ABSTRACT
Transplant renal artery stenosis is the most frequent vascular complication of transplantation. Early detection and correction reduce patients' morbidity and allograft dysfunction. Although noninvasive imaging can detect an underlying stenosis, angiography with subsequent angioplasty or stenting, or both, provides definitive diagnosis and treatment. With the introduction of alternative contrast agents and newer catheter and stent technology, these procedures can be performed safely with little risk of contrast-induced nephropathy or allograft loss.
Keywords: Kidney transplantation, balloon angioplasty, stents, renal artery stenosis, hypertension, angiography
Renal transplantation is the definitive therapy for end-stage renal disease and has especially flourished in the post–cyclosporine-mediated immunosuppression era. Rarely, renal autotransplantation is performed for other reasons, such as for trauma resulting in bilateral renal artery thrombosis.1 Historically, angiography has been performed for the evaluation for allograft rejection, delineating donor anatomy prior to surgery, and investigating the etiology of declining renal function after transplantation. At present, noninvasive imaging performed with ultrasonography and nuclear medicine in addition to renal biopsy has supplanted the routine use of potentially nephrotoxic contrast angiography for most renal transplantation evaluations. Newer modalities such as magnetic resonance angiography (MRA) and computed tomographic angiography in addition to carbon dioxide (CO2) digital subtraction angiography are now used routinely as well. Transplant renal artery stenosis (TRAS) is the most frequent vascular complication in renal transplantation with an incidence varying between 1 and 25%.2,3,4,5,6 The etiology and management of TRAS vary by location relative to the anastomosis, and it is therefore classified relative to this structure. TRAS can occur proximal to the anastomosis (preanastomotic), at the anastomosis, or within the donor artery (postanastomotic). TRAS may be a consequence of faulty surgical techniques, arterial damage during donor nephrectomy or kidney perfusion, kinking and compression of the renal artery, size discrepancy between donor and recipient renal arteries, and chronic rejection.5,7,8
CLINICAL PRESENTATION
Patients can present early or late in the post-transplantation period. They may present with poorly controlled hypertension, compromised function of the allograft, or rejection refractory to immunotherapy. Occasionally, a bruit in the iliac fossa can be heard; this finding is not specific. Although it is the most common long-term complication of renal transplantation, TRAS is the etiology of elevated blood pressure in only 3 to 12% of the hypertensive patients.9,10 Hypertension may occur suddenly or may have a more insidious onset. It is typified by refractoriness to multiple drug regimens. Progressive renal insufficiency may manifest itself and be exacerbated by excess diuresis or occur after use of angiotensin-converting enzyme (ACE) inhibitors. ACE inhibitors may cause a more rapid decline in renal function that may lead to profound hypertension and acute renal failure.11 A higher incidence of TRAS in transplants from cadavers (13.2–17.7%) compared with live donors (1.3–5.8%) has been reported.6,12 The interval between transplantation and diagnosis of TRAS ranges from 2 to 45 months.5,13 Acute rejection does not appear to correlate with the development of stenosis.5
ETIOLOGY
Mechanical or technical mechanisms of TRAS include atherosclerosis of the donor renal arteries or recipient iliac arteries, or both. In addition, trauma to the vessels during harvesting and transplantation, clamp injury, cannulation for organ perfusion, excess traction on the vessels, and suture techniques are other potential causes. Small subintimal flaps or dissections are thought to occur, leading to intimal scarring and hyperplasia and eventual anastomotic stenosis.14 Ostial stenosis is less frequent with a Carrel patch, wherein a cuff of donor aorta is harvested along with the renal artery, used frequently in transplants from cadaver donors.
Nontechnical mechanisms or immunologic causes have been suggested to contribute to TRAS. Intimal proliferation was associated with chronic rejection prior to the introduction of cyclosporine, but no consistent correlation has been found between acute rejection and TRAS.2,15,16 Cyclosporine has also been suggested as a cause for TRAS by means of inducing endothelial injury leading to the development of a stenosis.17 However, no studies have been conclusive.
Early (within a few months of surgery) TRAS is likely to be caused by mechanical or technical trauma, whereas TRAS developing remote (more than 6 months) from the time of transplantation cannot be explained and may be associated with progression of underlying atherosclerotic disease.
RENAL TRANSPLANT SURGICAL TECHNIQUE
Whether the transplant is from a living related or cadaver donor, the renal artery is anastomosed to the internal iliac artery as an end-to-end anastomosis or to the external iliac artery as an end-to-side anastomosis.18,19 The kidney is surgically implanted in either iliac fossa from a retroperitoneal approach through an oblique incision extending from the midline 3–4 cm from the inguinal ligament to a point just above the anterior superior iliac spine.20 When there are multiple vessels in a cadaver donor, a Carrel patch of aorta is used.21 With living related donors, multiple renal arteries are a surgical challenge because a patch of aorta containing the additional vessels cannot be obtained. If the additional vessel is small and perfuses only a small segment of kidney, it may be ligated. Larger accessory vessels may be anastomosed end to side to the main renal artery, although this may encroach on the main renal artery lumen. Two or more renal arteries can be anastomosed together side to side to create a “double-barrel anastomosis” preserving the lumen of each vessel.22 Other authors have suggested performing separate anastomoses to either the recipient internal or external iliac arteries or one renal branch to each. Another surgical option is to use the recipient's inferior epigastric artery for the accessory vessel.23 Pediatric cadaveric donors may have both kidneys with a segment of aorta transplanted en bloc through an anastomosis to the recipient iliac artery.24
The renal vein is usually anastomosed to a mobilized recipient iliac vein in an end-to-side fashion.18 When two or more renal veins are present, the smaller veins are typically ligated, resulting in a single anastomosis of the larger vein with drainage through an intrarenal venous anastomosis.20 When the veins are similar in size, the smaller of the two is first clamped to test for adequate venous collaterals.21 When the artery or vein is too short, grafting may be performed, usually with a saphenous vein or occasionally a gonadal vein.25 When significant vascular arterial or venous pathology is present, such as inferior vena cava agenesis, unusual surgical anastomoses may be needed.26 In children, the renal artery and vein may be anastomosed to the lower aorta and inferior vena cava, respectively. Three types of anastomoses are usually performed to create urinary continuity. The most common is the ureteroneocystostomy, which has the lowest incidence of extravasation. More involved transplant surgeries have now been performed in diabetics utilizing both kidney and pancreatic allografts.27,28
NONINVASIVE IMAGING EVALUATION
Evaluation of TRAS may be performed with both noninvasive and invasive imaging techniques. Color flow duplex ultrasound (CFDU) and MRA have now become the primary noninvasive imaging modalities for diagnosing TRAS. CFDU is highly dependent on visualization of the entire donor renal artery and the surgical anastomosis. This imaging modality is also limited by inability to visualize the iliac vessels completely to exclude inflow stenosis at this level, largely because of the patient's body habitus and bowel gas. Stenotic segments appear as regions of focal color aliasing, which with duplex Doppler techniques can be characterized and graded.29 Doppler criteria for significant stenosis include (1) peak systolic velocities greater than 2 m/sec (Fig. 1), (2) velocity gradient between stenotic and prestenotic segments of more than 2:1, and (3) marked distal turbulence (spectral broadening).30,31,32
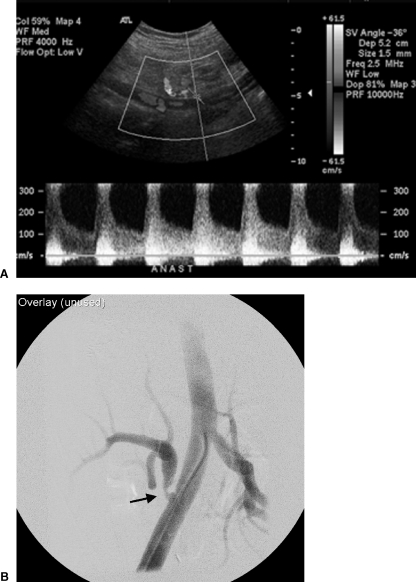
Figure 1.
