ABSTRACT
Injection sclerotherapy is an important primary and adjunctive therapy in the spectrum of care for superficial venous insufficiency. This article briefly reviews the history of the procedure, agents used, technique, and outcomes. The place of injection sclerotherapy in the treatment of superficial venous disease is discussed.
Keywords: Injection sclerotherapy, telangiectasia, varicose veins
Attempts at treating varicose veins have been documented as early as the fourth century BCE. Compression therapy, ligation, cautery, and phlebectomy were all reported by authors such as Celsus and Galen. The writings of Hippocrates (fourth century BCE) include a mention of treatment of varicose veins by thrombosis with a metal instrument. Given these observations, injection sclerotherapy appears to be a relative newcomer to the field of treatment for venous insufficiency.
Injection sclerotherapy is a technique or group of techniques for destruction of abnormal veins by injection of a medication that in some manner destroys the vein endothelium, leading to occlusion and subsequent fibrosis of the target vessel. The first attempts at sclerotherapy were reported in the 1680s,1 before the invention of the modern piston syringe. Modern sclerotherapy began in the first part of the 20th century with the techniques of Linser, Sicard, and others.2,3 Sclerotherapy was introduced to the United States in 1939 by McCausland, who reported an astounding series of 10,000 patients.4
Since the invention of synthetic sclerosants and the development of postsclerotherapy compression therapies in the 1940s and 1950s, injection sclerotherapy has assumed a major role in the treatment of venous insufficiency, particularly in the treatment of smaller veins such as telangiectasias and spider veins.
One can certainly query the need for an interventional radiologist to learn the technique of injection sclerotherapy. However, sclerotherapy is an important adjunct to other percutaneous treatment options for larger and deeper veins such as tributary varicosities and the saphenous veins and at times can be used to treat even these large vessels. Patients with superficial venous insufficiency often require multimodality treatments to obtain complete relief of their symptoms. The physician who limits him- or herself to one or a few techniques cannot offer the full range of therapies many patients may need, leading to frustration and disappointment in the patients and thus limiting practice development. Developing expertise and experience with injection sclerotherapy also opens new doors for the percutaneous treatment of vascular malformations and other entities, thereby widening the horizons of the practitioner.
SCLEROSANTS
The ideal sclerosant would cause full-thickness destruction of the wall of the vessel into which it was injected while creating minimal thrombus. Incomplete destruction of the vessel wall or local thrombosis may lead to recanalization. Thrombosis can also result in perivascular inflammation and hemosiderin staining of the overlying skin. The ideal agent would also be nontoxic, easily controlled, and painless. Unfortunately, no such agent presently exists, but currently available agents are fairly close approximations when used properly by experienced practitioners.
Sclerosants can be divided into three broad categories—osmotic agents, detergents, and irritant/corrosives.
Osmotic agents destroy the vessel wall by dehydration and cell wall disruption. Because they are rapidly diluted, their effect is typically very localized and their systemic toxicity is minimal. Unfortunately, they cause pain during injection as well as significant local damage if they are extravasated. The most commonly employed osmotic agents are hypertonic (23.4%) saline and Sclerodex™, a combination of hypertonic saline and dextrose.
Detergents act by disrupting cell membranes through the mechanism of protein theft denaturation, similar to the manner in which detergents are used to extract proteins in the chemistry laboratory. Detergents must be used at a concentration appropriate for the formation of micelles, which can then act on the cell membranes. Endothelial damage occurs within minutes of the administration of these agents and can spread farther from the injection site than can the damage caused by osmotic agents. The advantages of detergents are that their concentrations can be adjusted to match the size and type of vessel being treated and they can be made into foam, as discussed in more detail later. Sclerotherapy with foam enhances ultrasound image guidance and allows lower amounts of the active agent to be used with equivalent effect. Common detergent sclerosants include polidocanol, sodium tetradecyl sulfate, sodium morrhuate, and ethanolamine oleate. The latter two are used for endoscopic injection of bleeding varices and have complication profiles too high for safe use for varicose veins.
