ABSTRACT
This article describes the current state-of-the-art technique of percutaneous transplant renal biopsy. A brief overview of the history of transplant renal biopsy is given. The indications and contraindications are discussed, including pre- and postprocedure patient management. The technique of the procedure and the devices that are available in the market are described.
Keywords: Biopsy, transplant kidney, technique
Each year, some 12,000 patients undergo primary renal transplantation.1 The renal transplant survival rate has increased significantly with improvement in immunosuppressive medications.2 Several methods have been used to diagnose renal allograft dysfunction, including clinical evaluation and laboratory tests; however, core biopsy remains the “gold standard” for the diagnosis of renal transplant abnormality.
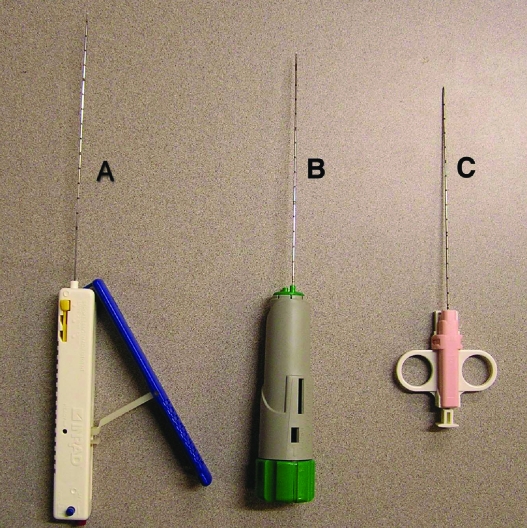
Iversen and Bran first introduced the percutaneous technique for renal biopsy over 50 years ago.3 Historically, renal biopsy has been performed with relatively large 14- or 15-gauge needles using a manual technique. The needles widely used in the past included the Jamshidi, Tru-Cut, and Silverman. Larger bore biopsy needles have been associated with higher complication rates. Automated and semiautomated biopsy devices have been introduced and are being used more commonly (Fig. 1).4
Figure 1.
(A) Express Core Biopsy Device, Inrad® (Kentwood, MI). The biopsy device comes with a blue handle, which is pulled as shown in the figure to cock the biopsy device. After the blue handle locks, it is pushed back to its resting position. The device is now cocked and ready to fire. The red button at the back of the device is the firing trigger. There is a safety lock seen as a blue button at the back of the handle. The device can be adjusted to have a 13-mm, 23-mm, and 33-mm throw. (B) Bard® Monopty® biopsy instrument (C. R. Bard Inc., Covington, GA). To cock the biopsy device, the green knob at the back of the handle is twisted twice in the clockwise direction. The pink button also at the back of the handle is pressed to fire the device. This device has a 22-mm throw. (C) Single Action Biopsy Device (US Biopsy, LLC., Franklin, IN). This device is cocked by pulling on the plunger at the back of the handle. The plunger is then advanced to a point of resistance. The device is cocked in this position and is fired by pushing the plunger all the way. This device has a 20-mm throw.
