and then readvanced. For fluoroscopic or ultrasound-guided biopsies, the position of the needle can be checked and appropriately readjusted during advancement. Although a vertical approach to the lesion is usually preferred, an alternative approach is sometimes necessary. For instance, subpleural lesions situated along the anterolateral or posterolateral lung are best approached obliquely through the anterior or posterior intercostal space3 (Fig. 8). This continuously situates the needle tip at the intended biopsy site, prevents respiratory misregistration (the needle tip and lesion move together), and ensures that the needle remains in the lung in case of a pneumothorax.
Figure 8.
Approach considerations. For superficial or subpleural lung target lesions, an oblique or tangential approach should continuously situate the needle tip near the lesion.
When the needle appears to have reached the target lesion, accurate placement of the needle tip should be confirmed. Due to partial volume averaging on CT, the needle tip may appear to be within the lesion when it is actually directly above or below. Misplacement of the needle tip can be avoided with the use of contiguous thin slices. These images should be obtained in at least a group of three, using a slice thickness less than one half the diameter of the nodule. In this manner, the portion of the nodule not containing the needle tip can be seen above and below the slice that shows the needle tip within the lesion.
Following needle tip confirmation, a reasonable specimen must be obtained. This can involve application of suction to the needle and withdrawal of the sample, as with aspiration techniques, or mechanical retrieval of the sample, as with automated devices. When TNB is performed on small lesions, even slight displacement of the needle can cause the tip to lie outside its intended position. Therefore, it has been recommended that needles with gridlines on their shafts or ones with attachable depth markers be used to control the depth of needle advancement. With aspiration techniques, the syringe should be large enough to achieve maximal suction while being easy to handle (usually either a 10- or 20-mL syringe). One study showed that the majority of carcinomas contain a reactive zone of variable thickness, representing ∼10% of the total tumor diameter,30 reinforcing the idea that multiple samples should be taken from several parts of a lesion. The wall of a necrotic or cavitary lesion should be carefully sampled. Because malignant cells tend to desquamate (especially in squamous cell carcinoma), aspiration of necrosis is also suggested.1 When the specimen has been obtained, the pathologist must advise the radiologist as to whether an adequate specimen has been attained. The presence of an on-site expert cytopathologist is extremely valuable as it enables rapid assessment of the sample so that repeated aspirations or cutting needle biopsy can be performed immediately if necessary.
COMPLICATIONS AND THEIR MANAGEMENT
The most common complications of percutaneous lung biopsy are pneumothorax and bleeding.1 Although single lung transplant recipients who undergo TNB of their donor lung are not at increased risk for developing complications per se, even a small pneumothorax or mild bleeding may cause significant respiratory compromise in these patients. Thus, special techniques for preventing and managing complications as they occur are of utmost importance in single lung transplant recipients.
Pneumothorax
Pneumothorax is the most common complication of TNB. The reported incidence of pneumothorax ranges from 0 to 61%, 20% in most recent large series, with 0.4 to 17% (average 7%) of patients requiring chest tube drainage.3 Factors reported to be associated with a higher incidence of pneumothorax and chest tube insertion include obstructive airways disease, increased lesion depth, decreased lesion size, a small angle of the needle with the thoracic pleura, multiple pleural punctures, fissure transgression, small and deeply situated lesions, longer procedure duration, and operator inexperience.23,31,32,33
Each of the factors implicated in producing pneumothorax should be minimized to the extent possible. Although there is generally not much that can be done about the status of the underlying lung parenchyma, it may be possible to choose a route for needle placement that minimizes the amount of diseased lung transgressed. When possible, large bullae should be avoided. The number of pleural punctures and the degree of needle manipulation within the lung should be minimized, and fissures should be avoided whenever possible, as they result in transgression of four pleural surfaces rather than two (Fig. 9). The time needed to perform the procedure must also be minimized. CT fluoroscopy, by shortening the length of the procedure and limiting the number of pleural punctures necessary to ensure accurate needle placement, may ultimately prove to decrease the incidence of pneumothorax and subsequent chest tube placement, although this has yet to be proved.