Irritant/corrosive agents act by a variety of mechanisms to destroy cell membranes. These agents vary greatly in chemical nature and properties, and some are quite dangerous. Most interventional radiologists are familiar with the destructive power of alcohols such as ethanol and phenol. Other irritant/corrosives include polyiodinated iodine (which can cause full-thickness vessel destruction in seconds), chromated glycerine, and glycerine/lidocaine/epinephrine (one of the mildest available agents in this category).
In clinical practice, the most commonly used sclerosants appear to be hypertonic saline, sodium tetradecyl sulfate, polidocanol, and glycerine/lidocaine/epinephrine. The former is approved by the Food and Drug Administration (FDA) for the treatment of hyponatremia, and its use as a sclerosant is off label. Sodium tetradecyl sulfate has recently been approved for sclerotherapy by the FDA. The other agents are not currently approved by the FDA. Their use in the United States, although widespread, requires an overseas source or the cooperation of a compounding pharmacy and may be challenged. The unapproved nature of these agents should be discussed with the patient before treatment.
FOAM SCLEROTHERAPY
At a minimum, the injected sclerosant must remain in contact with the vessel wall (dwell time) long enough to have the desired effect on the endothelium. Ideally, a sclerosant should stay in the target vessel until that vessel has fibrosed or should wash out from the vessel after a dwell time sufficient to damage the target without damaging any vessels into which it subsequently comes in contact. In the 1970s, a technique called “air block” was introduced.5,6 By injecting a small bubble of air into the target vessel and then injecting the sclerosant in the center of the bubble, a smaller bubble was formed at the beginning and end of the sclerosant column. This was thought to minimize the risk of extravasation and to decrease thrombus formation because the admixing of blood and sclerosant was minimized. Neither of these theoretical advantages has been definitively substantiated, and the air-block technique can be technically very difficult. After 10 years or so, it was largely abandoned.7
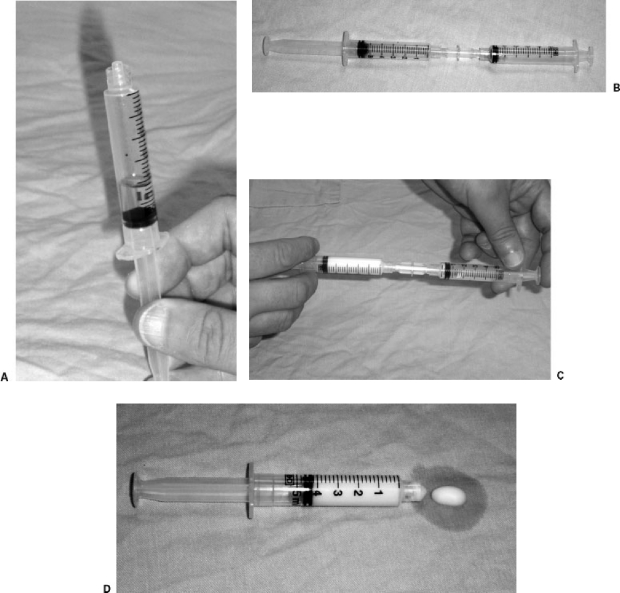
The successor to the air-block technique is microfoam sclerotherapy. Creating a foam with detergent sclerosants allows a more efficient sclerosant effect by increasing both dwell time and contact area while minimizing the admixture of sclerosant and blood. This increase in efficiency also allows lower sclerosant doses. Foam sclerotherapy was introduced in 1985,8 and several methods have been recommended for creating the foam. The method used by this author is a variant of the method of Tessari,9 who described the agitation of a mixture of sclerosant and air between two syringes attached to the arms of a three-way stopcock. The author uses a female-to-female Luer connector instead of a stopcock (Fig. 1). With either device, the operator mixes 1–2 mL of liquid sclerosant with 3–5 mL of air and vigorously squirts the mixture back and forth between syringes. Once the foam is created, it is stable and usable for times ranging up to several minutes, depending on the concentration of sclerosant used to make the foam.
Figure 1.
