Abstract
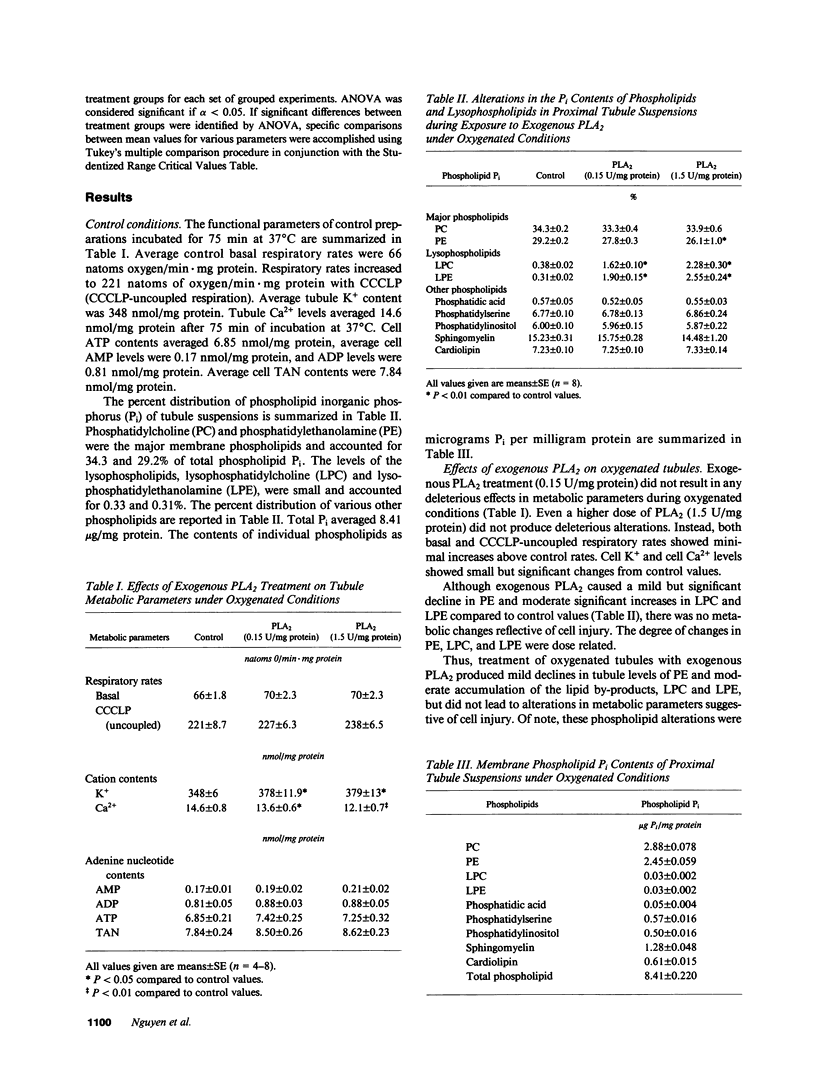
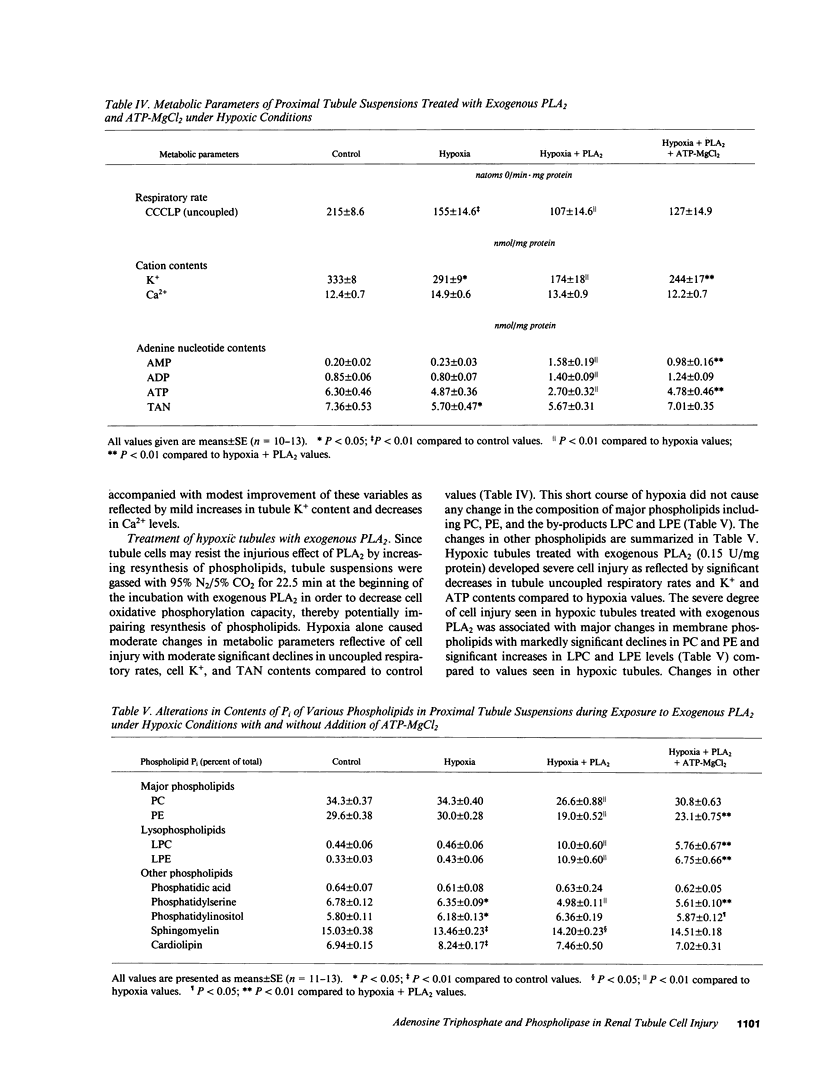
The pathogenesis of ischemic renal tubular cell injury involves a complex interaction of different processes, including membrane phospholipid alterations and depletion of high-energy phosphate stores. To assess the role of membrane phospholipid changes due to activation of phospholipases in renal tubule cell injury, suspensions enriched in rabbit renal proximal tubule segments were incubated with exogenous phospholipase A2 (PLA2). Exogenous PLA2 did not produce any significant change in various metabolic parameters reflective of cell injury in control nonhypoxic preparations despite a significant decrease in phosphatidylethanolamine (PE) and moderate increases in lysophosphatidylcholine (LPC) and lysophosphatidylethanolamine (LPE). In contrast, exogenous PLA2 treatment of hypoxic tubules resulted in a severe degree of cell injury, as demonstrated by marked declines in tubule K+ and ATP contents and significant decreases in tubule uncoupled respiratory rates, and was associated with significant phospholipid alterations, including marked declines in phosphatidylcholine (PC) and PE and significant rises in LPC, LPE, and free fatty acids (FFA). The injurious metabolic effects of exogenous PLA2 on hypoxic tubules were reversed by addition of ATP-MgCl2 to the tubules. The protective effect of ATP-MgCl2 was associated with increases in tubule PC and PE contents and declines in LPC, LPE, and FFA contents. These experiments thus indicate that an increase in exogenous PLA2 activity produces renal proximal tubule cell injury when cell ATP levels decline, at which point phospholipid resynthesis cannot keep pace with phospholipid degradation with resulting depletion of phospholipids and accumulation of lipid by-products. High-energy phosphate store depletion appears to be an important condition for exogenous PLA2 activity to induce renal tubule cell injury.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. M., Coffey A. K. Protection of kidneys from acute renal failure resulting from normothermic ischemia. Lab Invest. 1983 Jul;49(1):87–98. [PubMed] [Google Scholar]
- Arnsdorf M. F., Sawicki G. J. The effects of lysophosphatidylcholine, a toxic metabolite of ischemia, on the components of cardiac excitability in sheep Purkinje fibers. Circ Res. 1981 Jul;49(1):16–30. doi: 10.1161/01.res.49.1.16. [DOI] [PubMed] [Google Scholar]