Figure 9.
Approach considerations. Avoid crossing fissures. The rate of pneumothorax is directly related to the number of traversed pleural surfaces. Single image shows a pneumothorax as the needle crosses the superior aspect of the major fissure.
Aspiration of air through the indwelling biopsy needle or an angiocatheter has been reported to decrease the need for chest tube placement34,35 (Figs. 10 and 11). Another technique that has been reported variously as both useful and ineffective in reducing chest tube placement following TNB is the use of a blood patch.36,37 With this technique, a small amount of the patient's clotted blood is injected into the needle tract, as the needle is withdrawn.36 The blood serves to seal the tract of the biopsy needle, thereby, in theory, reducing the likelihood of air leakage. Lang et al reported a significantly decreased incidence of chest placement with the use of a blood clot seal in 100 randomly assigned patients undergoing TNB.36 Alternatively, other investigators have reported a decreased incidence of pneumothorax with insertion of a collagen plug following needle withdrawal.38 Positioning of the patient with the biopsy site dependent for at least 2 hours following TNB has been shown to reduce postbiopsy pneumothorax.3 In addition, supplemental nasal oxygen may be used to promote resorption of pleural air.34
Figure 10.
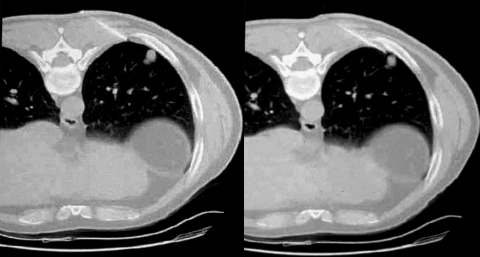
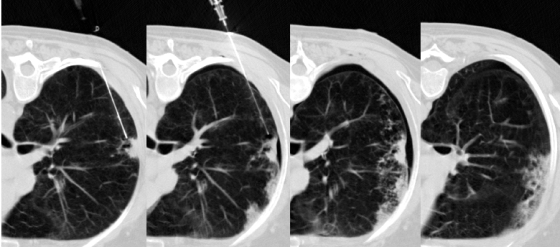
Pneumothorax management. Manual aspiration of pneumothoraces can be performed at the time of biopsy. With the evacuation of abnormally collected intrapleural air, normal coupling pressure between the visceral and parietal pleura is restored, usually enough to overcome an air leak through the pleural puncture. Intraprocedural images demonstrate placement of the biopsy needle at the intended site in a single allograft, development of a postbiopsy pneumothorax, and subsequent resolution of the pneumothorax following manual evacuation.
Figure 11.
Pneumothorax management. Intraprocedural pneumothoraces can be evacuated with a small-caliber catheter to preclude premature termination of intended transthoracic needle biopsy or immediately following the procedure with prolonged air leaks. Three intraprocedural images demonstrate development of a moderate pneumothorax following needle removal from a single allograft and placement of small-bore catheter to relieve the pneumothorax.
Most pneumothoraces are detected within the first hour after the procedure, do not require drainage, and can be managed conservatively with supplemental nasal oxygen.3 However, radiologists performing lung biopsy should have a low threshold for chest tube insertion in single lung transplant recipients and other patients with compromised lung function, as even a small pneumothorax may cause significant ventilatory compromise. In addition, any patient with a large (>20–30%) or symptomatic pneumothorax requires chest tube placement.