Preparation of sclerosant foam. (A) Approximately 1 mL of sclerosant solution (in this case 0.3% sodium tetradecyl sulfate) is drawn into a 5-mL disposable syringe, followed by 4–4.5 mL of room air. (B) The syringe containing the sclerosant solution and air is attached to a female-to-female Luer adapter, and an empty 5-mL syringe is attached to the other end of the adapter. (C) The contents are briskly agitated between the two syringes. Only three or four cycles are needed. (D) The finished microfoam is drawn into one of the syringes and ready for use. The stability of the microfoam varies with the concentration of the sclerosant.
In addition to superficial venous insufficiency, male varicocele and female pelvic congestion (ovarian vein) syndrome have been treated with sclerosant foam.10,11 The author has attempted to use a microfoam in one case of pelvic congestion syndrome, only to see visible embolization of the foam to the central veins (without any adverse sequelae).
INDICATIONS FOR AND CONTRAINDICATIONS TO SCLEROTHERAPY
In the hands of an experienced sclerotherapist, any abnormal vein, from the tiniest of spider veins to the largest of refluxing great saphenous veins, can be treated by sclerotherapy. The author uses injection sclerotherapy as a primary treatment for spider veins, telangiectasias, and reticular veins. Injection sclerotherapy is also used to treat larger veins that cannot be easily reached for phlebectomy or endovenous ablation, such as the spiral collaterals of the great saphenous vein seen as a recurrence after ligation and stripping.
As the size of the vessel increases, the amount and strength of sclerosant used must also increase. This, plus the increasing likelihood of spillover into the deep venous system, means that there is a greater risk of complications when one is treating larger veins. Contraindications to sclerotherapy include allergic sensitivity to the sclerosant, pregnancy, infection, deep venous thrombosis (DVT) (especially patients in whom the deep system is occluded and the extremity drained through the superficial system), and severe arterial disease. The patient must also be able and willing to comply with postsclerotherapy instructions regarding activity and use of compression bandages or hose.
METHOD
The concentration of sclerosant used in any application is determined by the size of the vessel being treated and the agent being employed. Small spider veins (red veins up to 1 mm in diameter) may be treated with hypertonic saline, glycerine/lidocaine/epinephrine (glycerine 72% mixed 2:1 with 1% lidocaine with epinephrine 1:100,000, prepared at physician's order by a compounding pharmacy) or 0.1% sodium tetradecyl sulfate (Fibro-Vein, STD Pharmaceuticals, Hereford, England, www.stdpharm.co.uk) as liquid or foam. Telangiectasias (bluish veins 1–3 mm in diameter, often occurring in fan-shaped arrays from a source reticular vein) can be treated with hypertonic saline or microfoams prepared from sodium tetradecyl sulfate in concentrations of 0.1% or 0.3% (for larger vessels). Reticular veins and tributary varicosities can be treated with hypertonic saline or microfoams prepared from 0.3% or 1% sodium tetradecyl sulfate, and saphenous veins or very large tributary varicosities are treated with microfoam prepared from 3% sodium tetradecyl sulfate. Veins larger than 4 mm are generally not responsive to hypertonic saline.
