ABSTRACT
The last four decades have seen tremendous advances in the field of pulmonary transplantation. Vast improvements in the areas of surgical transplantation techniques, immunosuppressive agents, and postoperative care have all contributed to improved survival of patients. Advances in noninvasive imaging and bronchoscopy have allowed the pulmonary transplant team to intervene early in patients presenting with airway complications, often using minimally invasive procedures such as endobronchial balloon dilation or stent placement, or both. Stent technology itself has also improved and stents may sometimes be customized for treatment of short airway lesions or to optimize continued airflow through the sides of stents by creating openings using balloons or bronchoscopically directed laser. Preliminary work with brachytherapy may be decreasing the need for secondary reinterventions. The authors present an overview of some of these conventional and novel approaches to the treatment of airway complications after lung transplantation.
Keywords: Lung transplantation, bronchus, stenosis
INTRODUCTION TO LUNG TRANSPLANTATION
The first successful human lung transplantation (LTx) was performed at the University of Mississippi Medical Center in April 1963.1,2 Over the subsequent four decades, surgical, medical, and immunosuppressive techniques have all contributed to the advancement of solid organ transplantation. LTx now offers a potential cure for many patients with end-stage pulmonary diseases such as cystic fibrosis, chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, emphysema, and primary pulmonary hypertension (Table 1).
Table 1.
Number of Transplants by Primary Diagnosis at Transplant (1988–2003)
| Primary Diagnosis | N | % |
|---|---|---|
| Based on Organ Procurement and Transplantation Network Data as of March 29, 2004. | ||
| Other | 325 | 2.9 |
| Cardiomyopathy | 3 | 0.0 |
| Coronary artery disease | 1 | 0.0 |
| Congenital heart disease | 222 | 2.0 |
| Retransplant/graft failure | 321 | 2.9 |
| Primary pulmonary hypertension | 646 | 5.8 |
| Cystic fibrosis | 1827 | 16.3 |
| Other lung disease | 891 | 8.0 |
| Idiopathic pulmonary fibrosis | 1699 | 15.2 |
| Alpha-1–antitrypsin deficiency | 1004 | 9.0 |
| Emphysema/COPD | 4246 | 38.0 |
| All | 11185 | 100.0 |
Unfortunately, a shortage of donor organs remains the most significant obstacle to potential transplant recipients. Data obtained from the United Network for Organ Sharing (UNOS) show that from a total of 82,000 awaiting organ transplantation in the United States, approximately 4000 (5%) are awaiting a lung transplant.3 Organ shortage, primarily due to a lack of donors, may result in the average lung transplant recipient waiting up to 2 years before a suitable organ becomes available. Living related LTx has opened up new avenues for some potential recipients, but living donor transplants constitute a small minority of all transplants.3,4 Approximately 1000 lung transplants are performed in the United States annually. Generally, about 20 (2%) of these are living related lung transplants3 (Table 2).
Table 2.
Number of Lung Transplants by Year and Type
| Donor Type |
|||
|---|---|---|---|
| Deceased | Living N (%) | Total | |
| Based on Organ Procurement and Transplantation Network Data as of March 29, 2004. | |||
| 1988 | 33 | 0 (0) | 33 |
| 1989 | 93 | 0 (0) | 93 |
| 1990 | 202 | 1 (0.5) | 203 |
| 1991 | 401 | 4 (1.0) | 405 |
| 1992 | 535 | 0 (0) | 535 |
| 1993 | 660 | 7 (1.0) | 667 |
| 1994 | 708 | 15 (2.1) | 723 |
| 1995 | 848 | 25 (2.9) | 873 |
| 1996 | 791 | 26 (3.2) | 817 |
| 1997 | 910 | 23 (2.5) | 933 |
| 1998 | 840 | 29 (3.3) | 869 |
| 1999 | 864 | 28 (3.1) | 892 |
| 2000 | 941 | 19 (2.0) | 960 |
| 2001 | 1034 | 21 (2.0) | 1055 |
| 2002 | 1029 | 13 (1.2) | 1042 |
| 2003 | 1070 | 15 (1.4) | 1085 |
| Total | 10959 | 226 (2.0) | 11185 |
SURGICAL PERSPECTIVE ON LUNG TRANSPLANTATION
The first lung transplant recipient expired within 18 days of transplantation. Over the next 20 years, only 40 more transplants were performed and only one of these patients survived as long as 10 months.5,6 However, it was during these early years that many surgical techniques related to LTx were described and improved upon.7 Among these critical advances included the crucial observation that shortening the donor bronchus improved airway healing because it became clear that postoperative bronchial ischemia played a major role in airway complications such as stenosis and bronchomalacia.8 Other methods of preventing bronchial ischemia, and its associated detrimental effects on the airway, would become a focal point in LTx surgical research in the ensuing decades.
There are several surgical techniques used in LTx.9,10,11,12,13 Patients requiring a single-lung transplant can receive either an entire single lung or a single lobe. Those requiring a double lung transplant may receive bilateral-sequential lung transplant, bilateral lobar transplant, or combined heart-lung transplant. Lobar transplantation techniques are employed in the case of cadaveric transplantation with a size mismatch or living donor transplantation. Various bronchial airway anastomotic techniques are employed, which may involve the use of continuous or noncontinuous suture and telescoped or nontelescoped bronchial anastomosis.
Along with improvements in surgical techniques, there were crucial developments in the field of immunosuppressive agents.14,15 The development of cyclosporine in the early 1980s allowed the use of lower dosages of steroids, which in turn allowed better control of the recipient's immune response without impairing wound healing.16
Improved postoperative surveillance for complications has also resulted in increased graft and patient survival along with decreased morbidity in the transplant recipient. Today, 1-, 3-, and 5-year patient survival rates after LTx have increased to 75, 58, and 45%, respectively.3
AIRWAY COMPLICATIONS OF LUNG TRANSPLANTATION
Despite the extensive surgical and postoperative management advances in this field, airway complications remain a common cause of morbidity and mortality; their timely recognition may improve long-term prognosis.17 Bronchiolitis obliterans is a leading cause of morbidity and mortality after LTx18,19 and may affect as many as 65% of recipients at 5 years.20 Over the past 30 years, the incidence of anastomotic airway complications has decreased from as high as 60%21,22 to ∼15%.22,23,24 Bronchial strictures make up the bulk of these complications.
Different classification systems have been described for postoperative airway complications. Kshettry et al22 divide postoperative complications into stenosis, bronchomalacia, exophytic granulation tissue, dehiscence, and anastomotic infections. Couraud et al25 have published a classification system to assess airway healing based on bronchoscopic appearance of the surgical anastomosis on the 15th postoperative day. Herrera et al23 have also described a modified classification system based on Courad's classification.