Hemoptysis and Pulmonary Hemorrhage
Hemorrhage, with or without hemoptysis, is the second most common complication of TNB. Although most bleeding is self-limited, hemorrhage is considered by most to be the most dangerous potential complication of percutaneous lung biopsy.1 Major intrathoracic hemorrhage causing cardiac tamponade and death has been reported.39,40
The risk of hemorrhage is reportedly increased with the use of cutting needles, in biopsy of mediastinal masses or vascular lesions such as metastatic renal cell carcinoma, and in patients with clotting disorders or pulmonary hypertension.2 The use of fine needles for aspiration and biopsy has reduced the incidence of significant bleeding to 0 to 10%, with most series reporting an incidence of less than 5%.3 In addition, small-gauge (18–20 gauge) automated cutting needles are now widely available and do not appear to be associated with a significantly increased risk of hemorrhage compared with aspirating needles, particularly when their use is confined to lesions in the peripheral third of the lung.41
If hemoptysis occurs, the patient should be placed with the biopsy site dependent to prevent transbronchial aspiration of blood. This maneuver usually suffices, and the hemoptysis usually resolves within minutes. If the hemorrhage continues or is more severe, bronchoscopic tamponade, arterial embolization, or surgery may be necessary.
Other Complications
Rare complications of TNB include systemic arterial air embolism, malignant seeding of the biopsy tract, and vasovagal reaction. Systemic air embolism usually arises as sudden deterioration in the patient's neurologic status or with coronary ischemia or arrhythmia. The mechanism is thought to be either air entry through the needle directly into a pulmonary vein or the iatrogenic creation of a bronchovenous or alveolovenous fistula along the biopsy needle. Predisposing factors cited include coughing, biopsy of consolidated lung, cavitary or vascular masses, and an associated vasculitis.2 To minimize the risk of air embolism, TNB should never be performed with the patient in an upright position, and the patient should be instructed not to cough.42 A styleted needle should be used to minimize exposure to the atmosphere.2 In the event of suspected air embolism, patients should be placed in either the left lateral decubitus position or the Trendelenburg position to prevent residual air within the left atrium from systemically embolizing. Oxygen should be administered by face mask to promote resorption of air bubbles, and blood pressure and ventilatory support should be provided. If possible, patients should be transferred immediately to a hyperbaric decompression chamber.43
Malignant seeding of the biopsy tract is another rare complication of TNB. An early report of two cases of pleural seeding following TNB led authors to recommend that TNB be reserved for inoperable lesions; however, subsequent large series have shown that this complication is extremely uncommon.44,45 A survey of the Society of Thoracic Radiology and literature review found an incidence of needle tract metastasis of 0.012%. Additional rare complications of TNB include vasovagal reaction and lung torsion.3
RESULTS
TNB is highly accurate for the diagnosis of intrathoracic malignancy, with most series reporting an overall sensitivity of 70 to 100%.1,3 A large study involving 12,000 transthoracic needle aspirations reported a sensitivity of 89%, specificity of 95%, positive predictive value of 99%, and negative predictive value of 70%.46 Even small lesions may be accurately evaluated, with Westcott et al reporting a sensitivity of 93% when sampling nodules <15 mm.47 The most common reasons for a false-negative biopsy are sampling error and inaccurate needle placement.1 Another study showed TNB to be highly accurate in the diagnosis of mediastinal and hilar lymph node metastases.48,49 This high success rate allows TNB to be offered as an alternative to transbronchial biopsy, mediastinoscopy, or thoracotomy in the nodal staging of lung cancer and confirmation of nodal metastases. With the use of cutting needles, TNB has a yield on cytology of ∼60 to 75% in the diagnosis of lymphoma.3 This technique provides material sufficient to guide therapy in 83 to 95% of cases.3
Perhaps the greatest limitation of TNB is in its ability to diagnose benign disease. Several large studies report an overall yield for benign disease of 88 to 97%; however, specific benign diagnoses were detected in only 16 to 68% of cases.3 This is a major drawback, as a “negative” biopsy cannot reliably exclude malignancy (and thereby prevent a thoracotomy) unless a specific benign diagnosis can be made. Nonetheless, with sampling of multiple portions of a lesion, the use of cutting needles when repeated aspirations fail to show definitive evidence of malignancy, and expert cytopathology, the yield for benign lesions is increased.3 Improvements in the design of cutting needles permit good samples to be obtained for histologic evaluation without significantly increasing patients' morbidity.2
TNB has long been used to diagnose pulmonary infection, particularly when other methods fail to isolate a causative organism. Castellino and Blank reported a diagnostic yield of 73% from 108 biopsies in 82 immunocompromised patients suspected to have focal infection.7 Conces et al identified the causative organism in 35 of 46 patients ultimately found to have infection.8 More recently, Grinan et al described fluoroscopically guided needle aspiration in 49 patients with lung abscess, with a diagnostic yield of 82%.50 The results of TNB led to a change in antibiotic therapy in 47% of these patients. TNB has also been shown to be of use in patients with acquired immunodeficiency syndrome (AIDS) and focal chest lesions that may be secondary to Kaposi's sarcoma, tuberculosis, and rarely pneumocystis pneumonia.51,52 Gruden et al determined the cause of a focal lesion by TNB in 27 of 32 patients with AIDS.51 Careful preparation of samples for microbiology is of utmost importance when TNB is performed in these patients.