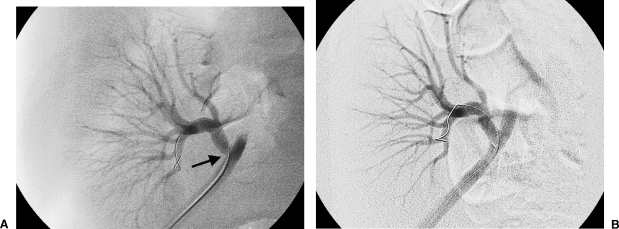
(A) Ultrasound Doppler examination of the renal artery anastomosis to the external iliac artery demonstrated velocities exceeding 3 m/sec. (B) Selective right external iliac artery angiogram confirms a focal moderately severe and eccentric stenosis at and just distal to the arterial anastomosis (arrow).
Although catheter-based angiography has conventionally been held as the “gold standard” in evaluation of arterial stenosis, three-dimensional gadolinium-enhanced MRA has demonstrated 100% sensitivity and 75–98% specificity for greater than 50% stenosis in small studies.33,34,35,36,37 This modality allows evaluation of the iliac arteries, donor artery, arterial anastomosis, and the renal vein (Fig. 2). Both these modalities allow imaging evaluation of the transplant kidney without the use of iodinated contrast agents. However, if there is a strong clinical suspicion of TRAS despite negative noninvasive imaging studies, proceeding to angiography should be considered.
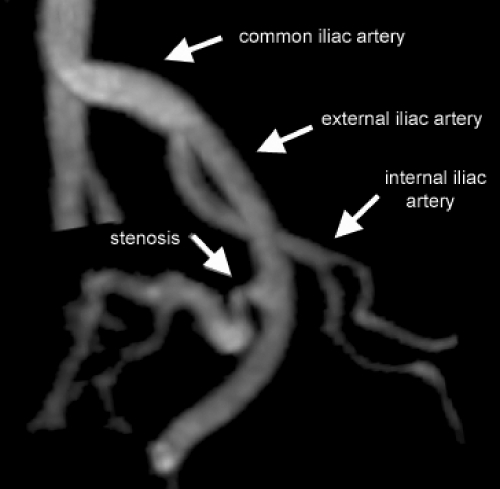
Figure 2.
Gadolinium-enhanced three-dimensional MRA reconstructed image demonstrates stenosis of the renal artery distal to the anastomosis. The iliac arteries are completely visualized.
RENAL TRANSPLANT ANGIOGRAPHY
Intra-arterial digital subtraction angiography has now replaced film screen (“cut film”) imaging for imaging renal transplants. Because of the risk of nephrotoxicity associated with iodinated contrast media, volume should be carefully tailored to the clinical question with careful consideration of the vascular anatomy, clinical scenario, and possible therapeutic intervention.24 Catheter and wire manipulation near the anastomosis or donor transplant renal artery should be kept to a minimum, especially when the transplantation has been recently performed. Consideration should be given to the use of nonionic contrast material because of its possible reduced nephrotoxicity, as its use appears to reduce contrast-induced nephropathy (CIN) by ∼50% compared with high-osmolality agents.38 Newer isosmolar contrast media (Visipaque, Amersham Health, Princeton, NJ) are considered to have less nephrotoxic effect than nonionic contrast media.39,40
Ipsilateral or contralateral approaches can be used for donor arteriography when the anastomosis is to the external iliac artery and a contralateral approach when the anastomosis is to the internal iliac artery. A Cobra configuration of catheter works well when performing a contralateral approach. Examination of the distal aorta and common iliac arteries should be performed in hypertensive patients because stenosis related to atherosclerosis or clamp injuries can cause an impediment to inflow,24,41,42 leading to local hypoperfusion.