Because a finite risk is associated with core renal biopsy, alternative noninvasive imaging techniques have been investigated to screen for and diagnose renal allograft dysfunction. Nampoory et al compared the predictive accuracy of Doppler ultrasonography, renal scintigraphy, and fine-needle aspiration (FNA) biopsy against the gold standard of core needle biopsy for the diagnosis of acute renal allograft rejection.5 The authors claimed the diagnostic accuracy for ultrasound, renal scintigram, and FNA biopsy to be 67.4%, 80.5%, and 74.4%, respectively. A higher diagnostic yield was credited to core renal biopsy compared with FNA biopsy and monoclonal antibody staining by McConnell et al.6
Histological markers of acute allograft rejection include intimal arteritis, interstitial inflammation, and tubulitis.7 For the diagnosis of cellular rejection, a FNA biopsy sample is examined for leukocyte infiltration and the antigen load. In comparing FNA biopsy with core biopsy, Brown et al indicated lack of diagnostic accuracy of the results obtained by FNA.8 Renowden et al studied renal allograft rejection with Doppler ultrasonography. The authors found an early to middiastolic notch in the arterial Doppler waveform of the patients who had biopsy proven cellular rejection. This finding was highly specific but not very sensitive for cellular rejection. The early diastolic notch was present even in patients with a normal resistive index of 0.7 or less.9
INDICATIONS AND CONTRAINDICATIONS OF TRANSPLANT RENAL BIOPSY
Transplant renal biopsy is generally performed when an acute or chronic renal allograft rejection is suspected. The main clinical indicator of renal allograft dysfunction is a trend toward increasing serum creatinine levels above a baseline value. A single abnormal laboratory value generally does not warrant renal biopsy. Some authors have suggested an increase in serum creatinine of 25% above the baseline as in indication for transplant renal biopsy.10
Other early clinical indicators of allograft rejection include unexplained fever, edema, hypertension, eosinophilia, oliguria, anuria, and proteinuria unrelated to glomerulonephritis. Clinical criteria alone cannot predict graft dysfunction in 50% to 70% of the patients; therefore, histological confirmation of the diagnosis is often required.11,12,13
Some authors have recommended protocol transplant biopsies at scheduled intervals despite normal renal function to diagnose subclinical allograft dysfunction. In one study, the authors performed 228 transplant biopsies on 108 patients. They concluded that by performing protocol transplant renal biopsies, their group was able to diagnose and treat clinically silent acute rejection in 13% and chronic rejection in 52% of their patients.14 Some authors believe that the presence of subclinical inflammation contributes to chronic graft sclerosis.15 However, the clinical significance of early detection of silent inflammatory infiltrates in stable allografts remains controversial.16
Although transplant renal biopsies have also been recommended to assess the response to antirejection therapy, the role of such an indication remains controversial.11,16 The difficulty in determining the response to antirejection therapy lies in the lack of understanding of the significance of silent inflammatory infiltrates in allograft biopsy specimens and the dearth of knowledge about the time frame involved in resolution of inflammatory infiltrates after successful antirejection therapy. Indeed, delayed resolution of histological changes consistent with allograft rejection and persistent inflammatory infiltrates have been found in up to 30% of protocol biopsies after clinical resolution of the symptoms.17
Hemorrhage is the major complication associated with renal transplant biopsy. The rate of both occult and frank hematuria ranges between 5 and 40%. Performance of biopsy in the presence of uncorrected coagulopathy predisposes the patient to a significantly higher risk of bleeding. Sharma et al studied the risk of bleeding in patients with a low platelet count undergoing solid organ biopsy. In a study involving 87 patients, the authors demonstrated that in the presence of otherwise normal clotting factors, a platelet count less than 60,000/mm3 predisposed patients to a significantly higher risk of bleeding.18 In the presence of a normal platelet count, an international normalized ratio (INR) of 1.3 or greater has been documented to increase bleeding complications significantly.19
In an attempt to manage bleeding complications in coagulopathic patients, authors have employed several techniques including injecting gel foam and fibrin in the needle tract as well as electrocautery of the needle tract.20,21,22,23,24,25
A relative contraindication to transplant renal biopsy is bacteremia. Biopsy of the transplanted kidney in the presence of bacteremia is controversial. Because infection may be a small complication of transplant renal biopsy, at our institute transplant renal biopsy in patients with bacteremia is delayed until the infection is brought under control.26
TECHNIQUE OF TRANSPLANT RENAL BIOPSY
Several techniques for transplant renal biopsies have been published. Use of real-time ultrasound guidance in the performance of percutaneous renal allograft biopsy is now almost universal; however, the wide-scale acceptance of this technique is relatively recent. Journal articles claiming superiority of real-time ultrasound guidance for renal biopsy over “blinded” techniques started being published in the early and mid-1990s.27,28 As late as the early 1990s, physicians routinely performed transplant renal biopsies without real-time imaging guidance.29 Blind biopsies were performed by nonradiologists using physical palpation, anatomical landmarks, or after the skin entry site was marked with ultrasound by a radiologist.
Although ultrasound guidance is now almost universal because of the ease of use and versatility of this technique, computed tomography (CT) has also been used to guide core needle biopsies in cases in which ultrasound guidance was suboptimal because of the patient's body habitus.30 In the author's opinion, CT guidance not only exposes the patient to unnecessary radiation but also is also more expensive and time consuming.