CONCLUSION
In summary, percutaneous TNB has become widely established in the diagnosis of focal pulmonary lesions, both infectious and neoplastic. Technical advances in imaging and guidance, needle design, and cytopathologic and histopathologic techniques continue to expand the applications of TNB and to reduce the incidence of experienced complications. Among patients more likely to require a lung biopsy eventually are lung transplant recipients, these patients also among the most likely to experience complications during and after the procedure. However, with the use of special techniques available to maximize diagnostic yield and minimize adverse effects, TNB may now be a viable option for lung transplant recipients, single or double.
REFERENCES
- Laurent F, Montaudon M, Latrabe V, et al. Percutaneous biopsy in lung cancer. Eur J Radiol. 2003;45:60–68. doi: 10.1016/s0720-048x(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Murphy J M, Gleeson F V, Flower C D. Percutaneous needle biopsy of the lung and its impact on patient management. World J Surg. 2001;25:373–380. doi: 10.1007/s002680020388. [DOI] [PubMed] [Google Scholar]
- Klein J S, Zarka M A. Transthoracic needle biopsy. Radiol Clin North Am. 2000;38:235–266. doi: 10.1016/s0033-8389(05)70161-5. [DOI] [PubMed] [Google Scholar]
- Collins J, Kazerooni E A, Lacomis J, et al. Bronchogenic carcinoma after lung transplantation: frequency, clinical characteristics, and imaging findings. Radiology. 2002;224:131–138. doi: 10.1148/radiol.2241011189. [DOI] [PubMed] [Google Scholar]
- DeMeo D L, Ginns L C. Lung transplantation at the turn of the century. Annu Rev Med. 2001;52:185–201. doi: 10.1146/annurev.med.52.1.185. [DOI] [PubMed] [Google Scholar]
- Charig M J, Stutley J E, Padley S PG, et al. The value of negative needle biopsy in suspected operable lung cancer. Clin Radiol. 1991;44(3):147–149. doi: 10.1016/s0009-9260(05)80856-6. [DOI] [PubMed] [Google Scholar]
- Castellino R A, Blank N. Etiologic diagnosis of focal pulmonary infection in immunocompromised patients by fluoroscopically guided percutaneous needle aspiration. Radiology. 1979;132:563–567. doi: 10.1148/132.3.563. [DOI] [PubMed] [Google Scholar]
- Conces D J, Clark S A, Tarver R D, et al. Transthoracic aspiration needle biopsy: value in the diagnosis of pulmonary infections. AJR Am J Roentgenol. 1989;152:31–34. doi: 10.2214/ajr.152.1.31. [DOI] [PubMed] [Google Scholar]
- Wallace M J, Krishnamurthy S, Broemeling L D, et al. CT-guided percutaneous fine-needle aspiration biopsy of small (< or = 1-cm) pulmonary lesions. Radiology. 2002;225:823–828. doi: 10.1148/radiol.2253011465. [DOI] [PubMed] [Google Scholar]
- Yankelevitz D F, Henschke C I, Koizumi J H, et al. CT-guided transthoracic needle biopsy of small solitary pulmonary nodules. Clin Imaging. 1997;21:107–110. doi: 10.1016/s0899-7071(96)00011-3. [DOI] [PubMed] [Google Scholar]
- Froelich J J, Ishaque N, Regn J, et al. Guidance of percutaneous pulmonary biopsies with real-time CT fluoroscopy. Eur J Radiol. 2002;42:74–79. doi: 10.1016/s0720-048x(01)00391-6. [DOI] [PubMed] [Google Scholar]