- Baker R., Dowdall M. J., Whittaker V. P. The incorporation of radioactive fatty acids and of radioactive derivatives of glucose into the phospholipids of subsynaptosomal fractions of cerebral cortex. Biochem J. 1976 Jan 15;154(1):65–75. doi: 10.1042/bj1540065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- Chien K. R., Han A., Sen A., Buja L. M., Willerson J. T. Accumulation of unesterified arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation-reacylation cycle and the depletion of membrane phospholipids. Circ Res. 1984 Mar;54(3):313–322. doi: 10.1161/01.res.54.3.313. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Reeves J. P., Buja L. M., Bonte F., Parkey R. W., Willerson J. T. Phospholipid alterations in canine ischemic myocardium. Temporal and topographical correlations with Tc-99m-PPi accumulation and an in vitro sarcolemmal Ca2+ permeability defect. Circ Res. 1981 May;48(5):711–719. doi: 10.1161/01.res.48.5.711. [DOI] [PubMed] [Google Scholar]
- Colvin R. A. Deinhibition of cardiac Na+-K+-ATPase after exposure to exogenous phospholipase A2. Am J Physiol. 1987 Jan;252(1 Pt 2):H32–H39. doi: 10.1152/ajpheart.1987.252.1.H32. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Gross R. W., Sobel B. E. Amphipathic metabolites and membrane dysfunction in ischemic myocardium. Circ Res. 1984 Aug;55(2):135–154. doi: 10.1161/01.res.55.2.135. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B., Hope M. J., Nayar R., Schmid S. L. Phospholipids and membrane transport. Can J Biochem. 1980 Oct;58(10):1091–1100. doi: 10.1139/o80-147. [DOI] [PubMed] [Google Scholar]
- Dahl J. L., Hokin L. E. The sodium-potassium adenosinetriphosphatase. Annu Rev Biochem. 1974;43(0):327–356. doi: 10.1146/annurev.bi.43.070174.001551. [DOI] [PubMed] [Google Scholar]
- Dalton S., Hughes B. P., Barritt G. J. Effects of lysophospholipids on Ca2+ transport in rat liver mitochondria incubated at physiological Ca2+ concentrations in the presence of Mg2+, phosphate and ATP at 37 degrees C. Biochem J. 1984 Dec 1;224(2):423–430. doi: 10.1042/bj2240423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Farber J. L., Young E. E. Accelerated phospholipid degradation in anoxic rat hepatocytes. Arch Biochem Biophys. 1981 Oct 1;211(1):312–320. doi: 10.1016/0003-9861(81)90459-8. [DOI] [PubMed] [Google Scholar]