Contrast injection rates depend on the location of the catheter tip and should be guided by fluoroscopic hand injections. Common iliac artery injections are performed at a rate of 7–15 mL/sec for a total of 12–30 mL and internal iliac artery injections are performed at 5 mL/sec for a total of 10 mL, depending on the type of imaging technique utilized. Selective injection into the renal artery is generally not required43 if image quality is optimal. Initial films can be obtained in the 30-degree left or right posterior oblique projection for the left- or right-sided transplant, respectively, unless a test injection dictates a different view. Multiple projections such as steep obliques or even a lateral projection are generally required to image the course of the arteries adequately and to visualize the in-profile anastomosis, which is typically placed anterior on the vessel. On average, three views are required to image the transplant adequately, although because of overlap of vessels, as many as six images may be required.44,45 The introduction of rotational angiography with three-dimensional reconstruction may allow savings in time, radiation, and contrast dose.46,47
The angiographic appearance of a normally functioning transplant is similar to that of a normal native kidney, but its size may vary. A normal transplant may measure up to 16 × 9 cm, and the size increases 10% in the first month and 1% a month for the first year.48,49 Complications associated with renal transplant angiography are typical of other similar diagnostic and interventional angiographic procedures. The risk of vascular injury is related to the risk and difficulty of the procedure, and contrast nephrotoxicity is related to underlying impairment of renal function.50,51
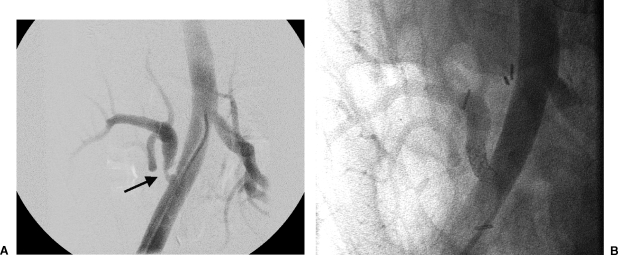
If the renal transplant is functioning poorly with an elevated creatinine level and contrast angiography is being considered, CO2 angiography may be used to confirm or exclude a diagnosis of a vascular abnormality52 (Fig. 3). CO2 acts as a negative contrast agent when combined with intra-arterial digital subtraction angiography and is cleared by the lungs rather than the kidneys.53 Current software allows “stacking” of images, producing image quality similar to that obtained with iodinated contrast agents. Because CO2 is not nephrotoxic, it can be used in patients with impaired renal function53,54,55 and has not been shown to change renal function significantly with selective injections.56 CO2 works well with renal transplants because the vessels are anteriorly located and the gas rises into the graft, allowing filling of the intraparenchymal arteries.56 A sufficient volume of CO2 has to be injected to avoid underestimating the diameter of vessels and vessel stenosis,57 but the amount that can be injected in subdiaphragmatic arterial vessels should be limited to less than 300 cm3.53 The potential for gas trapping or “vapor lock” causing reduced blood flow in the renal artery is eliminated if the minimal volumes for imaging are injected 1–2 minutes apart.52,53 Percutaneous interventions can be performed with CO2; however, this is more difficult because image quality may be adversely effected by the hardware introduced during the intervention. When CO2 angiography is inadequate, imaging can be supplemented with gadolinium-based agents.54
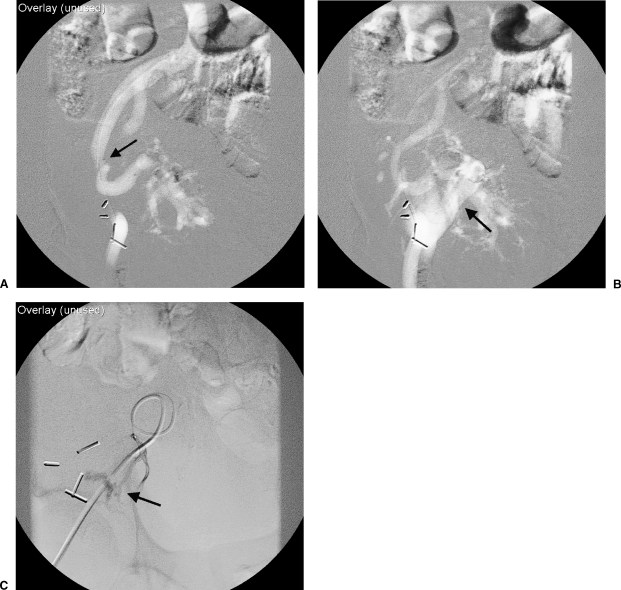
Figure 3.
(A) Carbon dioxide angiogram of the external iliac artery was performed demonstrating focal stenosis (arrow) of the proximal transplant renal artery as demonstrated in Figure 2. (B) Images obtained 0.2 seconds later demonstrate early opacification of the external iliac vein (arrow). (C) Selective injection of a renal segmental arterial branch verifies an arteriovenous fistula (arrow), later corrected with coil embolization.
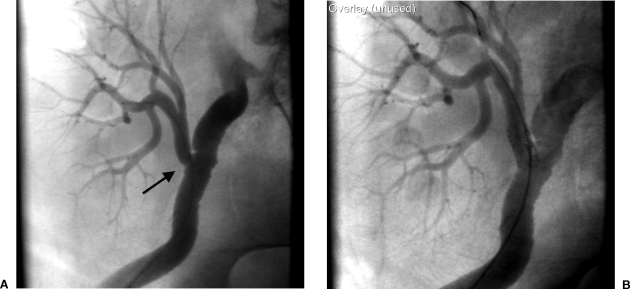
Gadolinium, a contrast agent primarily administered for contrast-enhanced magnetic resonance imaging, absorbs x-rays similarly to iodinated contrast material. The image quality is inferior to that with iodinated material because of the low concentration of gadolinium (0.5 mmol/mL) (Fig. 4). Image contrast with gadolinium is typically 25–50% that of images obtained with traditional full-strength contrast agents. In doses injected for magnetic resonance examinations (0.1 to 0.4 mmol/kg body weight) there has been no associated nephrotoxicity in patients with renal insufficiency.58,59,60 However, there has been a report of worsening renal function following a lower extremity angiogram using gadolinium when a dose of 0.44 mmol/kg was used.61 For this reason, we do not exceed a dose of 0.4 mmol/kg or, as a basic rule, we do not use more than 30 mL of gadolinium. Gadolinium can be hand or pump injected in a manner similar to injection of iodinated contrast material. It is advantageous to combine gadolinium imaging with CO2 to reduce the amount of gadolinium required for diagnostic images and therapeutic interventions.
Figure 4.
(A) Angiogram performed with gadolinium prior to intervention. (B) Following intervention, an angiogram was performed with a hand injection of iodinated contrast material demonstrating improved contrast resolution compared with gadolinium. The tip of a guiding sheath is visualized in the proximal renal artery.