An alternative technique of renal biopsy was used by Mimata et al.31 In a pilot study, the authors performed renal calyx puncture in a retrograde fashion, passing the wire transcutaneously using ureteroscopic guidance and then inserted a guiding needle over the wire. Through this guiding needle, a core biopsy device was inserted and biopsy performed. A 7 French catheter was inserted in the renal pelvis to achieve hemostasis by tamponading the tract. In this author's opinion, this technique is much more traumatic with a higher potential for complications and a low likelihood of a positive yield because the needle tract traverses the renal cortex at a right angle, minimizing the thickness of the cortex the biopsy device traverses. Another intriguing technique described by Leal involves transurethral biopsy of the native kidney.32 Theoretically, this technique could be employed in transplanted kidneys as well.
At the Indiana University hospital, the vast majority of renal biopsies are performed on an outpatient basis in the morning. The patients are admitted to the outpatient surgical unit after an overnight fast; preprocedure samples for laboratory studies are drawn (if not already available), an intravenous access is secured, and patients are observed after the procedure until discharge. In addition to review of the patient's medical records and analysis of laboratory results, a detailed history is obtained to ascertain the absence of familial or drug-induced coagulopathy and active infections. If such issues are present and it is clinically warranted, the biopsy is postponed until the issues are resolved. A physical examination is performed with emphasis on the assessment of cardiovascular and respiratory systems.
By convention, we require a minimum platelet count of 70,000/mm3 and an INR of 1.3 or less for solid organ biopsies including transplant renal biopsies. An uncorrectable coagulopathy is considered an absolute contraindication to renal biopsy. We attempt to correct thrombocytopenia by platelet transfusion tailored to the severity of the abnormality. To reduce the hemorrhagic risk of medication-induced thrombocythemia, aspirin is withheld for 3 to 5 days prior to the biopsy under elective circumstances. Outpatients with an INR ≥ 1.3 who are receiving warfarin (Coumadin®) have many options to reverse their coagulopathy transiently after withholding this agent: (1) anticoagulation with enoxaparin (Lovenox®) for 5–7 days and then withholding it 24 hours before and after biopsy and (2) vitamin K or fresh frozen plasma (FFP) administration, or both. For inpatients anticoagulated with intravenous heparin, the procedure is performed after interrupting heparin for 2 hours before and after the procedure without the need for activated partial thromboplastin time (aPTT) repetition because of heparin's pharmacokinetics. Bleeding time is not tested as its utility as a surrogate for hemorrhage risk associated with renal biopsy is unknown
Transplant renal biopsy is performed in the ultrasonography suite by an interventional radiologist. The operating physician scans the transplant kidney for any abnormality suggesting the etiology of transplant dysfunction such as hydronephrosis or a perinephric fluid collection. If no such cause of renal allograft dysfunction is found, the operator proceeds with the biopsy.
The transplanted kidney lies in the iliac fossa overlying the iliopsoas muscle in the extraperitoneal space. In patients receiving repeated renal transplants, the kidney may be placed intraperitoneally. The outer convex curvature of the kidney projects laterally, and the concave renal hilum projects medially. The anterior surface of the kidney is separated from the skin over the anterior abdominal wall by transversalis fascia, three layers of abdominal muscles, and subcutaneous tissue and is thus quite superficial depending on the thickness of the subcutaneous fat.
The skin over the transplanted kidney is prepared and draped in normal fashion. Vital signs are routinely monitored; however, conscious sedation is generally not administered to patients because of the lack of pain-sensitive surrounding fascia. The skin overlying the trajectory for the biopsy needle is infiltrated with 1% buffered lidocaine solution as a local anesthetic.
We perform biopsy at either the upper or lower pole of the transplanted kidney depending on the ease of access. The trajectory of the needle is chosen so that the entirety of its intrarenal course traverses only the renal cortex. This minimizes the risk of damage to major renal vessels (Fig. 2). The path of the needle in the renal parenchyma is oblique to the renal surface, maximizing the thickness of renal cortex that the needle traverses. Such a trajectory, in which the entirety of the needle passes only though the renal cortex, is possible with ease only at the renal poles.