Regardless of the classification system, it is evident that postoperative bronchial ischemia plays a major role in airway complications in the transplanted lung and probably contributes to both bronchial stenosis and bronchomalacia. There is also evidence that patients who undergo bronchial artery revascularization procedures may postpone the onset of bronchiolitis obliterans syndromes.26 The bronchial arteries provide the vascular supply to the distal trachea, carina, and bronchi.27 Bronchial artery anatomy is variable, although the arteries usually arise directly from the thoracic aorta with 41% of patients having one right and two left bronchial arteries. The bronchial arteries are traditionally severed during LTx. This can lead to postoperative perianastomotic ischemia until collaterals develop within the submucosal plexus from the pulmonary artery; such collaterals often take weeks to months to form adequately.28 Postoperative interstitial edema from poor graft preservation and reperfusion injury, organ rejection, prolonged positive pressure ventilation, and infection can all lead to increased arterial resistance and impair collateral formation, further contributing to anastomotic ischemic complications.29
Innovative techniques have been described to minimize this postoperative ischemia and improve collateral blood flow to this area. Direct bronchial artery revascularization techniques have been employed30 but are technically challenging, especially given the variable location and number of these vessels.31 Meticulous care has to be taken to preserve all these small vessels at the time of organ harvest. Vascularized omental wraps32 or intercostal muscle flaps33 placed around the anastomosis may hasten collateral ingrowth to the area. Reducing the length of the donor bronchus to within one to two cartilaginous rings above the takeoff of the upper lobe bronchus has been shown to reduce the degree of postoperative ischemia.34 Telescoping the donor bronchus into the recipient bronchus may also improve collateral blood flow. Pharmacological enhancement of blood flow to the site of bronchial healing has also been attempted with varying degrees of success.35
Nonsurgical endobronchial management of post-transplantation airway stenoses remains the mainstay of therapy.36 Balloon dilation alone is usually not effective treatment for such anastomotic lesions as there is often some component of bronchomalacia associated with granulation tissue formation. Softening of the bronchial walls from bronchomalacia leads to collapse of the airway in expiration. Reinforcement of the bronchus with metallic stents is effective therapy in isolated bronchomalacia.37,38
DIAGNOSTIC IMAGING IN LUNG TRANSPLANTATION
Lung transplant recipients typically undergo an extensive radiologic work-up prior to receiving a transplant, including chest radiograph, computed tomography (CT) scan, and ventilation-perfusion quantitative scanning. These scans become particularly important in the case of single-lung transplantation, both to determine the optimal side for transplantation and to assess for size discrepancies.39,40 Screening for lung and other cancers prior to transplantation is also important in these patients, who undergo extensive immunosuppression in the post-transplantation period.
Routine chest radiographs in the postoperative period may demonstrate signs of postoperative edema, infection, and pneumothorax. The transplanted organ is more prone to bacterial and viral infection because of immunosuppression and interruption of the lymphatic drainage pathways, continuous direct environmental exposure of the airways, and decreased mucociliary clearance.41 Development of pleural effusion, alveolar consolidation, atelectasis, or pneumonia may be a sign of an underlying airway complication. However, chest radiographs alone are of limited value in assessing for such airway complications. CT scans or direct visualization by bronchoscopy can assess for airway leaks, stenoses, and perianastomotic abscess formation. Advanced CT techniques involving multisection helical CT with three-dimensional reconstruction algorithms can be used to create virtual bronchoscopic views useful for assessment of anastomotic abnormalities.42 Such images may be comparable to those obtained by bronchoscopy and offer a noninvasive alternative to diagnostic bronchoscopy in certain circumstances (Fig. 1). CT can also identify post-transplantation lymphoproliferative disease (PTLD). PTLD manifests as pulmonary nodules, lymphadenopathy, and ground glass opacification around nodules on high-resolution CT. CT may also be used to evaluate recurrence of primary diseases following LTx.43 Bronchial defects and extraluminal air in the postoperative period may signify the presence of bronchial dehiscence, a rare (2–3%) but disastrous early complication of LTx.44,45
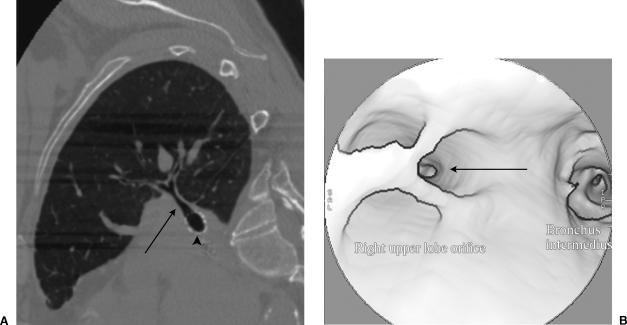
Figure 1.
CT of stent traversing right upper lobe bronchus. (A) Sagittal reformat of chest CT shows a stent (arrowhead) traversing the origin of the right upper lobe bronchus (arrow). (B) Endobronchial CT reformat shows the stent struts crossing the origin of the right upper lobe airway (arrow).
Thin-section expiratory CT may be helpful in detecting airway obstruction and air trapping in bronchiolitis obliterans.46
Pulmonary complications after lung transplantation can also occur in the native lung in the case of single-lung transplant recipients. As many as 15% of such recipients may develop complications such as infection or lung cancer in the native lung, resulting in significant morbidity and mortality in affected patients, and this may be diagnosed by CT.47
Pulmonary artery complications such as stenoses, occlusion, or pulmonary artery embolism may also occur after transplantation. Diagnostic imaging with CT pulmonary angiography or, in equivocal cases, catheter-directed pulmonary angiography is diagnostic. Catheter-directed pulmonary artery thrombolysis and thrombectomy may be used in certain cases and are technically feasible, but long-term follow-up in a significant number of cases is presently lacking. Pulmonary artery anastomotic stenoses or occlusions, or both, have been treated in limited cases with balloon angioplasty and stent placement.48,49
PULMONARY FUNCTION TESTS
In the absence of significant complications, physiological lung function generally improves to best baseline values within the first 6 months after transplantation as the thoracotomy wound heals, chest pain subsides, and ischemia-reperfusion injury resolves. Lung function, quantified as percent of predicted normal, has been shown to correlate with the individual patient's overall clinical outcome after LTx. When compromised by airway complications, pulmonary function generally improves significantly after treatment with balloon dilatation or stent placement.38 Pulmonary function tests have also been shown to improve after living related LTx.50
At our institution, serial pulmonary function tests are typically performed at 3-month intervals after transplantation. These tests are not only useful as surveillance for rejection or infection of the allograft but also may provide screening for bronchial anastomotic complications such as stricture formation, bronchomalacia, and endobronchial infection. A decline of 10% or greater from baseline forced expiratory volume in 1 second (FEV1) is an indication for further evaluation of the patient, including a chest radiograph and bronchoscopy with transbronchial biopsy and bronchoalveolar lavage. Of note, an inexorable decline in FEV1 without evidence of infection or large airway stenosis is indicative of bronchiolitis obliterans syndrome or chronic allograft rejection.51
DIAGNOSTIC BRONCHOSCOPY
The importance of the routine use of bronchoscopy cannot be overemphasized in the LTx population.52 Evaluation of the cadaveric donor pool by bronchoscopy demonstrated that only one third of potential cadaveric donors had a normal bronchoscopic examination prior to organ harvest.53 Up to 38% of cadaveric donors were found to have abnormal bronchoscopic examinations despite having a normal chest radiograph and oxygenation.53
Despite advances in noninvasive imaging techniques, bronchoscopy remains the "gold standard" in the follow-up care of lung transplant recipients. Indications for bronchoscopy include cough, shortness of breath, wheezing, stridor, fever, decreased pulmonary function tests, clinical pneumonia, and infiltrate on chest radiograph. However, routine surveillance bronchoscopy is also advocated in most (68%) active lung transplant centers and has been shown to influence patient management frequently even when patients are free of clinical symptoms because rejection may occur without affecting objective measurements of allograft function.54
At our institution, patients undergo surveillance bronchoscopy at 2 weeks after transplantation and then at approximately 3-month intervals for 2 years after transplantation. Additional diagnostic bronchoscopy is performed as warranted by clinical symptoms. In addition to visual survey of the airways and anastomosis, bronchoscopy allows the clinician to perform transbronchial biopsies to assess for histological evidence of rejection. Bronchoalveolar lavage can also be pursued to diagnose opportunistic infection.