Two supplemental medications can be administered to reduce the risk of CIN. Fenoldopam is similar to dopamine, acting on the DA-1 receptor. This medication is a potent renal vasodilator resulting in increased renal blood flow.62 Acetylcysteine, a thiol-containing antioxidant, has been shown to reduce CIN in high-risk patients.63,64 Dosing is 600 mg orally twice a day the day before and the day of contrast administration. Although these agents show promise, few studies validating their effectiveness have been published.
MANAGEMENT OF TRANSPLANT RENAL ARTERY STENOSIS
Three different treatment methods are available:
Medical management may be used to control hypertension with unknown effect on kidney function. This approach is indicated if the degree of stenosis is not considered hemodynamically significant or the risk of percutaneous intervention is considered high for graft loss, or both. Intervention is justified if there is a reasonable expectation of success in improving hypertension and renal function or stopping progressive renal deterioration.8,16,65
Surgical intervention with revascularization. This is considered a major operation with graft loss following vascular reconstruction approaching 30% with a recurrence rate of ∼12%.65,66,67 There is also an increased risk of ureteral injury with surgery. Surgical correction is the preferred method for treatment of kinking of the proximal transplant renal artery.68
Percutaneous endovascular management using transluminal angioplasty (PTA) or metallic stent placement, or both.
There are several locations that can develop stenoses. An arterial stenosis may be1 preanastomotic, which is usually due to atherosclerosis or a clamp injury18; at the anastomosis, which is usually due to a technical problem related to surgery19; just distal to the anastomosis, which is usually due to immune reactions or hemodynamic turbulence from the anastomosis causing an intimal hyperplasia20; or intrarenal due to chronic rejection.21,24,69 Postanastomotic strictures tend to be more severe and associated with end-to-side anastomoses as compared with end-to-end anastomoses, which tend to be at the anastomosis.69,70 Iliac artery stenosis ipsilateral and proximal to the renal artery anastomosis can also lead to allograft dysfunction, particularly in the elderly population.12,71,72,73,74
PTA is recognized as the initial treatment of choice for TRAS and does not preclude subsequent surgical correction.15,69,75,76,77,78 However, prior to the introduction of current catheter and wire technology, some investigators had high complication rates up to 20% with low clinical responses.65,79,80 Initial technical success of PTA has been reported to be over 80% and clinical success is ∼74 to 87%.2,70,75,77,78,81 Long-term clinical success, defined as either improvement in blood pressure control or stabilization or improvement in renal function, is between 53 and 70% at 1 year67,75,77 with the restenosis rate of PTA of TRAS to be in the range of 10 to 33%.15,67,75,76,78,79 Complication rates are generally low with the rate of graft loss lower for PTA than for surgery; however, Roberts et al reported their loss after PTA to be as high as 13%, higher than their surgical , which may have resulted from dated (1989) angioplasty catheters and wires compared with devices that are available today.16,65,69,70,75,81
PTA/stenting of an end-to-side anastomosis can be performed from the contralateral or ipsilateral side, depending on the angle of the anastomosis (Fig. 5). An end-to-end anastomosis, which is generally to the hypogastric artery, is performed from the contralateral side or by a left brachial artery approach. Serious complications, which may lead to graft loss, include acute thrombosis, arterial dissection, renal artery rupture, branch occlusion, distal embolization, and contrast-induced renal failure. As with any renal artery angioplasty, complications are reduced by utilizing adequate heparinization, vasodilators, minimal wire manipulations, proper balloon sizing, and inflation pressures. Because of the potential for a false-positive angiogram, Roberts et al suggested that an angiographic stenosis should demonstrate some physiological significance prior to performing an intervention such as a captopril-associated rise in creatinine or large pressure gradient.65
Figure 5.
(A) Prior to angiography, ultrasonography demonstrated a velocity of 6 m/sec at the external iliac artery–renal artery anastomosis. Gadolinium angiogram confirms stenosis (arrow) in this location. (B) Following angioplasty with an 8-mm balloon catheter, the pressure gradient dropped from 54 mm Hg to less than 10 mm Hg.
Metallic stents have been inserted for treatment of recurrent or ostial stenosis, although limited studies have been performed to date. Insertion is associated with high initial technical success and a good patency rate with minimal complications.82,83,84 Serious complications of PTA can be salvaged with stents. In addition, elastic recoil can lead to restenosis. Metallic stents are commonly used, primarily to treat ostial stenosis in native renal arteries and now in transplanted kidneys13,82,83 (Fig. 6). Complications related to stent insertion include thrombosis, peripheral embolization, transient renal failure, subintimal dissection, stent misplacement or migration, and restenosis related to myointimal hyperplasia from the normal healing process. Newman-Sanders et al reported stable allograft function in four patients with follow-up of 4 to 24 months,83 and Nicita et al reported a positive outcome using Palmaz® stents in five of seven patients at a median follow-up of 14.8 months.13 Bertoni et al implanted nine Palmaz® stents for TRAS with no complications with a significant decrease in peak blood flow velocity.82
Figure 6.
(A) Injection of gadolinium in the external iliac artery demonstrates a focal eccentric stenosis at the anastomosis (arrow) extending into the renal artery. (B) A balloon-expandable stent (4.5 mm × 12 mm length) was primarily deployed and expanded to 4.5 mm. Prior to stenting, the pressure gradient was 77 mm Hg. Following deployment, the gradient normalized.
When performing PTA/stenting, we attempt to place the vascular access sheath tip adjacent to the arterial anastomosis (Fig. 4). This allows monitoring the progress of the intervention by angiograms performed with wire access, either a 0.035 or 0.018 access, maintained across the lesion. The vascular sheath can also act as a buttress against a deployed stent to prevent stent migration when removing the deployment balloon. Our indications for PTA include greater than 50% luminal narrowing with angiography or a systolic pressure gradient of 10 mm Hg across the stenotic segment, or both.85 Angioplasty balloon and stent sizing may be obtained with the aid of software found on most modern digital subtraction angiography units. Indications for stent use at our institutions include greater than 30% residual stenosis following PTA, persistent systolic pressure gradient greater than 10 mm Hg following angioplasty, flow limiting dissection, and early recurrent clinically significant stenosis within 3 months of angioplasty. We prefer to use balloon-expandable stents for higher radial strength and accurate placement compared with self-expanding stents (Fig. 7). Previous limitations of balloon-expandable stents have now been resolved with the introduction of multiple new stent platforms by a variety of medical device companies; these have a reduced profile and good trackability over the aortic bifurcation and are premounted, preventing migration of the stent off the deployment balloon.