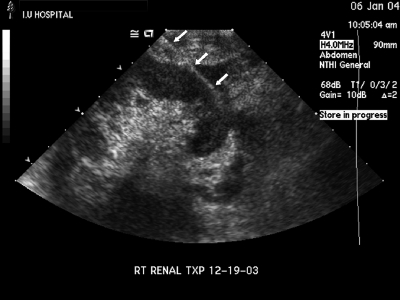
Figure 2.
The entire needle tract from the skin to the needle tip is demonstrated (arrowheads). The tract traverses the hypoechoic peripheral rind of renal cortex. The hyperechoic renal sinus complex situated more centrally is completely avoided. The figure also demonstrates the greater thickness of cortex available at the renal poles. An oblique needle approach as depicted in this figure traverses the greatest thickness of the cortex, thus maximizing the diagnostic yield, and at the same time avoids the larger vessels situated in the renal sinus complex.
The use of a guide mounted on the ultrasound probe that predicts the path of the needle is a matter of personal preference. It restricts the number of potential trajectories but maybe useful for those uncomfortable with the freehand technique of ultrasound-guided biopsy. A small dermatotomy with a no. 11 blade is created to allow the biopsy needle to be inserted in the subcutaneous tissue. The intracorporeal course of the needle is monitored in real time with ultrasound. The tip of the biopsy needle is introduced a few millimeters into the renal cortex before the gun is fired to avoid ricocheting off the tough fibrous renal pseudocapsule. Usually, a second pass is made in a similar fashion to obtain adequate tissue for histological analysis. The biopsy specimen is then examined visually as well as under a dissecting microscope for assessment of the adequacy of the biopsy sample by the cytopathologist or technologist. Postprocedure ultrasound assessment is performed immediately before termination of the procedure to identify any potential complication such as a perinephric or intraparenchymal hematoma, after which patients are returned to the day care surgery unit for postprocedure monitored convalescence that entails 4 hours of bed rest and analgesia as required. If this is uneventful, they are discharged without further examination. Patients are warned that they may experience mild transient hematuria and discomfort at the biopsy site. If patients complain of worsening pain or experience gross hematuria, they are brought back to the ultrasound suite and are scanned again. Appropriate treatment is prescribed according to the ultrasound findings and the clinical scenario.
SAMPLE ADEQUACY
A minimum of 5–10 intact glomeruli are required to make an adequate histological diagnosis.33,34 In a study involving 108 subjects, Yoshinari et al demonstrated that each 10-mm length of a 0.5-mm-diameter specimen contained a mean of 11.9 ± 5 glomeruli. Therefore, an average 1-cm length of specimen is required for adequate tissue diagnosis.35 The authors also suggest that an on-site microscope be available to determine the adequacy of the biopsy specimen.
In a study performed with 16-gauge biopsy needles, Chan et al demonstrated that radiologists performing the procedure were able to predict the adequacy of the sample in 88.4% of cases by visual inspection of the biopsy sample only. However, they recommend the complementary presence of a histopathology technologist during the procedure to virtually eliminate the need for repeated biopsy because of inadequate sampling.36
In a study performed on autopsy kidney specimens, Hopper et al demonstrated that the larger 14-gauge Tru-Cut and Jamshidi needles yielded a larger number of intact glomeruli but were also associated with a greater crush artifact than with smaller 18-gauge needles.37 They concluded that biopsy specimens obtained by 18-gauge automated biopsy devices yielded an adequate number of glomeruli for histological diagnosis. Boyvat et al compared the adequacy of biopsy samples in 250 consecutive transplant renal biopsies. Of these, 102 biopsies were performed with conventional 14-gauge needles and the subsequent 148 biopsies were performed with an automated 18-gauge biopsy device. They found that the yield of glomeruli was slightly higher with the smaller 18-gauge needle and the rate of complications was lower. However, neither of these results reached statistical significance.38
COMPLICATIONS OF TRANSPLANT RENAL BIOPSY
The rate of complication secondary to transplant renal biopsy has been variously reported between 0.06 and 13%.27,41,42,43,44,45 The variation in the frequency of the reported complications depends on multiple factors including the experience of the operator, utilization of imaging guidance, gauge of the biopsy needle, and a proactive effort on the part of the operator to pursue subclinical complications by follow-up imaging.