Although there is evidence that diagnostic bronchoscopy is of value in the immunosuppressed host with respiratory symptoms, there are arguably no set standards for the optimal use and timing of surveillance bronchoscopy and biopsy of the lung allograft following LTx. We believe the early diagnosis of chronic rejection may affect outcomes. The addition of aerosolized cyclosporin to patients with proven bronchiolitis obliterans has been shown to provide a survival advantage over conventional therapy alone.55,56 Baz et al advocate the routine use of surveillance bronchoscopy for the first few postoperative months.57 They acknowledge that patients without evidence of clinically significant rejection or cytomegalovirus pneumonitis by 4 months may be spared additional routine bronchoscopy unless dictated clinically.
In their small series, Kelly et al58 have shown that it is common to see ischemic changes on bronchoscopy in the postoperative period, but these do not necessarily predict significant differences in clinical outcomes. As opposed to the accurate diagnosis of bronchomalacia and strictures after transplantation, visual inspection of the airways is neither sensitive nor specific in reference to diagnosing infection or allograft rejection.
INTERVENTIONAL RADIOLOGY AND BRONCHOSCOPY
General Principles
Bronchial strictures are identified as areas of greater than 50% fixed endoluminal airway narrowing and remain the most common airway complication after LTx. Fixed stenoses are most commonly related to scarring or granulation tissue or are seen as a component of bronchiolitis obliterans. Bronchomalacia is defined as dynamic airway narrowing of greater than 50% during expiration.38 Once a lung transplant recipient is diagnosed with an airway-limiting lesion, multiple treatment options exist including balloon dilation, laser débridement, endobronchial stent placement, and surgical revision.
Although individual clinical services are often called upon to deal with these problems, it is essential to have a close working relationship between the various services involved with the care of the LTx patient. Transplant cardiothoracic surgeons, pulmonary and critical care medicine physicians, and interventional radiologists each bring their own clinical and technical expertise to the table. Working as a team ultimately results in better patient care and improved long-term results.
At our institution, the pulmonary transplant medicine service's technical expertise in bronchoscopy and extensive clinical expertise and experience in the medical aspects of the management of lung transplant patients mesh nicely with the interventional radiology (IR) experience with balloon angioplasty and stent placement in other organs. We had little difficulty in adapting standard balloon dilatation and stent techniques to bronchoplasty and fluoroscopy-guided endobronchial stent placement. Combined bronchoscopic and fluoroscopic guidance for such interventions allowed our team to achieve better, more cost-effective outcomes working together than either service could working separately.
Interventional Radiology Suite and Technique of Endobronchial Intervention
We initially chose to treat all patients at our institution in the IR suite to perform endobronchial procedures. This location was chosen because of the availability of better fluoroscopic guidance (Siemens MultiStar, Siemens AG, Munich, Germany), ease of obtaining assistance of the anesthesiology department in the IR suite, and ready access to various balloons and catheters. All patients were treated under general anesthesia through a no. 8 or larger endotracheal tube (ETT). It is important to consider that if patients are put under general anesthesia with inhaled anesthetics, some of the inhaled agent may escape during introduction of various diagnostic and interventional devices into the airways and adversely affect the operator. This can be avoided by using airtight adapters on the ETT. At our institution, we use a 9Fr sheath placed through a Y connector attached to the ETT to allow airtight catheter exchanges and prevent significant air leaks. Also, intravenous anesthetics could be used to avoid the technical difficulties associated with gaseous anesthetic agents. Muscle relaxants are also of paramount importance; coughing during stent placement may result in suboptimal stent position.
The same techniques employed in crossing vascular stenoses are applicable to treatment of endobronchial lesions. We have found the standard or heavy-duty Glidewire (Boston Scientific, Natick, MA) to be our wire of choice for crossing severe bronchial stenoses. Care must be taken to keep the wire well lubricated with saline because the wire may dry out relatively quickly in the airway. Advancement of a guidewire through a bronchoscope can also be helpful in directing the guidewire to and through specific areas of concern. We frequently use a 0.035-inch Amplatz Super Stiff guidewire (Boston Scientific) advanced through the biopsy channel of the P30 bronchoscope (Olympus, Lake Success, NY) for direct selection of specific bronchi for treatment. The bronchoscope is then carefully withdrawn, leaving the exchange wire in place for passage of the catheter. We chose the Amplatz guidewire because the distal end is quite floppy and atraumatic and the proximal guidewire provided enough rigidity to pass catheters, balloons, and stents. Once the lesion has been crossed, bronchography is performed using nonionic contrast media (Optiray 320, Mallinckrodt, St. Louis, MO) delivered through a multisidehole catheter and digital subtraction or native digital imaging (Fig. 2). Very small amounts of contrast agent are necessary for bronchography (3–5 mL per injection). Percentage of narrowing and airway size is determined using digital calipers. Bronchoscopic visualization is also used to assess the area of narrowing for clinical significance.
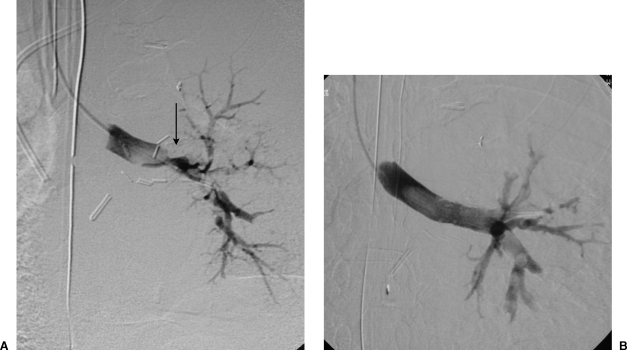
Figure 2.
Endobronchial stent placement for anastomotic stricture. (A) Left lung bronchogram shows a severe anastomotic stenosis (arrow). Bronchoscope could not be advanced through the lesion. (B) After endobronchial stent placement, repeated bronchography shows a widely patent bronchus that now easily accommodated a bronchoscope.
Differentiation between fixed stenosis and bronchomalacia is important to determine appropriate therapy. In fixed stenoses, bronchoplasty is our initial procedure of choice and is performed using standard or high-pressure angioplasty balloons such as the Marshall or Blue Max balloons (Boston Scientific). When airways are treated just proximal to bifurcation points, it becomes important to use balloons with shorter shoulders. The balloon is left inflated for a period of 30–60 seconds. If large balloons are used in proximal airways, the IR team, and the anesthesiologist in particular, should be monitoring oxygenation status because an entire lung may be deprived of oxygen for the duration of the bronchoplasty. This is especially important in single-lung recipients.
Results are evaluated by both bronchography and bronchoscopy. Persistent fixed areas of narrowing are either dilated further if the balloon was felt to be undersized or stented as necessary (Fig. 3). Upon completion of the bronchograms, the contrast agent may frequently be suctioned using the bronchoscope. This may be helpful in lessening postprocedural bronchospasm.