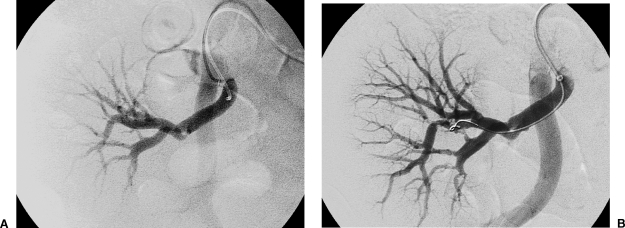
Figure 7.
(A) Iliac angiogram demonstrates a focal osteal or anastomotic stenosis (arrow) of the transplant renal artery. (B) The lesion was primarily stented with a Palmaz 154 stent dilated to 6 mm. Use of a balloon-expandable stent allowed accurate placement.
CONCLUSION
Advances in diagnostic and device technology have allowed improved diagnosis and percutaneous management of TRAS. The use of alternative contrast agents and supplemental medications reduces the likelihood of CIN. Percutaneous transluminal angioplasty with or without intravascular stenting has high technical success with an acceptable complication rate and is the preferred initial therapy in these patients. The introduction of newer technology such as lower profile balloon catheters, premounted balloon-expandable stents, and cutting angioplasty balloon catheters is likely to improve the outcome of percutaneous interventions for TRAS. The success of these interventions requires appropriate selection of patients and attention to meticulous technique.
ABBREVIATIONS
TRAS transplant renal artery stenosis
PTA percutaneous transluminal angioplasty
CFDU color flow duplex ultrasound
MRA magnetic resonance angiography
CO2 carbon dioxide
CIN contrast-induced nephropathy
REFERENCES
- Brunetti D R, Sasaki T M, Friedlander G, et al. Successful renal autotransplantation in a patient with bilateral renal artery thrombosis. Urology. 1994;43:235–237. doi: 10.1016/0090-4295(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Wong W, Fynn S P, Higgins R M, et al. Transplant renal artery stenosis in 77 patients—does it have an immunological cause? Transplantation. 1996;61:215–219. doi: 10.1097/00007890-199601270-00009. [DOI] [PubMed] [Google Scholar]
- Chan H W, Ho Y W, Chan C M, Yiu T F, Tong M K, Wong P H. Treatment of anastomotic ostial allograft and renal artery stenosis with the Palmaz stent. Transplantation. 1995;59:436–439. [PubMed] [Google Scholar]
- Loubeyre P, Cahen R, Grozel F, et al. Transplant renal artery stenosis. Evaluation of diagnosis with magnetic resonance angiography compared with color duplex sonography and arteriography. Transplantation. 1996;62:446–450. doi: 10.1097/00007890-199608270-00004. [DOI] [PubMed] [Google Scholar]
- Sutherland R S, Spees E K, Jones J W, Fink D W. Renal artery stenosis after renal transplantation: the impact of the hypogastric artery anastomosis. J Urol. 1993;149:980–985. doi: 10.1016/s0022-5347(17)36273-0. [DOI] [PubMed] [Google Scholar]
- Sankari B R, Geisinger M, Zelch M, Brouhard B, Cunningham R, Novick A C. Post-transplant renal artery stenosis: impact of therapy on long-term kidney function and blood pressure control. J Urol. 1996;155:1860–1864. doi: 10.1016/s0022-5347(01)66030-0. [DOI] [PubMed] [Google Scholar]
- Kauffman H M, Sampson D, Fox P S, Doyle T J, Maddison F E. Prevention of transplant renal artery stenosis. Surgery. 1977;81:161–167. [PubMed] [Google Scholar]
- Tilney N L, Rocha A, Strom T B, Kirkman R L. Renal artery stenosis in transplant patients. Ann Surg. 1984;199:454–460. doi: 10.1097/00000658-198404000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R B, Cosimi A B, Lordon R, Thompson A L, Ehrlich R M. Diagnosis and management of arterial stenosis causing hypertension after successful renal transplantation. J Urol. 1976;115:639–642. doi: 10.1016/s0022-5347(17)59318-0. [DOI] [PubMed] [Google Scholar]
- Munda R, Alexander J W, Miller S, First M R, Fidler J P. Renal allograft artery stenosis. Am J Surg. 1977;134:400–403. doi: 10.1016/0002-9610(77)90415-9. [DOI] [PubMed] [Google Scholar]
- Curtis J J, Luke R G, Whelchel J D, Diethelm A G, Jones P, Dustan H P. Inhibition of angiotensin-converting enzyme in renal-transplant recipients with hypertension. N Engl J Med. 1983;308:377–381. doi: 10.1056/NEJM198302173080707. [DOI] [PubMed] [Google Scholar]
- Lacombe M. Arterial stenosis complicating renal allotransplantation in man: a study of 38 cases. Ann Surg. 1975;181:283–288. doi: 10.1097/00000658-197503000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicita G, Villari D, Marzocco M, Li Marzi V, Trippitelli A, Santoro G. Endoluminal stent placement after percutaneous transluminal angioplasty in the treatment of post-transplant renal artery stenosis. J Urol. 1998;159:34–37. doi: 10.1016/s0022-5347(01)64002-3. [DOI] [PubMed] [Google Scholar]
- Smellie W A, Vinik M, Hume D M. Angiographic investigation of hypertension complicating human renal transplantation. Surg Gynecol Obstet. 1969;128:963–968. [PubMed] [Google Scholar]
- Fauchald P, Vatne K, Paulsen D, et al. Long-term clinical results of percutaneous transluminal angioplasty in transplant renal artery stenosis. Nephrol Dial Transplant. 1992;7:256–259. doi: 10.1093/oxfordjournals.ndt.a092116. [DOI] [PubMed] [Google Scholar]
- Merkus J W, Huysmans F T, Hoitsma A J, Buskens F G, Skotnicki S H, Koene R A. Renal allograft artery stenosis: results of medical treatment and intervention. A retrospective analysis. Transpl Int. 1993;6:111–115. doi: 10.1007/BF00336655. [DOI] [PubMed] [Google Scholar]
- Sawaya B, Provenzano R, Kupin W L, Venkat K K. Cyclosporine-induced renal macroangiopathy. Am J Kidney Dis. 1988;12:534–537. doi: 10.1016/s0272-6386(88)80107-0. [DOI] [PubMed] [Google Scholar]
- Hanto D W, Simmons R L. Renal transplantation: clinical considerations. Radiol Clin North Am. 1987;25:239–248. [PubMed] [Google Scholar]
- Flye M. Renal Transplantation. Philadelphia: WB Saunders; 1989.