For each patient, the benefit of histological confirmation of allograft rejection needs to be weighed against the risks of procedure-related complications. Hemorrhage is the predominant complication related to transplant biopsy and may occur acutely as microscopic or gross hematuria or subcapsular hematoma. The rate of frank and occult hematuria secondary to renal biopsy has been reported to be between 5 and 40%.39,40 Arterial injury may also result in arteriovenous fistulae or a pseudoaneurysm.. A subcapsular fluid collection is generally managed conservatively but may require percutaneous or operative intervention if there is evidence of compromised renal function. A small minority of patients with a decrease in their hemoglobin levels or hemodynamic instability may even require blood product transfusion. Loss of a renal allograft as a complication of biopsy is rare.
Transplant renal biopsy carries a lower complication rate than native renal biopsy.4 Among the 458 patients studied, with 315 in the native kidney arm and 143 in the transplant kidney arm, the complication rate was 28.9% in the native kidney arm and 19.5% in the transplant kidney arm. The transplanted kidney is much more superficial than the native kidney, separated from the skin only by a thin layer of muscle, fascia, and subcutaneous tissue of the anterior abdominal wall, and is therefore much better visualized because of less acoustic impedance of these structures. In addition, because the biopsy needle has to travel through a thinner layer of tissue, the operator has better directional control.
Many authors have documented the safety and efficacy of the smaller 18-gauge automated biopsy needle compared with the conventional Tru-Cut biopsy needles.27,38,45 In the pediatric patient population, the safety and efficacy of percutaneous renal biopsy using a smaller 18-gauge needle over conventional larger needles has been proved by Kersnik Levart et al.43
Given the higher complication rate of previous renal biopsy techniques, patients were traditionally admitted to the hospital overnight after the procedure for observation. However, with the advent of safer biopsy devices with smaller gauges, combined with improvements in renal localization and real-time visualization of the process of tissue acquisition, the complication rates have decreased.27,45 This, aided by increasing health care costs, has prompted many physicians to perform renal biopsy as an outpatient procedure.44 The safety and cost benefits of renal biopsy as an outpatient procedure have been well described.46,47,48,49,50 Therefore, as the number of renal transplant patients increases, so will the utilization of this technique in care of these patients.51,52,53,54
ABBREVIATIONS
aPTT activated partial thromboplastin time
CT computed tomography
INR international normalized ratio
FFP fresh frozen plasma
FNA fine-needle aspiration
PT prothrombin time
REFERENCES
- Report, O.S.A. UNOS: URREA; 2002.
- Cecka J. In: Cecka E, Terasaki, editor. Clinical Transplants. Los Angeles: UCLA Immunogenetics Center; 2002. The UNOS renal transplant registry.
- Iversen P, Bran C. Aspiration biopsy of the kidney. Am J Med. 1951;11:324. doi: 10.1016/0002-9343(51)90169-6. [DOI] [PubMed] [Google Scholar]
- Riehl J, Maigatter S, Kierdorf H, Schmitt H, Maurin N, Sieberth H. Percutaneous renal biopsy: comparison of manual and automated puncture techniques with native and transplanted kidney. Nephrol Dial Transplant. 1994;9:1568–1574. [PubMed] [Google Scholar]