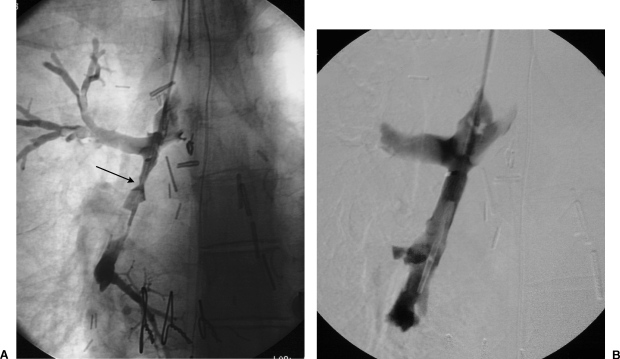
Figure 3.
Endobronchial stent placement for severe stenosis of bronchus intermedius. (A) Right lung bronchogram demonstrates severe stenosis of bronchus intermedius (arrow). (B) Widely patent bronchus intermedius after placement of self-expanding nitinol stent.
Bronchoscopy Suite Procedure
As members of the interventional bronchoscopy team began to feel more comfortable doing some of these procedures without the use of general anesthesia, we elected to perform some of the more straightforward procedures in the bronchoscopy suite using a C-Arm (Siemens AG, Munich, Germany) instead of in the IR suite. This allowed patients to be treated during the same visit as their diagnostic bronchoscopy. The lack of general anesthesia also allows substantially decreased cost and faster recovery of the patient. Appropriate stents and balloons are stocked in the bronchoscopy suite. Diagnostic and therapeutic bronchoscopy is performed with topical anesthesia using 2% lidocaine liquid and moderate conscious sedation with a combination of an anxiolytic agent (midazolam HCl) and pain medication (fentanyl citrate). Balloon dilatation or stent deployment is facilitated by the use of external metallic markers fixed to the skin to mark the location of the lesion so that interventions can be performed without the use of bronchography. Larger proximal lesions can be easily treated in this fashion. More distal or complex lesions still require general anesthesia and the better fluoroscopic guidance and bronchography usually available in a full IR suite. External markers are prone to parallax error and respiratory motion and should not be relied upon for stent placement when there is little room for error.
Choice of Stents
Traditionally, plastic and silicone stents have been used for treatment of bronchial strictures. These stents are placed bronchoscopically. In general, these stents have a much smaller ratio of internal lumen to external lumen diameter, tend to migrate easily, prevent mucociliary clearance through the stent, and may occlude side branches. For bronchial applications, these stents have largely been replaced by metallic stents placed under fluoroscopic guidance.59,60,61,62,63,64,65,66 Metal stents allow epithelialization of the stent to occur, helping to anchor the stent in place and prevent migration. In addition, because of this epithelialization, mucociliary clearance of secretions is enhanced.
The unique characteristics of bronchial strictures make the choice of an ideal stent problematic. Bronchi change diameter with phase of respiration and lesions may be fixed or dynamic. There are multiple side branches of varying size, and preservation of airflow to all regions is paramount because pulmonary reserve is frequently limited in this population. The Wallstent (Boston Scientific) is a self-expanding metal stent approved by the Food and Drug Administration for tracheobronchial indications. It comes in a variety of diameters and lengths and is the only stent that can be recovered and moved after it is partially deployed. However, because of the significant foreshortening that occurs during deployment, it can be difficult to place this stent with the same accuracy as nitinol or balloon-expandable stents. Also, the tight mesh of this stent may have a greater negative impact on mucociliary clearance than other stents with a wider cell size. In our institution, we have found the Symphony (Boston Scientific) stent to epithelialize rapidly. The large cell size of this stent appears to be more conducive to drainage of secretions. However, in our experience, this rigid stent has been prone to fracture, especially in patients with severe bronchomalacia and a strong cough reflex.37 Self-expanding stents are mandatory in the treatment of bronchomalacia. Their radial expansile force allows self-recovery after deformation that occurs with violent coughing.
Balloon-expandable stents generally offer more crush resistance than self-expanding stents; however, once crushed they do not recover and may become dislodged.67 Metal stents may also be dislodged iatrogenically, by bronchoscopy, deep airway suctioning, or ETT placement.37 Removal and repositioning of both self-expanding68 and balloon-expandable stents have been described in the literature.69 Biopsy forceps are generally used to grasp the stents and adjust or remove them. Rigid bronchoscopy may be necessary in some cases. We have had some success in stent retrieval by placing snares around the stent and constricting them before pulling them out through an ETT (Fig. 4).
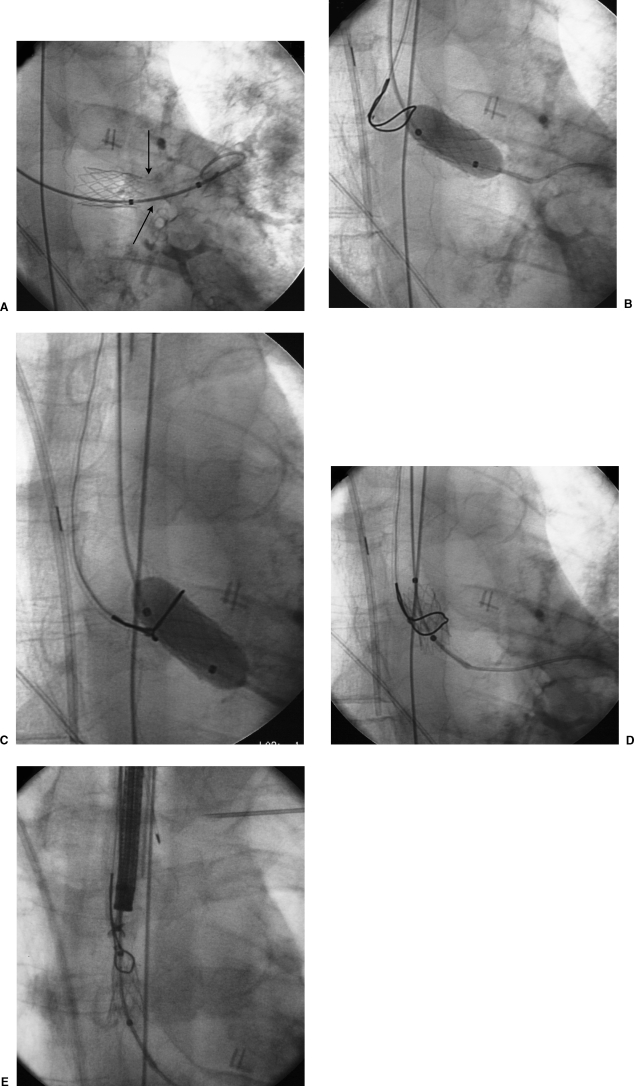
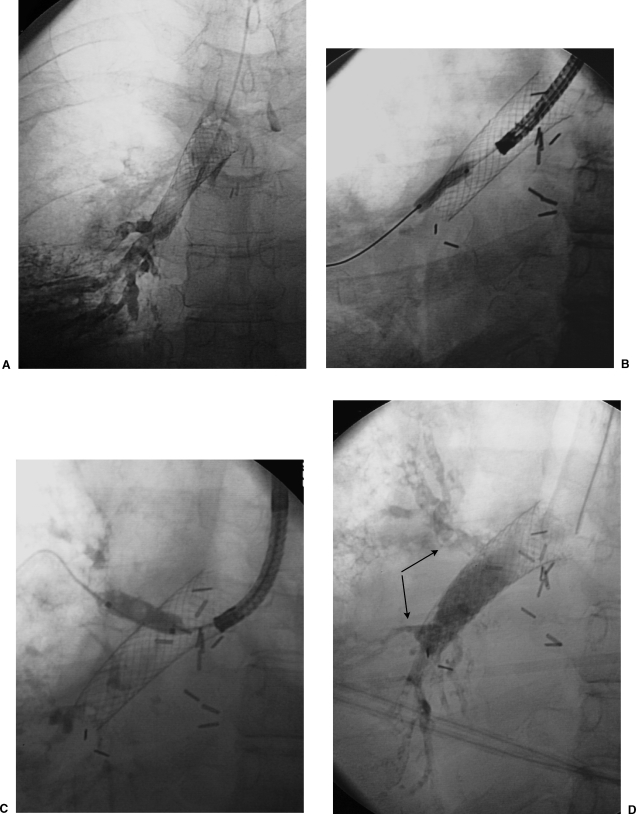
Figure 4.