- Lee H. In: Morris P, editor. Kidney Transplantation. Philadelphia: WB Saunders; 1988. Surgical techniques in renal transplantation. pp. 215–233.
- Belzer F, Glass N, Sollingerr H. In: Morris P, editor. Kidney Transplantation. Philadelphia: WB Saunders; 1988. Technical complications after renal transplantation. pp. 511–522.
- Guerra E E, Didone E C, Zanotelli M L, et al. Renal transplants with multiple arteries. Transplant Proc. 1992;24:1868. [PubMed] [Google Scholar]
- Ganesan K S, Huilgol A K, Sundar S, Chandrashekar V, Prasad S, Raviraj K G. Management of multiple arteries in renal transplantation. Transplant Proc. 1994;26:2101–2102. [PubMed] [Google Scholar]
- Crain M, Ditmanson P, Finlay D. Vascular complications of renal transplantation: angiographic diagnosis and intervention. Semin Interv Radiol. 1992;9:235–245. [Google Scholar]
- Nghiem D D. Spiral gonadal vein graft extension of right renal vein in living renal transplantation. J Urol. 1989;142:1525. doi: 10.1016/s0022-5347(17)39148-6. [DOI] [PubMed] [Google Scholar]
- Talbot-Wright R, Carretero P, Alcaraz A, Vilardell J. Complex renal transplant for vascular reasons. Transplant Proc. 1992;24:1865–1866. [PubMed] [Google Scholar]
- Perez R, Navarro M D, del Castillo D, et al. Simultaneous pancreas-kidney transplant compared with kidney transplant in type I diabetic patients with end-stage renal disease. Transplant Proc. 2001;33:3494–3495. doi: 10.1016/s0041-1345(01)02412-5. [DOI] [PubMed] [Google Scholar]
- Lo A, Stratta R J, Hathaway D K, et al. Long-term outcomes in simultaneous kidney-pancreas transplant recipients with portal-enteric versus systemic-bladder drainage. Am J Kidney Dis. 2001;38:132–143. doi: 10.1053/ajkd.2001.25207. [DOI] [PubMed] [Google Scholar]
- Tublin M E, Dodd G D., III Sonography of renal transplantation. Radiol Clin North Am. 1995;33:447–459. [PubMed] [Google Scholar]
- Snider J F, Hunter D W, Moradian G P, Castaneda-Zuniga W R, Letourneau J G. Transplant renal artery stenosis: evaluation with duplex sonography. Radiology. 1989;172:1027–1030. doi: 10.1148/172.3.1027. [DOI] [PubMed] [Google Scholar]
- Taylor K J, Morse S S, Rigsby C M, Bia M, Schiff M. Vascular complications in renal allografts: detection with duplex Doppler US. Radiology. 1987;162:31–38. doi: 10.1148/radiology.162.1.3538150. [DOI] [PubMed] [Google Scholar]
- de Morais R H, Muglia V F, Mamere A E, et al. Duplex Doppler sonography of transplant renal artery stenosis. J Clin Ultrasound. 2003;31:135–141. doi: 10.1002/jcu.10147. [DOI] [PubMed] [Google Scholar]
- Kelekis N L, Semelka R C, Worawattanakul S, Molina P L, Mauro M A. Magnetic resonance imaging of the abdominal aorta and iliac vessels using combined 3-D gadolinium-enhanced MRA and gadolinium-enhanced fat-suppressed spoiled gradient echo sequences. Magn Reson Imaging. 1999;17:641–651. doi: 10.1016/s0730-725x(99)00020-x. [DOI] [PubMed] [Google Scholar]
- Huber A, Heuck A, Scheidler J, et al. Contrast-enhanced MR angiography in patients after kidney transplantation. Eur Radiol. 2001;11:2488–2495. doi: 10.1007/s003300100992. [DOI] [PubMed] [Google Scholar]
- Chan Y L, Leung C B, Yu S C, Yeung D K, Li P K. Comparison of non-breath-hold high resolution gadolinium-enhanced MRA with digital subtraction angiography in the evaluation on allograft renal artery stenosis. Clin Radiol. 2001;56:127–132. doi: 10.1053/crad.2000.0590. [DOI] [PubMed] [Google Scholar]
- Ferreiros J, Mendez R, Jorquera M, et al. Using gadolinium-enhanced three-dimensional MR angiography to assess arterial inflow stenosis after kidney transplantation. AJR Am J Roentgenol. 1999;172:751–757. doi: 10.2214/ajr.172.3.10063875. [DOI] [PubMed] [Google Scholar]
- Luk S H, Chan J H, Kwan T H, Tsui W C, Cheung Y K, Yuen M K. Breath-hold 3D gadolinium-enhanced subtraction MRA in the detection of transplant renal artery stenosis. Clin Radiol. 1999;54:651–654. doi: 10.1016/s0009-9260(99)91085-1. [DOI] [PubMed] [Google Scholar]
- Barrett B J, Carlisle E J. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology. 1993;188:171–178. doi: 10.1148/radiology.188.1.8511292. [DOI] [PubMed] [Google Scholar]
- Chalmers N, Jackson R W. Comparison of iodixanol and iohexol in renal impairment. Br J Radiol. 1999;72:701–703. doi: 10.1259/bjr.72.859.10624328. [DOI] [PubMed] [Google Scholar]
- Davidson C J, Laskey W K, Hermiller J B, et al. Randomized trial of contrast media utilization in high-risk PTCA: the COURT trial. Circulation. 2000;101:2172–2177. doi: 10.1161/01.cir.101.18.2172. [DOI] [PubMed] [Google Scholar]
- Gossmann J, Liermann D, Scheuermann E H, Lenz T. Curable hypertensive renal failure due to iliac artery stenosis in a kidney transplant recipient. Nephrol Dial Transplant. 1997;12:596–598. doi: 10.1093/ndt/12.3.596. [DOI] [PubMed] [Google Scholar]