- Nampoory M, Das D, Johny K, et al. Comparative study of ultrasonogram, renogram, and fine needle aspiration cytology in diagnosis of acute allograft rejection. Transplant Proc. 1997;29:2807–2811. doi: 10.1016/s0041-1345(97)00688-x. [DOI] [PubMed] [Google Scholar]
- McConnell J, Sagalowsky A, Lewis S, et al. Prospective evaluation of renal allograft dysfunction with 99mtechnetium-diethylenetriaminepentaacetic acid renal scans. J Urol. 1984;131:875–879. doi: 10.1016/s0022-5347(17)50691-6. [DOI] [PubMed] [Google Scholar]
- Solez K, Axelsen R, Benediktsson H, et al. International standardization of criteria for the histologic classification of kidney transplant pathology. Kidney Int. 1993;44:411–422. doi: 10.1038/ki.1993.259. [DOI] [PubMed] [Google Scholar]
- Brown S, Horsburgh T, Veitch P, Bell P. Comparison of fine-needle aspiration biopsy and Tru-cut biopsy performed under ultrasound guidance. Transplant Proc. 1988;20:595–596. [PubMed] [Google Scholar]
- Renowden S, Griffiths D, Nair S, Krishnan H, Cochlin D. Renal transplant sonography: correlation of Doppler and biopsy results in cellular rejection. Clin Radiol. 1992;46:265–269. doi: 10.1016/s0009-9260(05)80168-0. [DOI] [PubMed] [Google Scholar]
- Mazzali M, Ribeiro-Alves M A, Alves Filho G. Percutaneous renal graft biopsy: a clinical laboratory and pathological analysis. Sao Paulo Med J. 1999;117:57–62. doi: 10.1590/s1516-31801999000200003. [DOI] [PubMed] [Google Scholar]
- Parfrey P, Kuo Y, Hanley J, et al. The diagnostic and prognostic value of renal allograft biopsy. Transplantation. 1984;38:586–590. doi: 10.1097/00007890-198412000-00007. [DOI] [PubMed] [Google Scholar]
- Al-Awwa I, Hariharan S, First M. The importance of allograft biopsy in renal transplant recipients. Am J Kidney Dis. 1998;31:S15–S18. doi: 10.1053/ajkd.1998.v31.pm9631859. [DOI] [PubMed] [Google Scholar]
- Kiss D, Landmann J, Mihatsch M, Huser B, Brunner F, Thiel G. Risks and benefits of graft biopsy in renal transplantation under cyclosporine-A. Clin Nephrol. 1992;38:132–134. [PubMed] [Google Scholar]
- Schwartz A, Mengel M, Gwinner W, et al. Protocol biopsy program after renal transplantation: structure and first results. Transplant Proc. 2002;34:2238–2239. doi: 10.1016/s0041-1345(02)03286-4. [DOI] [PubMed] [Google Scholar]
- Rush D, Jeffery J, Gough J. Sequential protocol biopsies in renal transplant patients: clinico-pathological correlations using the Banff schema. Transplantation. 1995;59:511–514. [PubMed] [Google Scholar]
- Gaber L. Role of renal allograft biopsy in multicentre clinical trials in transplantation. Am J Kidney Dis. 1998;31:S19–S25. doi: 10.1053/ajkd.1998.v31.pm9631860. [DOI] [PubMed] [Google Scholar]
- Chavers B, Reinsmoen N, Mauer S, Najarian J, Maltas A. High incidence of histologically-defined renal allograft rejection in asymptomatic patients following treatment. Presented at the American Society of Transplant Physicians 12th Annual Meeting; 1993.
- Sharma P, McDonald G B, Banaji M. The risk of bleeding after percutaneous liver biopsy: relation to platelet count. J Clin Gastroenterol. 1982;4:451–453. doi: 10.1097/00004836-198210000-00011. [DOI] [PubMed] [Google Scholar]
- Gilmore I T, Burroughs A, Murray-Lyon I M, Williams R, Jenkins D, Hopkins A. Percutaneous liver biopsy in England and Wales; an audit by the British Society of Gastroenterology and Royal College of Physicians of London. Gut. 1995;36:437–441. doi: 10.1136/gut.36.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falstrom J, Moore M M, Caldwell S H, Matsumoto A H, Abbott R D, Spotnitz W D. Use of fibrin sealant to reduce bleeding after needle liver biopsy in an anticoagulated canine model: work in progress. J Vasc Interv Radiol. 1999;10:457–462. doi: 10.1016/s1051-0443(99)70065-5. [DOI] [PubMed] [Google Scholar]