- Finkelstein S. D., Gilfor D., Farber J. L. Alterations in the metabolism of lipids in ischemia of the liver and kidney. J Lipid Res. 1985 Jun;26(6):726–734. [PubMed] [Google Scholar]
- Gaudio K. M., Taylor M. R., Chaudry I. H., Kashgarian M., Siegel N. J. Accelerated recovery of single nephron function by the postischemic infusion of ATP-MgCl2. Kidney Int. 1982 Jul;22(1):13–20. doi: 10.1038/ki.1982.126. [DOI] [PubMed] [Google Scholar]
- Green D. E., Fry M., Blondin G. A. Phospholipids as the molecular instruments of ion and solute transport in biological membranes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):257–261. doi: 10.1073/pnas.77.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. I., Balaban R. S., Barrett L., Mandel L. J. Mitochondrial respiratory capacity and Na+- and K+-dependent adenosine triphosphatase-mediated ion transport in the intact renal cell. J Biol Chem. 1981 Oct 25;256(20):10319–10328. [PubMed] [Google Scholar]
- Hattori M., Ogawa K., Satake T., Sugiyama S., Ozawa T. Depletion of membrane phospholipid and mitochondrial dysfunction associated with coronary reperfusion. Basic Res Cardiol. 1985 May-Jun;80(3):241–250. doi: 10.1007/BF01907900. [DOI] [PubMed] [Google Scholar]
- Humes H. D., Hunt D. A., White M. D. Direct toxic effect of the radiocontrast agent diatrizoate on renal proximal tubule cells. Am J Physiol. 1987 Feb;252(2 Pt 2):F246–F255. doi: 10.1152/ajprenal.1987.252.2.F246. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Messineo F. C. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res. 1981 Jan;48(1):1–16. doi: 10.1161/01.res.48.1.1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsubara C., Nishikawa Y., Yoshida Y., Takamura K. A spectrophotometric method for the determination of free fatty acid in serum using acyl-coenzyme A synthetase and acyl-coenzyme A oxidase. Anal Biochem. 1983 Apr 1;130(1):128–133. doi: 10.1016/0003-2697(83)90659-0. [DOI] [PubMed] [Google Scholar]
- Matthys E., Patel Y., Kreisberg J., Stewart J. H., Venkatachalam M. Lipid alterations induced by renal ischemia: pathogenic factor in membrane damage. Kidney Int. 1984 Aug;26(2):153–161. doi: 10.1038/ki.1984.149. [DOI] [PubMed] [Google Scholar]
- Mittnacht S., Jr, Farber J. L. Reversal of ischemic mitochondrial dysfunction. J Biol Chem. 1981 Apr 10;256(7):3199–3206. [PubMed] [Google Scholar]