Retrieval of malpositioned stent. (A) A balloon-expandable stent was placed for a severe stenosis at the left bronchial anastomosis. During stent deployment, the stent was inadvertently displaced medially (“watermelon seeded”) from the stenosis into the donor mainstem bronchus proximal to the level of the anastomosis (arrows). (B) A balloon was expanded within the stent to immobilize the stent and facilitate capture with a snare. (C) An Amplatz snare (Microvena, Great Bear Lake, MN) was placed around the balloon and stent. (D) The balloon was deflated and the snare tightened around the stent. (E) The stent was compressed using the snare and removed through the endotracheal tube.
Creating Side Holes in Stents
In some cases it may be advantageous to place a stent across the origin of a bronchus, such as the upper lobe bronchus when treating the mainstem bronchus. Creating a hole in the stent at the side branch origin can enhance patency of the side branch. Depending on the stent type used, this may be possible either with a bronchoscope-directed laser or with a balloon. Creating a hole with a balloon may be accomplished in Wallstents by passing a 0.018-inch guidewire through the interstices of the stent and then using a small balloon to dilate open the struts (Fig. 5). The Wallstent may also be perforated using a bronchoscopy-guided laser. However, in our experience, we have found the integrity of Wallstents and their inherent radial force to be diminished after creating side holes using a laser. This is especially true in shorter (2 cm) Wallstents.
Figure 5.
Creation of openings in a Wallstent occluding right upper and lower lobe bronchi. (A) A Wallstent has been placed extending from right mainstem bronchus into bronchus intermedius for treatment of severe bronchomalacia of both airway segments. The stent traversed the right upper lobe bronchus and a right lower lobe segmental bronchus, causing functional obstruction of the airways. (B,C) Openings were created in the side of the Wallstent to enhance drainage of secretions and airflow from the right lower and upper lobes by passing a 0.018-inch guidewire through the stent and then dilating the interstices of the stent with a low-profile balloon (Symmetry, Boston Scientific, Natick, MA). Passing the balloon through the biopsy channel of the bronchoscope helps give stability and pushing force to the balloon. (D) Final bronchograms, showing good flow into the upper and lower lobe bronchi (arrows).
Creation of side holes using a balloon cannot be done with nitinol stents and most balloon-expandable stents; nitinol stents revert back to their original shape, and advancing a balloon through the struts of most balloon-expandable stents carries the risk of getting the balloon stuck in the stent.
Complications of Balloon Angioplasty and Stent Placement
The potential complications related to balloon dilation and endobronchial stent placement are analogous to complications seen in the vascular or biliary system (Table 3).
Table 3.
Complications Related to Bronchoplasty and Airway Stent Placement
| Complications related to bronchoplasty |
| Bleeding |
| Bronchial rupture |
| Occlusion of side branches |
| Complications related to stent placement |
| Misplaced/malpositioned stents |
| Stent narrowing |
| Stent crush/fracture |
| Stent migration |
| Side branch airway occlusion |
| Iatrogenic damage to stent |
| Tracheobronchitis |
In general, periprocedural complications are rare and are most often related to anesthesia or postoperative bronchospasm. Bronchospasm may be due to airway irritation from bronchography or airway manipulation from the bronchoscope, catheters, or balloons. Many of these patients have depressed pulmonary function and may be considered at high risk for general anesthesia. It is imperative that the anesthesiologist be thoroughly familiar with treatment of the lung transplant population.
Long-term complications are mainly related to stent migration, deformation, and recurrent stenosis. In our experience, the long-term patencies of balloon dilatation and endobronchial stent placement are remarkably similar when treating granulomatous or fixed stenoses. This is probably due to the inability of metallic stents to prevent granulomatous ingrowth through the cells of the stents. Tighter meshwork may prevent this to an extent, but this tighter mesh is counterproductive to side branch airflow and pulmonary toilet. Covered stents may enhance long-term patency for fixed stenoses but, again, at the expense of side branches. More investigation is needed in this area as a greater variety of covered stents become available.
Results
Experience at our institution has demonstrated a significant improvement in lung function and reduction in incidence of pulmonary infections in patients who were treated with airway stents for stenoses and bronchomalacia. In a 2-year data set of 30 patients undergoing a total of 75 interventions (50 stent insertions and 25 balloon dilations), Burns et al38 demonstrated an improvement in FEV1 after stent placement compared with baseline through 12 months mean follow-up. The pulmonary infection rate also decreased significantly when the 12-month period preceding stent insertion was compared with the corresponding period after stent insertion (p = 0.018). Recurrent stenoses occurred in 17.3%, but these were generally amenable to repeated intervention. No life-threatening complications occurred after stent placement and no deaths were attributed to stent malfunction or malposition.
Saad et al70 described placement of self-expanding metallic stents in 12 patients after LTx with a mean follow-up of 20 months. Eighty-three percent of the patients experienced immediate resolution of symptoms. There was a significant improvement in FEV1 after intervention; the overall 5-year survival rate in treated patients was similar to that in the rest of the lung transplant recipients.
NON–AIRWAY-RELATED COMPLICATIONS OF LUNG TRANSPLANTATION
Pneumonia in the transplanted or contralateral lung may lead to an associated pleural effusion and empyema. The radiologist may be called upon to perform image-guided thoracentesis or drain placement in some of these collections under CT or ultrasonography.
Pulmonary artery complications such as stenoses or pulmonary artery embolism may occur after transplantation. Up to 20% of lung transplant patients may have a pulmonary embolus on CT that was not suspected clinically.71 Diagnostic imaging with CT angiography augmented by catheter-directed angiography in equivocal cases could be employed to make the diagnosis. Catheter-directed pulmonary artery thrombolysis and thrombectomy might be used in certain cases, such as acute massive pulmonary embolism with severe respiratory compromise. There are rare case reports of pulmonary artery anastomotic stenoses or occlusions, or both, having been treated with balloon angioplasty and stent placement.48,49,72,73
Hemoptysis in the lung recipient can pose a challenging clinical condition. Bronchial artery bleeding is the usual cause of hemoptysis in the native lungs. However, these vessels have usually been severed in the transplant process. Restoration of bronchial artery circulation after LTx in the canine model has been described.74 There is at least one case report of bronchial artery embolization successfully treating hemoptysis even though the bronchial artery had not been reimplanted at the time of surgery.75
Future Horizons
Recurrent narrowing and granulation tissue formation are seen in most patients despite maximal endobronchial therapy with stents and lasers. High-dose brachytherapy has been used in a few selected patients with promising early results. Kennedy et al76 have shown symptomatic improvement in two patients after receiving brachytherapy. Halkos et al77 have also shown promising results in two of four patients treated with high-dose endobronchial brachytherapy. Further evaluation and studies are under way at many institutions including our own.