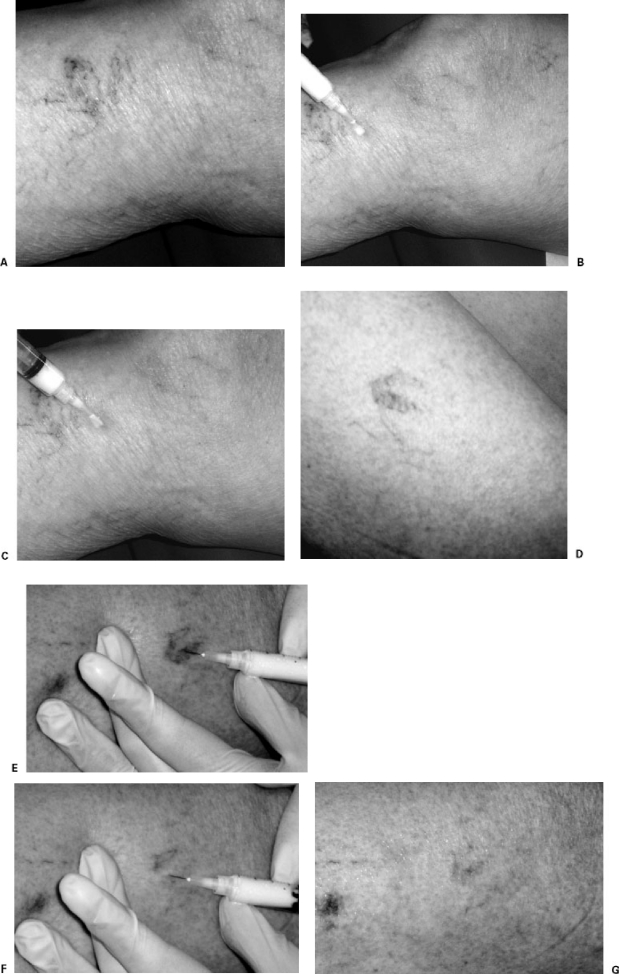
Once the appropriate sclerosant is drawn into a 3- or 5-mL disposable syringe, a 30G needle is attached and (at the operator's preference) may be bent at a shallow angle. The patient is positioned in a way that is comfortable and allows access to the target veins, and the skin is prepared with alcohol. The target vein is entered with the needle as close to parallel with the skin as possible and the sclerosant injected. Most practitioners advocate verifying an intravascular location of the needle tip by aspirating a small amount of blood into the hub of the needle before injection. This is easier with larger vessels. Sclerosant is injected into the vein until the area around the puncture site blanches or resistance is felt, and the injection is immediately discontinued if there is evidence of extravasation (most often manifest as the development of a wheal). Most individual injections utilize between 0.1 and 0.5 mL, although larger volumes are required for larger veins. In most superficial vessels, the replacement of the blood content by sclerosant can be followed visually (Fig. 2).
Figure 2.
Injection sclerotherapy. (A) Patient with painful small varicosities and telangiectasias. Note the varicosity along the lateral aspect of the proximal lower leg. (B) After skin preparation the vessel has been punctured with a 30G needle. The syringe contains microfoam prepared from 0.3% sodium tetradecyl sulfate. (C) After injection of ∼1 mL of microfoam, note that the blood in the varicosity has been replaced by microfoam, changing the color of the vessel. The telangiectasias were treated with microfoam prepared from 0.1% sodium tetradecyl sulfate. (D) Patient with telangiectasia of the thigh. Pretreatment photograph. (E) A vessel of the lesion is cannulated with a 30G needle. The syringe contains microfoam prepared from 0.1% sodium tetradecyl sulfate. (F) After injection, there is clearing of the lesion as the blood is replaced by microfoam. (G) After two injections the lesion is completely filled with microfoam.
Fairly large areas can be treated by multiple injections at any one session, depending on the tolerance of the patient and hand fatigue of the treating physician. No more than 10 mL of glycerine/lidocaine/epinephrine should be used at any one session, or patients may develop transient hematuria. This is harmless but can be disconcerting. There is no generally recognized maximum dose for hypertonic saline or sodium tetradecyl sulfate, with recommendations for the latter ranging from 120 to 300 mg (4–10 mL of 3% stock solution). The author usually limits himself to a maximum of 15 mL of microfoam per treatment session.