- Rehncrona S., Westerberg E., Akesson B., Siesjö B. K. Brain cortical fatty acids and phospholipids during and following complete and severe incomplete ischemia. J Neurochem. 1982 Jan;38(1):84–93. doi: 10.1111/j.1471-4159.1982.tb10857.x. [DOI] [PubMed] [Google Scholar]
- Rouser G., Simon G., Kritchevsky G. Species variations in phospholipid class distribution of organs. I. Kidney, liver and spleen. Lipids. 1969 Nov;4(6):599–606. doi: 10.1007/BF02531047. [DOI] [PubMed] [Google Scholar]
- Rubalcava B., Rodbell M. The role of acidic phospholipids in glucagon action on rat liver adenylate cyclase. J Biol Chem. 1973 Jun 10;248(11):3831–3837. [PubMed] [Google Scholar]
- Shug A. L., Shrago E., Bittar N., Folts J. D., Koke J. R. Acyl-CoA inhibition of adenine nucleotide translocation in ischemic myocardium. Am J Physiol. 1975 Mar;228(3):689–692. doi: 10.1152/ajplegacy.1975.228.3.689. [DOI] [PubMed] [Google Scholar]
- Siegel N. J., Glazier W. B., Chaudry I. H., Gaudio K. M., Lytton B., Baue A. E., Kashgarian M. Enhanced recovery from acute renal failure by the postischemic infusin of adenine nucleotides and magnesium chloride in rats. Kidney Int. 1980 Mar;17(3):338–349. doi: 10.1038/ki.1980.39. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Snowdowne K. W., Freudenrich C. C., Borle A. B. The effects of anoxia on cytosolic free calcium, calcium fluxes, and cellular ATP levels in cultured kidney cells. J Biol Chem. 1985 Sep 25;260(21):11619–11626. [PubMed] [Google Scholar]
- Troyer D. A., Kreisberg J. I., Venkatachalam M. A. Lipid alterations in LLC-PK1 cells exposed to mercuric chloride. Kidney Int. 1986 Feb;29(2):530–538. doi: 10.1038/ki.1986.31. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Harding P. G., Humes H. D. Alterations in renal cortex cation homeostasis during mercuric chloride and gentamicin nephrotoxicity. Exp Mol Pathol. 1983 Aug;39(1):43–60. doi: 10.1016/0014-4800(83)90040-0. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Humes H. D. Increases of cell ATP produced by exogenous adenine nucleotides in isolated rabbit kidney tubules. Am J Physiol. 1986 Apr;250(4 Pt 2):F720–F733. doi: 10.1152/ajprenal.1986.250.4.F720. [DOI] [PubMed] [Google Scholar]
- van der Vusse G. J., Roemen T. H., Prinzen F. W., Coumans W. A., Reneman R. S. Uptake and tissue content of fatty acids in dog myocardium under normoxic and ischemic conditions. Circ Res. 1982 Apr;50(4):538–546. doi: 10.1161/01.res.50.4.538. [DOI] [PubMed] [Google Scholar]