CONCLUSION
Lung transplantation offers a ray of hope to many patients with end-stage lung disease. The radiologist has to be attuned to assessing for specific complications related to this field. The interventional radiologist has at his or her disposal many diagnostic and therapeutic technologies to alter the natural progression of some of these complications. This can result in an improved overall prognosis by the use of minimally invasive modalities to maintain airway patency and avoid major surgical intervention for some of the previously described complications.
REFERENCES
- Hardy J, Webb W, Dalton M, et al. Lung homotransplantations in man. JAMA. 1963;186:1065–1074. doi: 10.1001/jama.1963.63710120001010. [DOI] [PubMed] [Google Scholar]
- Hardy J D. The first lung transplant in man (1963) and the first heart transplant in man (1964) Transplant Proc. 1999;31:25–29. doi: 10.1016/s0041-1345(98)02059-4. [DOI] [PubMed] [Google Scholar]
- Based on Organ Procurement and Transplantation Network data as of January 12. 2004.
- Barr M L, Schenkel R G, Cohen R G, et al. Recipient and donor outcomes in living related and unrelated lobar transplantation. Transplant Proc. 1998;30:2261–2263. doi: 10.1016/s0041-1345(98)00612-5. [DOI] [PubMed] [Google Scholar]
- Grover F L, Fullerton D A, Zamora M R, et al. The past, present, and future of lung transplantation. Am J Surg. 1997;173:523–533. doi: 10.1016/s0002-9610(97)00004-4. [DOI] [PubMed] [Google Scholar]
- Derom F, Barbier F, Ringoi S, et al. Ten month survival after lung homotransplantations in man. J Thorac Cardiovasc Surg. 1971;61:835–846. [PubMed] [Google Scholar]
- Veith F J, Kambolg S L, Mollenkopf F P, Montefusco C M. Lung transplantation. Transplantation. 1983;35:271–278. doi: 10.1097/00007890-198304000-00001. [DOI] [PubMed] [Google Scholar]
- Schafers H J, Haydock D A, Cooper J D. The prevalence and management of bronchial anastomotic complications in lung transplantation. J Thorac Cardiovasc Surg. 1991;101:1044–1052. [PubMed] [Google Scholar]
- Lau C L, Patterson G A. Technical considerations in lung transplantation. Chest Surg Clin N Am. 2003;13:463–483. doi: 10.1016/s1052-3359(03)00059-0. [DOI] [PubMed] [Google Scholar]
- Meyers B F, Patterson G A. Technical aspects of adult lung transplantation. Semin Thorac Cardiovasc Surg. 1998;10:213–220. doi: 10.1016/s1043-0679(98)70039-5. [DOI] [PubMed] [Google Scholar]
- Patterson G A. Bilateral lung transplant: indications and technique. Semin Thorac Cardiovasc Surg. 1992;4:95–100. [PubMed] [Google Scholar]
- Patterson G A, Cooper J D, Goldman B, et al. Technique of successful clinical double-lung transplantation. Ann Thorac Surg. 1988;45:626–633. doi: 10.1016/s0003-4975(10)64763-7. [DOI] [PubMed] [Google Scholar]
- Cooper J D, Pearson F G, Patterson G A, et al. Technique of successful lung transplantation in humans. J Thorac Cardiovasc Surg. 1987;93:173–181. [PubMed] [Google Scholar]
- den Berg J WK van, Postma D S, Koeter G H, der Bij W van. New immunosuppressive drugs and lung transplantation: last or least? Thorax. 1999;54:550–553. doi: 10.1136/thx.54.6.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima O, Cooper J D, Peters W J, et al. Effects of methylprednisolone and azathioprine on bronchial healing following lung autotransplantation. J Thorac Cardiovasc Surg. 1981;82:211–215. [PubMed] [Google Scholar]
- Goldberg M, Lima O, Morgan E, et al. A comparison between cyclosporine A and methylprednisolone plus azathioprine on bronchial healing following canine lung autotransplantation. J Thorac Cardiovasc Surg. 1983;85:821–826. [PubMed] [Google Scholar]
- Dransfield M T, Garver R I, Weill D. Standardized guidelines for surveillance bronchoscopy reduce complications in lung transplant recipients. J Heart Lung Transplant. 2004;23:110–114. doi: 10.1016/s1053-2498(03)00098-6. [DOI] [PubMed] [Google Scholar]
- Westall G P, Michaelides A, Williams T J, Snell G I, Kotsimbos T C. Bronchiolitis obliterans syndrome and early human cytomegalovirus DNAaemia dynamics after lung transplantation. Transplantation. 2003;75:2064–2068. doi: 10.1097/01.TP.0000069234.04901.A3. [DOI] [PubMed] [Google Scholar]
- Corris P A. Lung transplantation. Bronchiolitis obliterans syndrome. Chest Surg Clin N Am. 2003;13:543–557. doi: 10.1016/s1052-3359(03)00052-8. [DOI] [PubMed] [Google Scholar]
- Boehler A, Estenne M. Obliterative bronchiolitis after lung transplantation. Curr Opin Pulm Med. 2000;6:133–139. doi: 10.1097/00063198-200003000-00009. [DOI] [PubMed] [Google Scholar]
- Wildevuur C R, Benfield J R. A review of 23 human lung transplantations by 20 surgeons. Ann Thorac Surg. 1970;9:489–515. doi: 10.1016/s0003-4975(10)65544-0. [DOI] [PubMed] [Google Scholar]
- Kshettry V R, Kroshus T J, Hertz M I, et al. Early and late airway complications after lung transplantation: incidence and management. Ann Thorac Surg. 1997;63:1576–1583. doi: 10.1016/s0003-4975(97)83852-0. [DOI] [PubMed] [Google Scholar]
- Herrera J M, McNeil K D, Higgins R SD, et al. Airway complications after lung transplantation: treatment and long-term outcome. Ann Thorac Surg. 2001;71:989–994. doi: 10.1016/s0003-4975(00)02127-5. [DOI] [PubMed] [Google Scholar]
- Date H, Trulock E P, Arcidi J M, Sundaresan S, Cooper J D, Patterson G A. Improved airway healing after lung transplantation. An analysis of 348 bronchial anastomoses. J Thorac Cardiovasc Surg. 1995;110:1424–1433. doi: 10.1016/S0022-5223(95)70065-X. [DOI] [PubMed] [Google Scholar]
- Couraud L, Nashef S A, Nicolini P, Jougon J. Classification of airway anastomotic healing. Eur J Cardiothorac Surg. 1992;6:496–497. doi: 10.1016/1010-7940(92)90247-u. [DOI] [PubMed] [Google Scholar]
- Norgaard M A, Andersen C B, Pettersson G. Does bronchial artery revascularization influence results concerning bronchiolitis obliterans syndrome and/or obliterative bronchiolitis after lung transplantation? Eur J Cardiothorac Surg. 1998;14:311–318. doi: 10.1016/s1010-7940(98)00182-1. [DOI] [PubMed] [Google Scholar]