- Kim E H, Kopecky K K, Cummings O W, Dreesen R G, Pound D C. Electrocautery of the tract after needle biopsy of the liver to reduce blood loss: experience in the canine model. Invest Radiol. 1993;28:228–230. doi: 10.1097/00004424-199303000-00008. [DOI] [PubMed] [Google Scholar]
- Chisholm R, Jones S, Lees W. Fibrin sealant as a plug for the post liver biopsy needle track. Clin Radiol. 1989;40:627–628. doi: 10.1016/s0009-9260(89)80326-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez Fuchs C A, Bruno M. Plugging liver biopsy sites with coagulation factors. Lancet. 1987;2:1087. doi: 10.1016/s0140-6736(87)91512-1. [DOI] [PubMed] [Google Scholar]
- Tabuse Y, Tabuse K, Mori K, et al. Percutaneous microwave tissue coagulation in liver biopsy: experimental and clinical studies. Nippon Geka Hokan. 1986;55:381–392. [PubMed] [Google Scholar]
- Riley S A, Ellis W R, Irving H C, Lintott D J, Axon A T, Losowsky M S. Percutaneous liver biopsy with plugging of needle track: a safe method for use in patients with impaired coagulation. Lancet. 1984;2:436. doi: 10.1016/s0140-6736(84)92910-6. [DOI] [PubMed] [Google Scholar]
- Klauser R, Zlabinger G J, Traindl O, et al. Influence of immunosuppressive therapy on infectious complications in renal transplant recipients. Transplant Proc. 1992;24:292–294. [PubMed] [Google Scholar]
- Nyman R, Cappelen-Smith J, Al Suhaibani H, Alfurayh O, Shakweer W, Akhtar M. Yield and complications in percutaneous renal biopsy. A comparison between ultrasound-guided gun-biopsy and manual techniques in native and transplant kidneys. Acta Radiol. 1997;38:431–436. doi: 10.1080/02841859709172096. [DOI] [PubMed] [Google Scholar]
- Cozens M, Murchison J, Allen P, Winney R. Conventional 15 G needle technique for renal biopsy compared with ultrasound-guided spring-loaded 18 G needle biopsy. Br J Radiol. 1992;65:594–597. doi: 10.1259/0007-1285-65-775-594. [DOI] [PubMed] [Google Scholar]
- Wennberg L, Miyahara S, Wilczek H. Percutaneous core-needle biopsy of renal transplant performed safely without radiographic aid: a prospective study. Transplant Proc. 1994;26:1769–1770. [PubMed] [Google Scholar]
- Kudryk B, Martinez G, Gunasekeran S, Ramirez G. CT guided renal biopsy using coaxial technique and an automated biopsy gun. South Med J. 1995;88:543–546. doi: 10.1097/00007611-199505000-00007. [DOI] [PubMed] [Google Scholar]
- Mimata H, Kasagi Y, Nasu N, et al. Renal needle biopsy along the retrograde puncture line of the renal calyx: A new promising technique. Urol Int. 1998;60:165–168. doi: 10.1159/000030243. [DOI] [PubMed] [Google Scholar]
- Leal J. A new technique for renal biopsy: the transurethral approach. J Urol. 1993;149:1061–1063. doi: 10.1016/s0022-5347(17)36296-1. [DOI] [PubMed] [Google Scholar]
- Pirani C. In: Tisher BBC, editor. Renal Pathology with Clinical and Functional Correlations. Philadelphia: Lippincott; 1989. Evaluation of kidney biopsy specimens. pp. 11–42.
- Sakai H, Abe K, Kobayash Y, et al. Clinical guidelines of IgA nephropathy. Nippon Jinzo Gakkai Shi. 1995;37:417–421. [PubMed] [Google Scholar]
- Yoshinari M, Suzuki R, Watanabe K, Katoh T, Watanabe T. How long is enough: length of renal needle biopsy specimen for histological diagnosis. Am J Nephrol. 2002;22:402. doi: 10.1159/000065237. [DOI] [PubMed] [Google Scholar]
- Chan R, Common A, Marcuzzi D. Ultrasound guided renal biopsy: experience using an automated core biopsy system. Can Assoc Radiol J. 2000;51:107–113. [PubMed] [Google Scholar]
- Hopper K D, Abendroth C, Sturtz K, Matthews Y, Shirk S, Stevens L. Blinded comparison of biopsy needles and automated devices on vitro: 2. Biopsy of medical renal disease. AJR Am J Roentgenol. 1993;161:1299–1301. doi: 10.2214/ajr.161.6.8249746. [DOI] [PubMed] [Google Scholar]