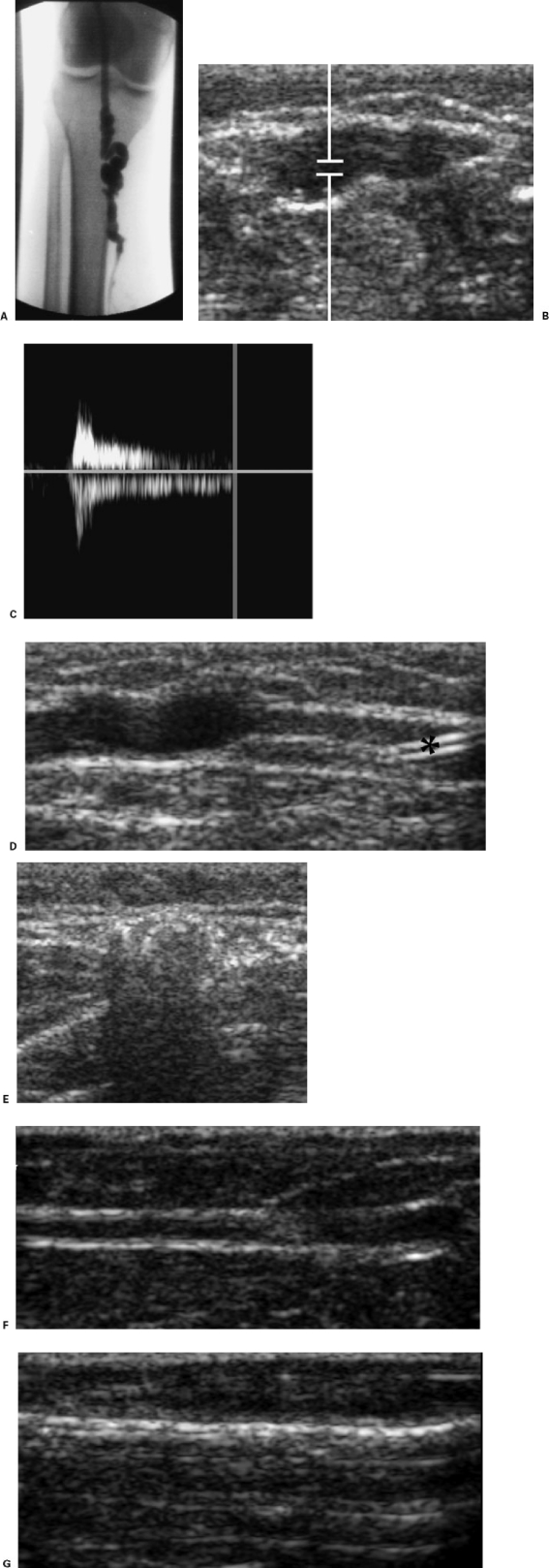
Larger, deeper veins, such as the saphenous veins, should be treated under ultrasound guidance. Access to the vessel with a needle or a micropuncture access set is performed with continuous ultrasound imaging. Sclerosant foam is particularly useful in such applications, both because of its larger surface contact area and because it is so clearly demonstrated sonographically. When the treatment area approximates a deep vein or other nontarget vessel (such as the saphenofemoral or saphenopopliteal junction or a perforating vein), the ultrasound probe can be used to occlude the communication point to prevent spillover into the deep system (Fig. 3).
Figure 3.
Ultrasound-guided injection sclerotherapy. (A) Patient with refluxing short saphenous vein (SSV). Attempted radiofrequency ablation of the vein was unsuccessful because the vessel was too tortuous for catheterization. (B) Transverse ultrasound image of the SSV caudal to the saphenopopliteal junction before injection sclerotherapy. (C) Doppler tracing shows prolonged reflux after augmentation. (D) Longitudinal ultrasound image of the SSV shows a 20G Angiocath (*) inserted under real-time ultrasound guidance. Approximately 3 mL of microfoam created from 3% sodium tetradecyl sulfate 1:5 with room air was injected under real-time sonographic monitoring. When the column of microfoam approached the saphenopopliteal junction, this point of potential communication was compressed for 5 minutes to prevent the spilling of microfoam into the popliteal vein. (E) Transverse image of the SSV at about the same level as (B) showing echogenic microfoam filling the vessel lumen. (F) In a different patient, longitudinal image of residual great saphenous vein lumen after attempted endovenous ablation. Reflux was still present by color flow. (G) Note echogenic microfoam filling lumen after injection of microfoam prepared from 3% sodium tetradecyl sulfate.
POSTPROCEDURE CARE
After injection sclerotherapy, prolonged compression of the treated veins is essential to help with the healing process. Compression keeps the treated vessel walls apposed, limiting the likelihood of recanalization. When the treated veins fill with blood before they reach a state of fibrosis, the likelihood of coagulum formation, with attendant pain, skin staining, and ultimate failure of occlusion, is increased. This is discussed subsequently.
At minimum, the treated areas should be dressed with a long-stretch bandage (e.g., Ace). Focal pressure over the treated veins can be increased with pads or cotton balls. If a patient has surgical compression hose (as is often the case when injection sclerotherapy is used as an adjunct to endovenous vein ablation), these should also be worn. The author currently instructs patients to wear the long-stretch bandage or compression hose continually for at least 24 hours after injection and whenever out of bed for 10–14 days thereafter. At minimum, patients must wear the compression bandage or hose for 3–5 days.