- Katada K, Kato R, Anno H, et al. Guidance with real-time CT fluoroscopy: early clinical experience. Radiology. 1996;200:851–856. doi: 10.1148/radiology.200.3.8756943. [DOI] [PubMed] [Google Scholar]
- Moore E H. Technical aspects of needle aspiration lung biopsy: a personal perspective. Radiology. 1998;208:303–318. doi: 10.1148/radiology.208.2.9680552. [DOI] [PubMed] [Google Scholar]
- Li H, Boiselle P M, Shepard J O. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: comparison of small and large pulmonary nodules. AJR Am J Roentgenol. 1996;167:105–109. doi: 10.2214/ajr.167.1.8659351. [DOI] [PubMed] [Google Scholar]
- Haramati L B. CT-guided automated needle biopsy of the chest. AJR Am J Roentgenol. 1995;165:53–55. doi: 10.2214/ajr.165.1.7785631. [DOI] [PubMed] [Google Scholar]
- Greif J, Marmur S, Schwarz Y, et al. Percutaneous core cutting needle biopsy compared with fine-needle aspiration in the diagnosis of peripheral lung malignant lesions: results in 156 patients. Cancer. 1998;84:144–147. doi: 10.1002/(sici)1097-0142(19980625)84:3<144::aid-cncr4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Klein J S, Schultz S. Interventional chest radiology. Curr Probl Diagn Radiol. 1992;21:219–277. doi: 10.1016/0363-0188(92)90005-z. [DOI] [PubMed] [Google Scholar]
- Klein J S, Salomon G, Stewart E A. Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle: results in 122 patients. Radiology. 1996;198:715–720. doi: 10.1148/radiology.198.3.8628859. [DOI] [PubMed] [Google Scholar]
- Greif J, Marmur S, Schwarz Y, et al. Percutaneous core needle biopsy vs. fine-needle aspiration in diagnosing benign lung lesions. Acta Cytol. 1999;43:756–760. doi: 10.1159/000331287. [DOI] [PubMed] [Google Scholar]
- Staroselsky A N, Schwarz Y, Man A, et al. Additional information from percutaneous cutting needle biopsy following fine-needle aspiration in the diagnosis of chest lesions. Chest. 1998;113:1522–1525. doi: 10.1378/chest.113.6.1522. [DOI] [PubMed] [Google Scholar]
- McLoud T C. Should cutting needles replace needle aspiration of lung lesions? Radiology. 1998;207:569–570. doi: 10.1148/radiology.207.3.9609875. [DOI] [PubMed] [Google Scholar]
- Yankelevitz D F, Vazquez M, Henschke C I. Special techniques in transthoracic needle biopsy of pulmonary nodules. Radiol Clin North Am. 2000;38:267–279. doi: 10.1016/s0033-8389(05)70162-7. [DOI] [PubMed] [Google Scholar]
- Haramati L B, Austin J HM. Complications after CT-guided needle biopsy through aerated versus nonaerated lung. Radiology. 1991;181:778. doi: 10.1148/radiology.181.3.1947096. [DOI] [PubMed] [Google Scholar]
- Stern E J, Webb W R, Gamsu G. CT gantry tilt: utility in transthoracic fine-needle aspiration biopsy. Radiology. 1993;187:873–874. doi: 10.1148/radiology.187.3.8497650. [DOI] [PubMed] [Google Scholar]
- Yankelevitz D F, Henschke C I, Davis S D. Percutaneous CT biopsy of chest lesions: an in vitro analysis of the effect of partial volume averaging on needle positioning. AJR Am J Roentgenol. 1993;161:273–278. doi: 10.2214/ajr.161.2.8333360. [DOI] [PubMed] [Google Scholar]
- Sandhu J, Meglin A J, Trerotola S O. Thoracic and Visceral Nonvascular Interventions. Fairfax, VA: The Society of Cardiovascular and Interventional Radiology; 1997. pp. 159–170.