- Norgaard M A, Andersen C B, Pettersson G. Airway epithelium of transplanted lungs with and without direct bronchial artery revascularization. Eur J Cardiothorac Surg. 1999;15:37–44. doi: 10.1016/s1010-7940(98)00292-9. [DOI] [PubMed] [Google Scholar]
- Siegelmann S S, Hagstrom J WC, Koerner S K, Veith F J. Restoration of bronchial artery circulation after canine lung allograft transplantation. J Thorac Cardiovasc Surg. 1977;73:792–795. [PubMed] [Google Scholar]
- Mulligan M S. Endoscopic management of airway complications after lung transplantation. Chest Surg Clin N Am. 2001;11:907–915. [PubMed] [Google Scholar]
- Couraud L, Bauded E, Nashef S AM, et al. Lung transplantation with bronchial revascularization. Eur J Cardiothorac Surg. 1992;6:490–495. doi: 10.1016/1010-7940(92)90246-t. [DOI] [PubMed] [Google Scholar]
- Cauldwell E W, Siekert R G, Lininger R E, Anson B J. The bronchial arteries: an anatomic study of 150 human cadavers. Surg Gynecol Obstet. 1948;86:395–412. [PubMed] [Google Scholar]
- Morgan E, Lima O, Goldberg M, Ferdman A, Luk S K, Cooper J D. Successful revascularization of totally ischemic bronchial autograft with omental pedicle flaps in dogs. J Thorac Cardiovasc Surg. 1982;84:204–210. [PubMed] [Google Scholar]
- Fell S C, Mollenkopf F P, Montefusco C M, et al. Revascularization of ischemic bronchial anastomosis by an intercostal pedicle flap. J Thorac Cardiovasc Surg. 1985;90:172–178. [PubMed] [Google Scholar]
- Pinkser K L, Koerner S K, Kamholz S L, Hagstrom J WC, Veith F J. Effect of donor bronchial length on healing: a canine model to evaluate bronchial anastomotic problems in lung transplantation. J Thorac Cardiovasc Surg. 1979;77:669–673. [PubMed] [Google Scholar]
- Schafers J H, Haverich A, Wagner T OF, Wahler T, Alken A, Borst H G. Decreased incidence of bronchial complications following lung transplantation. Eur J Cardiothorac Surg. 1992;6:174–179. doi: 10.1016/1010-7940(92)90213-h. [DOI] [PubMed] [Google Scholar]
- Chhajed P N, Malouf M A, Tamm M, Spratt P, Glanville A R. Interventional bronchoscopy for the management of airway complications following lung transplantation. Chest. 2001;120:1894–1899. doi: 10.1378/chest.120.6.1894. [DOI] [PubMed] [Google Scholar]
- Orons P D, Amesur N B, Dauber J H, Zajko A B, Keenan R J, Iacono A T. Balloon dilation and endobronchial stent placement for bronchial strictures after lung transplantation. J Vasc Interv Radiol. 2000;11:89–99. doi: 10.1016/s1051-0443(07)61288-3. [DOI] [PubMed] [Google Scholar]
- Burns K E, Orons P D, Dauber J H, et al. Endobronchial metallic stent placement for airway complications after lung transplantation: longitudinal results. Ann Thorac Surg. 2002;74:1934–1941. doi: 10.1016/s0003-4975(02)04033-x. [DOI] [PubMed] [Google Scholar]
- Kazerooni E A, Chow L C, Whyte R I, et al. Preoperative examination of lung transplant candidates: value of chest CT compared with chest radiography. AJR Am J Roentgenol. 1995;165:1343–1348. doi: 10.2214/ajr.165.6.7484560. [DOI] [PubMed] [Google Scholar]
- Collins J. Imaging of the chest after lung transplantation. J Thorac Imaging. 2002;17:102–112. doi: 10.1097/00005382-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Collins J, Kuhlman J E, Love R B. Acute, life-threatening complications of lung transplantation. Radiographics. 1998;18:21–43. doi: 10.1148/radiographics.18.1.9460107. [DOI] [PubMed] [Google Scholar]
- Konen E, Yellin A, Greenber I, et al. Complications of tracheal and thoracic surgery: the role of multisection helical CT and computerized reformations. Clin Radiol. 2003;58:341–350. doi: 10.1016/s0009-9260(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Collins J, Hartman M J, Warner T F, et al. Frequency and CT findings of recurrent disease after lung transplantation. Radiology. 2001;219:503–509. doi: 10.1148/radiology.219.2.r01ma12503. [DOI] [PubMed] [Google Scholar]
- Schlueter F J, Semenkovich J W, Glazer H S, Arcidi J M, Trulock E P, Patterson G A. Bronchial dehiscence after lung transplantation: correlation of CT findings with clinical outcomes. Radiology. 1996;199:849–854. doi: 10.1148/radiology.199.3.8638016. [DOI] [PubMed] [Google Scholar]
- Semenkovich J W, Glazer H S, Anderson D J, Arcidi J, Cooper J D, Patterson G A. Bronchial dehiscence in lung transplantation: CT evaluation. Radiology. 1995;194:205–208. doi: 10.1148/radiology.194.1.7997554. [DOI] [PubMed] [Google Scholar]
- Siegel M J, Bhalla S, Gutierrez F R, Hildebolt C, Sweet S. Post-lung transplantation bronchiolitis obliterans syndrome: usefulness of expiratory thin- section CT for diagnosis. Radiology. 2001;220:455–462. doi: 10.1148/radiology.220.2.r01au19455. [DOI] [PubMed] [Google Scholar]
- McAdams H P, Erasmus J J, Palmer S M. Complications (excluding hyperinflation) involving the native lung after single-lung transplantation: incidence, radiologic features, and clinical importance. Radiology. 2001;218:233–241. doi: 10.1148/radiology.218.1.r01ja45233. [DOI] [PubMed] [Google Scholar]
- Hearne S E, O'Laughlin M P, Davis R D, Baker W A, Bashore T M, Harrison J K. Total pulmonary artery occlusion immediately after lung transplantation: successful revascularization with intravascular stents. J Heart Lung Transplant. 1996;15:532–535. [PubMed] [Google Scholar]
- Berger H, Steiner W, Schmidt D, Forst H, Dienemann H. Stent-angioplasty of an anastomotic stenosis of the pulmonary artery after lung transplantation. Eur J Cardiothorac Surg. 1994;8:103–105. doi: 10.1016/1010-7940(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Yoon H, Huddleston C B, Miyoshi S, Matsuda H, Kamiike W, Patterson G A. Pulmonary function after living donor lung transplantation. Transplant Proc. 2001;33:1626–1627. doi: 10.1016/s0041-1345(00)02619-1. [DOI] [PubMed] [Google Scholar]
- Sritippayawan S, Keens T G, Horn M V, Starnes V A, Woo M S. What are the best pulmonary function test parameters for early detection of post-lung transplant bronchiolitis obliterans syndrome in children? Pediatr Transplant. 2003;7:200–203. doi: 10.1034/j.1399-3046.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- Trulock E P. Flexible bronchoscopy in lung transplantation. Clin Chest Med. 1999;20:77–87. doi: 10.1016/s0272-5231(05)70128-3. [DOI] [PubMed] [Google Scholar]