- Butterworth P C, Bolia A, Nicholson M L. Recurrent pulmonary oedema caused by iliac artery stenosis in a renal transplant recipient. Nephron. 1998;79:119–120. doi: 10.1159/000045010. [DOI] [PubMed] [Google Scholar]
- Thomsen H S, Dorph S, Mygind T, Holm H H, Munck O, Damgaard-Pedersen K. The transplanted kidney. Diagnostic and interventional radiology. Acta Radiol Diagn (Stockh) 1985;26:353–367. doi: 10.1177/028418518502600401. [DOI] [PubMed] [Google Scholar]
- Gedroyc W M, Reidy J F, Saxton H M. Arteriography of renal transplantation. Clin Radiol. 1987;38:239–243. doi: 10.1016/s0009-9260(87)80055-7. [DOI] [PubMed] [Google Scholar]
- Picus D, Neeley J P, McClennan B L, Weyman P J, Heiken J P. Intraarterial digital subtraction angiography of renal transplants. AJR Am J Roentgenol. 1985;145:93–96. doi: 10.2214/ajr.145.1.93. [DOI] [PubMed] [Google Scholar]
- Seymour H R, Matson M B, Belli A M, Morgan R, Kyriou J, Patel U. Rotational digital subtraction angiography of the renal arteries: technique and evaluation in the study of native and transplant renal arteries. Br J Radiol. 2001;74:134–141. doi: 10.1259/bjr.74.878.740134. [DOI] [PubMed] [Google Scholar]
- Hagen G, Wadstrom J, Magnusson A. 3D rotational angiography of transplanted kidneys. Acta Radiol. 2003;44:193–198. doi: 10.1080/j.1600-0455.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- Burgener F A, Schabel S I. The radiographic size of renal transplants. Radiology. 1975;117:547–550. doi: 10.1148/117.3.547. [DOI] [PubMed] [Google Scholar]
- Fletcher E W, Lecky J W. The radiological size of renal transplants—a retrospective study. Br J Radiol. 1969;42:892–898. doi: 10.1259/0007-1285-42-504-892. [DOI] [PubMed] [Google Scholar]
- Waybill M M, Waybill P N. Contrast media-induced nephrotoxicity: identification of patients at risk and algorithms for prevention. J Vasc Interv Radiol. 2001;12:3–9. doi: 10.1016/s1051-0443(07)61394-3. [DOI] [PubMed] [Google Scholar]
- Briguori C, Tavano D, Colombo A. Contrast agent–associated nephrotoxicity. Prog Cardiovasc Dis. 2003;45:493–503. doi: 10.1053/pcad.2003.YPCAD16. [DOI] [PubMed] [Google Scholar]
- Moresco K P, Patel N H, Namyslowski Y, Shah H, Johnson M S, Trerotola S O. Carbon dioxide angiography of the transplanted kidney: technical considerations and imaging findings. AJR Am J Roentgenol. 1998;171:1271–1276. doi: 10.2214/ajr.171.5.9798859. [DOI] [PubMed] [Google Scholar]
- Kerns S R, Hawkins I F, Jr, Sabatelli F W. Current status of carbon dioxide angiography. Radiol Clin North Am. 1995;33:15–29. [PubMed] [Google Scholar]
- Spinosa D J, Matsumoto A H, Angle J F, et al. Gadolinium-based contrast and carbon dioxide angiography to evaluate renal transplants for vascular causes of renal insufficiency and accelerated hypertension. J Vasc Interv Radiol. 1998;9:909–916. doi: 10.1016/s1051-0443(98)70421-x. [DOI] [PubMed] [Google Scholar]
- Hawkins I F, Jr, Wilcox C S, Kerns S R, Sabatelli F W. CO2 digital angiography: a safer contrast agent for renal vascular imaging? Am J Kidney Dis. 1994;24:685–694. doi: 10.1016/s0272-6386(12)80232-0. [DOI] [PubMed] [Google Scholar]
- Harward T R, Smith S, Hawkins I F, Seeger J M. Follow-up evaluation after renal artery bypass surgery with use of carbon dioxide arteriography and color-flow duplex scanning. J Vasc Surg. 1993;18:23–30. doi: 10.1067/mva.1993.41752. [DOI] [PubMed] [Google Scholar]
- Caridi J G, Hawkins I F., Jr CO2 digital subtraction angiography: potential complications and their prevention. J Vasc Interv Radiol. 1997;8:383–391. doi: 10.1016/s1051-0443(97)70577-3. [DOI] [PubMed] [Google Scholar]
- Weissleder R. Can gadolinium be safely given in renal failure? AJR Am J Roentgenol. 1996;167:278–279. doi: 10.2214/ajr.167.1.8659405. [DOI] [PubMed] [Google Scholar]
- Prince M R, Arnoldus C, Frisoli J K. Nephrotoxicity of high-dose gadolinium compared with iodinated contrast. J Magn Reson Imaging. 1996;6:162–166. doi: 10.1002/jmri.1880060129. [DOI] [PubMed] [Google Scholar]
- Niendorf H P, Haustein J, Cornelius I, Alhassan A, Clauss W. Safety of gadolinium-DTPA: extended clinical experience. Magn Reson Med. 1991;22:222–228. doi: 10.1002/mrm.1910220212. [DOI] [PubMed] [Google Scholar]
- Gemery J, Idelson B, Reid S, et al. Acute renal failure after arteriography with a gadolinium-based contrast agent. AJR Am J Roentgenol. 1998;171:1277–1278. doi: 10.2214/ajr.171.5.9798860. [DOI] [PubMed] [Google Scholar]
- Bakris G L, Lass N A, Glock D. Renal hemodynamics in radiocontrast medium-induced renal dysfunction: a role for dopamine-1 receptors. Kidney Int. 1999;56:206–210. doi: 10.1046/j.1523-1755.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- Tepel M, der Giet M van, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–184. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- Briguori C, Manganelli F, Scarpato P, et al. Acetylcysteine and contrast agent-associated nephrotoxicity. J Am Coll Cardiol. 2002;40:298–303. doi: 10.1016/s0735-1097(02)01958-7. [DOI] [PubMed] [Google Scholar]