Patients should be encouraged to go about normal activities, excluding heavy lifting or exertion, after injection sclerotherapy. They are seen in the office 1–2 weeks after injection sclerotherapy to assess for trapping of coagulum and any complications. Additional veins in other areas can be treated at this time, but retreatment of any single area should be delayed for 6–8 weeks to allow the treated veins to heal fully; in this manner, unnecessary retreatment of an effectively sclerosed vein is not performed.
ADVERSE SEQUELAE AND COMPLICATIONS OF INJECTION SCLEROTHERAPY
All treated veins contain some extent of thrombus after treatment. In some cases, this causes a local superficial phlebitis with symptoms of discomfort and discoloration. The thrombus usually begins to liquefy within 1–2 weeks of injection and should be drained if there is visible trapping of blood within the vein or if the patient complains of discomfort. Drainage of trapped coagulum is a simple procedure: the skin overlying the target vein is prepared with alcohol and the target vein punctured with an 18G hypodermic needle. Liquid and semiliquid coagulum is manually expressed by pressure on the vein and surrounding tissues. Drainage of coagulum may need to be repeated, especially from larger veins.
Postsclerotherapy hyperpigmentation is the occurrence of brown-black staining of the skin overlying the treated veins. It is common, with reported incidences ranging from 2% to 80%, and appears to depend upon the choice and concentration of sclerosant solution, vessel size, injection technique, and postprocedure care. Skin staining is caused by the deposition of hemosiderin in the tissues around the treated vein.12 Skin staining can be worsened by the presence of undrained coagulum. Skin staining usually fades with time and is most often resolved within 6–12 months of treatment. On rare occasions it can persist beyond a year. Unfortunately, there is no reliable treatment for persistent skin staining, although transcutaneous laser treatment appears to have the greatest success.
Telangiectatic matting is the appearance of a complex of fine red veins around a treated vein after sclerotherapy. It is probably due to neovascularization of the treated tissues and occurs in ∼16% of patients.13 Matting is a source of frustration to both patient and physician but (like skin staining) usually resolves without therapy. If persistent, it can be treated by further injection sclerotherapy or transcutaneous laser, or both.
Skin necrosis can occur at puncture sites, especially if there has been extravasation of sclerosant. This is usually very focal and heals without therapy in a short time. More worrisome is the possibility of inadvertent injection of sclerosant into an arteriole. This rare complication may result in areas of ischemia and skin necrosis that may be larger but usually heal. There are a few reports of arteriolar or arterial injection leading to large areas of ischemia and necrosis with disastrous results.14
Overspill of sclerosant into the deep system can cause DVT if the concentration of sclerosant is high enough to damage the endothelium of the deep veins. This is of particular concern when treating vessels in the knee and thigh, where the deep vessels in question (femoral and popliteal veins) are unpaired and thus without available collaterals. More central complications of nontarget sclerotherapy are rare. Nonetheless, there have been a few reports of patients experiencing transient scotomata15 after being treated with sclerosing foam. The etiology of this experience is unclear, but it may be due to small amounts of foam crossing a clinically silent atrial septal defect. The author has seen this occur in one patient, who had complete resolution of symptoms within a few minutes.
If one accepts that the accumulation of coagulum and occasional skin staining are not true complications but rather expected sequelae of injection sclerotherapy, complications are rare and usually minor.16 To date, the author has had no severe (SIR classification grade C–F) complications, and the incidence of minor complications has been under 5% (most requiring no therapy—SIR classification A).
RESULTS
There have been several studies reporting the results of sclerotherapy in small series of patients. The majority of these show clinical success rates of 80–90% for the resolution of injected vessels. Using microfoam techniques, over 90% of treated vessels resolve, especially telangiectasias, reticular veins, and small tributary varicosities.16
CONCLUSIONS
When properly applied, injection sclerotherapy can be successful in resolving 90% or more of treated vessels. It is well tolerated, rapid, and has relatively low morbidity. For some vessels it is clearly the best therapy available, and it is a valuable option for many others. Injection sclerotherapy is a necessary tool in the kit of any physician seeking to develop a practice treating superficial venous disease.