- Boyvat F, Tarhan N, Coskun M, Agidere A, Tutar N, Bilgin N. Comparison of two biopsy techniques for renal transplant assessment. Transplant Proc. 1998;30:777–779. doi: 10.1016/s0041-1345(98)00044-x. [DOI] [PubMed] [Google Scholar]
- Hlatky M. Is renal biopsy necessary in adults with nephrotic syndrome? Lancet. 1982;2:1264–1268. doi: 10.1016/s0140-6736(82)90116-7. [DOI] [PubMed] [Google Scholar]
- Rosenbaum R, Hoffsten P, Stanley R, Klahr S. Use of computerized tomography to diagnose complications of percutaneous renal biopsy. Kidney Int. 1978;14:87–92. doi: 10.1038/ki.1978.93. [DOI] [PubMed] [Google Scholar]
- Diaz-Buxo J, Donadio J V., Jr Complication of percutaneous renal biopsy: an analysis of 1000 consecutive biopsies. Clin Nephrol. 1975;4:223–227. [PubMed] [Google Scholar]
- Stiles K, Yuan C, Chung E, Lyon R, Lane J, Abbott K. Renal biopsy in high-risk patients with medical disease of the kidney. Am J Kidney Dis. 2000;36:419–433. doi: 10.1053/ajkd.2000.8998. [DOI] [PubMed] [Google Scholar]
- Kersnik Levart T, Kenig A, Buturovic Ponikvar J, Ferluga D, Avgustin Cavic M, Kenda R. Real-time ultrasound-guided renal biopsy with a biopsy gun in children: safety and efficacy. Acta Paediatr. 2001;90:1394–1397. doi: 10.1080/08035250152708789. [DOI] [PubMed] [Google Scholar]
- Chesney D, Brouhard B, Cunningham R. Safety and cost effectiveness of pediatric percutaneous renal biopsy. Pediatr Nephrol. 1996;10:493–495. doi: 10.1007/s004670050146. [DOI] [PubMed] [Google Scholar]
- Mahoney M, Racadio J, Merhar G, First M. Safety and efficacy of kidney transplant biopsy: Tru-Cut needle vs sonographically guided biopsy gun. AJR Am J Roentgenol. 1993;160:325–326. doi: 10.2214/ajr.160.2.8424343. [DOI] [PubMed] [Google Scholar]
- Murphy B, Macisaac A. Percutaneous renal biopsy as a day-patient procedure. Am J Kidney Dis. 1989;14:77. doi: 10.1016/s0272-6386(89)80100-3. [DOI] [PubMed] [Google Scholar]
- Hussain F, Watson A, Hayes J. Standards for renal biopsies: comparison of inpatient and day care procedures. Pediatr Nephrol. 2003;18:53–56. doi: 10.1007/s00467-002-1003-2. [DOI] [PubMed] [Google Scholar]
- Fraser I R, Fairley K. Renal biopsy as an outpatient procedure. Am J Kidney Dis. 1995;25:876–878. doi: 10.1016/0272-6386(95)90569-3. [DOI] [PubMed] [Google Scholar]
- Maddux F, Maddux D, Sterling J, Baker W, Moore M. Outpatient renal biopsy is a safe procedure in the community setting. J Am Soc Nephrol. 1992;3:345. Abstract. [Google Scholar]
- Al-Wakeel J. Outcome of early ambulation after renal biopsy using automated biopsy needle by inexperienced trainees. Int Urol Nephrol. 1998;30:399–405. doi: 10.1007/BF02550217. [DOI] [PubMed] [Google Scholar]
- White R, Poole C. Day care renal biopsy. Pediatr Nephrol. 1996;10:408–411. doi: 10.1007/s004670050130. [DOI] [PubMed] [Google Scholar]
- Khajehdehi K, Junaid S, Salinas-Madrgal L, Schmitz P, Bastani B. Percutaneous renal biopsy in the 1990's: safety, value and implication for early hospital discharge. Am J Kidney Dis. 1999;34:92–97. doi: 10.1016/s0272-6386(99)70113-7. [DOI] [PubMed] [Google Scholar]
- Davis I, Ochlenschlager W, O'Riordan M, Avner E. Pediatric renal biopsy: should this procedure be performed in an outpatient setting? Pediatr Nephrol. 1998;12:96–100. doi: 10.1007/s004670050412. [DOI] [PubMed] [Google Scholar]
- Chesney D, Brouhard B, Cunningham R. Safety and cost effectiveness of pediatric percutaneous renal biopsy. Pediatr Nephrol. 1996;10:493–495. doi: 10.1007/s004670050146. [DOI] [PubMed] [Google Scholar]