- Roberts J P, Ascher N L, Fryd D S, et al. Transplant renal artery stenosis. Transplantation. 1989;48:580–583. [PubMed] [Google Scholar]
- Hohnke C, Abendroth D, Schleibner S, Land W. Vascular complications in 1,200 kidney transplantations. Transplant Proc. 1987;19:3691–3692. [PubMed] [Google Scholar]
- Benoit G, Moukarzel M, Hiesse C, Verdelli G, Charpentier B, Fries D. Transplant renal artery stenosis: experience and comparative results between surgery and angioplasty. Transpl Int. 1990;3:137–140. doi: 10.1007/BF00355459. [DOI] [PubMed] [Google Scholar]
- Gray D. Graft renal artery stenosis in the transplanted kidney. Transpl Rev. 1994;8:15–21. [Google Scholar]
- Sniderman K W, Sprayregen S, Sos T A, et al. Percutaneous transluminal dilation in renal transplant arterial stenosis. Transplantation. 1980;30:440–444. doi: 10.1097/00007890-198012000-00011. [DOI] [PubMed] [Google Scholar]
- Grossman R A, Dafoe D C, Shoenfeld R B, et al. Percutaneous transluminal angioplasty treatment of renal transplant artery stenosis. Transplantation. 1982;34:339–343. doi: 10.1097/00007890-198212000-00005. [DOI] [PubMed] [Google Scholar]
- Becker B N, Odorico J S, Becker Y T, et al. Peripheral vascular disease and renal transplant artery stenosis: a reappraisal of transplant renovascular disease. Clin Transplant. 1999;13:349–355. doi: 10.1034/j.1399-0012.1999.130412.x. [DOI] [PubMed] [Google Scholar]
- Braun W E. Long-term complications of renal transplantation. Kidney Int. 1990;37:1363–1378. doi: 10.1038/ki.1990.123. [DOI] [PubMed] [Google Scholar]
- Raine A E, Carter R, Mann J I, Morris P J. Adverse effect of cyclosporin on plasma cholesterol in renal transplant recipients. Nephrol Dial Transplant. 1988;3:458–463. doi: 10.1093/oxfordjournals.ndt.a091698. [DOI] [PubMed] [Google Scholar]
- Weigele J B. Iliac artery stenosis causing renal allograft-mediated hypertension: angiographic diagnosis and treatment. AJR Am J Roentgenol. 1991;157:513–515. doi: 10.2214/ajr.157.3.1831319. [DOI] [PubMed] [Google Scholar]
- Raynaud A, Bedrossian J, Remy P, Brisset J M, Angel C Y, Gaux J C. Percutaneous transluminal angioplasty of renal transplant arterial stenoses. AJR Am J Roentgenol. 1986;146:853–857. doi: 10.2214/ajr.146.4.853. [DOI] [PubMed] [Google Scholar]
- Greenstein S M, Verstandig A, McLean G K, et al. Percutaneous transluminal angioplasty. The procedure of choice in the hypertensive renal allograft recipient with renal artery stenosis. Transplantation. 1987;43:29–32. [PubMed] [Google Scholar]
- Patel N H, Jindal R M, Wilkin T, et al. Renal arterial stenosis in renal allografts: retrospective study of predisposing factors and outcome after percutaneous transluminal angioplasty. Radiology. 2001;219:663–667. doi: 10.1148/radiology.219.3.r01jn30663. [DOI] [PubMed] [Google Scholar]
- Halimi J M, Al-Najjar A, Buchler M, et al. Transplant renal artery stenosis: potential role of ischemia/reperfusion injury and long-term outcome following angioplasty. J Urol. 1999;161:28–32. doi: 10.1016/s0022-5347(01)62051-2. [DOI] [PubMed] [Google Scholar]
- Clements R, Evans C, Salaman J R. Percutaneous transluminal angioplasty of renal transplant artery stenosis. Clin Radiol. 1987;38:235–237. doi: 10.1016/s0009-9260(87)80052-1. [DOI] [PubMed] [Google Scholar]
- Chandrasoma P, Aberle A M. Anastomotic line renal artery stenosis after transplantation. J Urol. 1986;135:1159–1162. doi: 10.1016/s0022-5347(17)46023-x. [DOI] [PubMed] [Google Scholar]
- Matalon T A, Thompson M J, Patel S K, Brunner M C, Merkel F K, Jensik S C. Percutaneous transluminal angioplasty for transplant renal artery stenosis. J Vasc Interv Radiol. 1992;3:55–58. doi: 10.1016/s1051-0443(92)72186-1. [DOI] [PubMed] [Google Scholar]
- Bertoni E, Zanazzi M, Rosat A, et al. Efficacy and safety of Palmaz stent insertion in the treatment of renal artery stenosis in kidney transplantation. Transpl Int. 2000;13(suppl 1):S425–S430. doi: 10.1007/s001470050376. [DOI] [PubMed] [Google Scholar]
- Newman-Sanders A P, Gedroyc W G, al-Kutoubi M A, Koo C, Taube D. The use of expandable metal stents in transplant renal artery stenosis. Clin Radiol. 1995;50:245–250. doi: 10.1016/s0009-9260(05)83479-8. [DOI] [PubMed] [Google Scholar]
- Sierre S D, Raynaud A C, Carreres T, Sapoval M R, Beyssen B M, Gaux J C. Treatment of recurrent transplant renal artery stenosis with metallic stents. J Vasc Interv Radiol. 1998;9:639–644. doi: 10.1016/s1051-0443(98)70335-5. [DOI] [PubMed] [Google Scholar]
- Martin L G, Rundback J H, Sacks D, et al. Quality improvement guidelines for angiography, angioplasty, and stent placement in the diagnosis and treatment of renal artery stenosis in adults. J Vasc Interv Radiol. 2002;13:1069–1083. doi: 10.1016/s1051-0443(07)61947-2. [DOI] [PubMed] [Google Scholar]