REFERENCES
- Goldman M P, Bergan J J. Sclerotherapy: Treatment of Varicose and Telangiectatic Leg Veins. 3rd ed. St. Louis: Mosby; 2001. pp. 1–6.
- Linser P. Über die Konservative Behandlung der Varicen. Med Klin. 1916;12:897–902. [Google Scholar]
- Sicard J A, Roger H. Traitement des varices par injections intraveineusses locales de carbonate de soude. Marseille Med. 1921;4:97. [Google Scholar]
- McCausland S. The modern treatment of varicose veins. Med Press Circ. 1939;201:404–410. [Google Scholar]
- Foley W T. The eradication of venous blemishes. Cutis. 1975;15:665–668. [Google Scholar]
- Alderman D B. Therapy for essential cutaneous telangiectasias. Postgrad Med. 1977;61:91–95. doi: 10.1080/00325481.1977.11714509. [DOI] [PubMed] [Google Scholar]
- Duffy D M. Small vessel sclerotherapy: an overview. Adv Dermatol. 1988;3:221–242. [PubMed] [Google Scholar]
- Green A R, Morgan B DG. Sclerotherapy for venous flare. Br J Plast Surg. 1985;38:241–242. doi: 10.1016/0007-1226(85)90057-8. [DOI] [PubMed] [Google Scholar]
- Tessari L. Metodique extemporaine de la préparation de la “scléromousse” en syringe de plastique monousage. Paris, France: Presented at the French Society of Phlebology, December 11; 1999.
- Gandini R, Reale C A, Spinelli A, Konda D, Pendenza G, Simonetti G. Transcatheter embolization of male varicocele with fibro-vein mousse infusion: experience with 230 patients. Barcelona, Spain: Presented at Annual Meeting and Postgraduate Course of the Cardiovascular and Interventional Radiology Society of Europe (CIRSE), September 25–29; 2004.
- Gandini R, Fabiano S, Spinelli A, et al. Ovarian varices treatment by transcatheter embolization. Lucerne, Switzerland: Presented at Annual Meeting and Postgraduate Course of the Cardiovascular and Interventional Radiology Society of Europe (CIRSE), October 5–9; 2002.
- Goldman M P, Kaplan R P, Duffy D M. Postsclerotherapy hyperpigmentation: a histologic evaluation. J Dermatol Surg Oncol. 1987;13:547–550. doi: 10.1111/j.1524-4725.1987.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Davis L T, Duffy D M. Determination of incidence and risk factors for postsclerotherapy telangiectatic matting of the lower extremity: a retrospective analysis. J Dermatol Surg Oncol. 1990;16:327–330. doi: 10.1111/j.1524-4725.1990.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Bergan J J, Weiss R A, Goldman M P. Extensive tissue necrosis following high-concentration sclerotherapy for varicose veins. Dermatol Surg. 2000;26:535–542. doi: 10.1046/j.1524-4725.2000.00033.x. [DOI] [PubMed] [Google Scholar]
- Cabrera J, Cabrera J, Garcia-Olmedo M A. Sclerosants in microfoam: a new approach in angiology. Int Angiol. 2001;20:322–329. [PubMed] [Google Scholar]
- Frullini A, Cavezzi A. Sclerosing foam in the treatment of varicose veins and telangiectases: history and analysis of safety and complications. Dermatol Surg. 2002;28:11–15. doi: 10.1046/j.1524-4725.2002.01182.x. [DOI] [PubMed] [Google Scholar]
SUGGESTED READINGS
- American College of Phlebology Web site Available at: www.phlebology.org Available at: www.phlebology.org
- Goldman M P, Bergan J J. Sclerotherapy: Treatment of Varicose and Telangiectatic Leg Veins. 3rd ed. St. Louis: Mosby; 2001.
- Weiss R A, Feied C, Weiss M A. Vein Diagnosis and Treatment: A Comprehensive Approach. New York: McGraw-Hill; 2001.