- Rio B, Guesde R, Jacquens Y, et al. Fiberoptic bronchoscopy in brain-dead organ donors. Am J Respir Crit Care Med. 1994;150:558–560. doi: 10.1164/ajrccm.150.2.8049847. [DOI] [PubMed] [Google Scholar]
- Kukafka D S, O'Brien G M, Furukawa S, Criner G. Surveillance bronchoscopy in lung transplant recipients. Chest. 1997;111:377–381. doi: 10.1378/chest.111.2.377. [DOI] [PubMed] [Google Scholar]
- Iacono A T, Corcoran T E, Griffith B P, et al. Aerosol cyclosporin therapy in lung transplant recipients with bronchiolitis obliterans. Eur Respir J. 2004;23:384–390. doi: 10.1183/09031936.04.00058504. [DOI] [PubMed] [Google Scholar]
- Corcoran T E, Smaldone G C, Dauber J H, et al. Preservation of post-transplant lung function with aerosol cyclosporine. Eur Respir J. 2004;23:378–383. doi: 10.1183/09031936.04.00059204. [DOI] [PubMed] [Google Scholar]
- Baz M A, Layish D T, Govert J A, et al. Diagnostic yield of bronchoscopies after isolated lung transplantation. Chest. 1996;110:84–88. doi: 10.1378/chest.110.1.84. [DOI] [PubMed] [Google Scholar]
- Kelly W F, Sanghani S, Barnett S D, Burton N, Nathan S. Significance of early bronchoscopic airway abnormalities after lung transplantation. J Heart Lung Transplant. 2003;22:583–586. doi: 10.1016/s1053-2498(02)00572-7. [DOI] [PubMed] [Google Scholar]
- Spatenka J, Khaghani A, Irving J D, Theodoropoulos S, Slavik Z, Yacoub M H. Gianturco self-expanding metallic stents in treatment of tracheobronchial stenosis after single lung and heart and lung transplantation. Eur J Cardiothorac Surg. 1991;5:648–652. doi: 10.1016/1010-7940(91)90121-y. [DOI] [PubMed] [Google Scholar]
- Carre P, Rousseau H, Lombart L, et al. Balloon dilatation and self-expanding metal Wallstent insertion. For management of bronchostenosis following lung transplantation. The Toulouse Lung Transplantation Group. Chest. 1994;105:343–348. doi: 10.1378/chest.105.2.343. [DOI] [PubMed] [Google Scholar]
- Higgins R, McNeil K, Dennis C, et al. Airway stenoses after lung transplantation: management with expanding metal stents. J Heart Lung Transplant. 1994;13:774–778. [PubMed] [Google Scholar]
- Sonett J R, Keenan R J, Ferson P F, Griffith B P, Landreneau R J. Endobronchial management of benign, malignant, and lung transplantation airway stenoses. Ann Thorac Surg. 1995;59:1417–1422. doi: 10.1016/0003-4975(95)00216-8. [DOI] [PubMed] [Google Scholar]
- Susanto I, Peters J I, Levine S M, Sako E Y, Anzueto A, Bryan C L. Use of balloon-expandable metallic stents in the management of bronchial stenosis and bronchomalacia after lung transplantation. Chest. 1998;114:1330–1335. doi: 10.1378/chest.114.5.1330. [DOI] [PubMed] [Google Scholar]
- Lonchyna V A, Arcidi J M, Jr, Garrity E R, Jr, et al. Refractory post-transplant airway strictures: successful management with wire stents. Eur J Cardiothorac Surg. 1999;15:842–849. doi: 10.1016/s1010-7940(99)00091-3. [DOI] [PubMed] [Google Scholar]
- Mulligan M S. Endoscopic management of airway complications after lung transplantation. Chest Surg Clin N Am. 2001;11:907–915. [PubMed] [Google Scholar]
- Chhajed P N, Malouf M A, Tamm M, Glanville A R. Ultraflex stents for the management of airway complications in lung transplant recipients. Respirology. 2003;8:59–64. doi: 10.1046/j.1440-1843.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- Perini S, Gordon R, Golden J, et al. Deformation and migration of Palmaz stents after placement in the tracheobronchial tree. J Vasc Interv Radiol. 1999;10:209–215. doi: 10.1016/s1051-0443(99)70466-5. [DOI] [PubMed] [Google Scholar]
- Sonett J R, Conte J V, Orens J, Krasna M. Removal and repositioning of “permanent” expandable wire stents in bronchial airway stenosis after lung transplantation. J Heart Lung Transplant. 1998;17:328–330. [PubMed] [Google Scholar]
- Susanto I, Peters J I, Levine S M, Sako E Y, Anzueto A, Bryan C L. Use of balloon-expandable metallic stents in the management of bronchial stenosis and bronchomalacia after lung transplantation. Chest. 1998;114:1330–1335. doi: 10.1378/chest.114.5.1330. [DOI] [PubMed] [Google Scholar]
- Saad C P, Ghamande S A, Minai O A, et al. The role of self-expandable metallic stents for the treatment of airway complications after lung transplantation. Transplantation. 2003;75:1532–1538. doi: 10.1097/01.TP.0000061229.83500.A0. [DOI] [PubMed] [Google Scholar]
- Burns K E, Iacono A T. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation. 2003;76:964–968. doi: 10.1097/01.TP.0000084523.58610.BA. [DOI] [PubMed] [Google Scholar]
- Clark S C, Levine A J, Hasan A, Hilton C J, Forty J, Dark J H. Vascular complications of lung transplantation. Ann Thorac Surg. 1996;61:1079–1082. doi: 10.1016/0003-4975(96)00003-3. [DOI] [PubMed] [Google Scholar]
- Waurick P E, Kleber F X, Ewert R, et al. Pulmonary artery stenosis 5 years after single lung transplantation in primary pulmonary hypertension. J Heart Lung Transplant. 1999;18:1243–1245. doi: 10.1016/s1053-2498(99)00091-1. [DOI] [PubMed] [Google Scholar]
- Pearson F G, Goldberg M, Stone R M, Colapinto R F. Bronchial arterial circulation restored after reimplantation of canine lung. Can J Surg. 1970;13:243–250. [PubMed] [Google Scholar]
- Schoenberger J A, Darcy M D. Bronchial artery embolization for hemoptysis in a lung transplant recipient. J Vasc Interv Radiol. 1995;6:354–356. doi: 10.1016/s1051-0443(95)72822-6. [DOI] [PubMed] [Google Scholar]
- Kennedy A S, Sonett J R, Orens J B, King K. High dose rate brachytherapy to prevent recurrent benign hyperplasia in lung transplant bronchi: theoretical and clinical considerations. J Heart Lung Transplant. 2000;19:155–159. doi: 10.1016/s1053-2498(99)00117-5. [DOI] [PubMed] [Google Scholar]
- Halkos M E, Godette K D, Lawrence E C, Miller J I., Jr High dose rate brachytherapy in the management of lung transplant airway stenosis. Ann Thorac Surg. 2003;76:381–384. doi: 10.1016/s0003-4975(03)00466-1. [DOI] [PubMed] [Google Scholar]