- Klose K C. CT-guided large-bore biopsy: extrapleural injection of saline for safe transpleural access to pulmonary lesions. Cardiovasc Intervent Radiol. 1993;16:259–261. doi: 10.1007/BF02602975. [DOI] [PubMed] [Google Scholar]
- Horton J A, Bank W O, Kerber C W. Guiding the thin spinal needle. AJR Am J Roentgenol. 1980;134:845–846. doi: 10.2214/ajr.134.4.845. [DOI] [PubMed] [Google Scholar]
- Yankelevitz D F, Davis S D, Chiarella D, et al. Needle-tip repositioning during computed-tomography-guided transthoracic needle aspiration biopsy of small deep pulmonary lesions: minor adjustments make a big difference. J Thorac Imaging. 1996;11:279–282. doi: 10.1097/00005382-199623000-00006. [DOI] [PubMed] [Google Scholar]
- Layfield L J, Liu K, Erasmus J J. Radiologically determined diameter, pathologic diameter, and reactive zone surrounding pulmonary neoplasms: implications for transthoracic fine-needle aspiration of pulmonary neoplasms. Diagn Cytopathol. 1999;21:250–252. doi: 10.1002/(sici)1097-0339(199910)21:4<250::aid-dc4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Moore E H, Shepard J O, McLoud T C, et al. Positional precautions in needle aspiration lung biopsy. Radiology. 1990;175:733–735. doi: 10.1148/radiology.175.3.2343123. [DOI] [PubMed] [Google Scholar]
- Berquist T H, Bailey P B, Cortese D A, et al. Transthoracic needle biopsy: accuracy and complications in relation to location and type of lesion. Mayo Clin Proc. 1980;55:475–481. [PubMed] [Google Scholar]
- Fish G D, Stanley J H, Miller S, et al. Postbiopsy pneumothorax: estimating the risk by chest radiography and pulmonary function tests. AJR Am J Roentgenol. 1988;150:71–74. doi: 10.2214/ajr.150.1.71. [DOI] [PubMed] [Google Scholar]
- Yankelevitz D F, Davis S D, Henschke C I. Aspiration of a large pneumothorax resulting from transthoracic needle biopsy. Radiology. 1996;200:695–697. doi: 10.1148/radiology.200.3.8756917. [DOI] [PubMed] [Google Scholar]
- Yamagami T, Nakamura T, Iida S, et al. Management of pneumothorax after percutaneous CT-guided lung biopsy. Chest. 2002;121:1159–1164. doi: 10.1378/chest.121.4.1159. [DOI] [PubMed] [Google Scholar]
- Lang E K, Ghavami R, Schreiner V C, et al. Autologous blood clot seal to prevent pneumothorax at CT-guided lung biopsy. Radiology. 2000;216:93–96. doi: 10.1148/radiology.216.1.r00jl3293. [DOI] [PubMed] [Google Scholar]
- Herman S J, Weisbrod G L. Usefulness of the blood patch technique after transthoracic needle aspiration biopsy. Radiology. 1990;176:395–397. doi: 10.1148/radiology.176.2.2367653. [DOI] [PubMed] [Google Scholar]
- Engeler C E, Hunter D W, Castaneda-Zuniga W, et al. Pneumothorax after lung biopsy: prevention with transpleural placement of compressed collagen foam plugs. Radiology. 1992;184:787–789. doi: 10.1148/radiology.184.3.1509068. [DOI] [PubMed] [Google Scholar]
- Man A, Schwarz Y, Greif J. Case report: cardiac tamponade following fine needle aspiration (FNA) of a mediastinal mass. Clin Radiol. 1998;53:151–152. doi: 10.1016/s0009-9260(98)80065-2. [DOI] [PubMed] [Google Scholar]
- Milner L B, Ryan K, Gullo J. Fatal intrathoracic hemorrhage after percutaneous aspiration lung biopsy. AJR Am J Roentgenol. 1979;132:280–281. doi: 10.2214/ajr.132.2.280. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Nakajima Y, Kurihara H, et al. CT-guided transthoracic needle biopsy: a comparison between automated biopsy gun and fine needle aspiration. Clin Radiol. 1996;51:503–506. doi: 10.1016/s0009-9260(96)80191-7. [DOI] [PubMed] [Google Scholar]
- Aberle D R, Gamsu G, Golden J A. Fatal systemic arterial air embolism following lung needle aspiration. Radiology. 1987;165:351–353. doi: 10.1148/radiology.165.2.3659355. [DOI] [PubMed] [Google Scholar]
- Murphy B P, Harford F J, Cramer F S. Cerebral air embolism resulting from invasive medical procedures: treatment and hyperbaric oxygen. Ann Surg. 1985;201:242–245. doi: 10.1097/00000658-198502000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger R L, Dargan E L, Huang B L. Dissemination of cancer cells by needle biopsy of the lung. J Thorac Cardiovasc Surg. 1972;63:430–432. [PubMed] [Google Scholar]
- Khouri N F, Stitik F P, Erozan Y S, et al. Transthoracic needle aspiration biopsy of benign and malignant lung lesions. AJR Am J Roentgenol. 1985;144:281–288. doi: 10.2214/ajr.144.2.281. [DOI] [PubMed] [Google Scholar]
- Zarbo R J, Fenoglio-Presier C M. Inter-institutional database for comparison of performance in lung fine-needle aspiration cytology: a College of American Pathologists Q-probe study of 5264 cases with histologic correlation. Arch Pathol Lab Med. 1992;116:463–470. [PubMed] [Google Scholar]
- Westcott J L, Najmussaqib R, Colley D P. Transthoracic needle biopsy of small pulmonary nodules. Radiology. 1997;202:97–103. doi: 10.1148/radiology.202.1.8988197. [DOI] [PubMed] [Google Scholar]
- Belfiore G, Camera L, Moggio G, et al. Middle mediastinal lesions: preliminary experience with CT-guided fine-needle aspiration biopsy with a supra-sternal approach. Radiology. 1997;202:870–873. doi: 10.1148/radiology.202.3.9051049. [DOI] [PubMed] [Google Scholar]
- Protopapas Z, Westcott J L. Transthoracic needle biopsy of mediastinal lymph nodes for staging lung and other cancers. Radiology. 1996;199:489–496. doi: 10.1148/radiology.199.2.8668801. [DOI] [PubMed] [Google Scholar]
- Grinan N P, Lucena F M, Romero J V, et al. Yield of percutaneous needle lung aspiration in lung abscess. Chest. 1990;97:69–74. doi: 10.1378/chest.97.1.69. [DOI] [PubMed] [Google Scholar]
- Gruden J F, Klein J S, Webb W R. Percutaneous transthoracic needle biopsy in AIDS: analysis in 32 patients. Radiology. 1993;189:567–571. doi: 10.1148/radiology.189.2.8105506. [DOI] [PubMed] [Google Scholar]
- Scott W W, Kuhlman J E. Focal pulmonary lesions in patients with AIDS: percutaneous transthoracic needle biopsy. Radiology. 1991;180:419–421. doi: 10.1148/radiology.180.2.2068304. [DOI] [PubMed] [Google Scholar]