ABSTRACT
Chronic venous insufficiency is a complex condition, with widely varied clinical manifestations, etiologies, and underlying pathophysiology. An orderly workup is mandatory to assess the nature of a patient's underlying venous disease. This begins in the office setting with a careful medical history, physical examination, and bedside diagnostic tests. These are augmented by confirmatory diagnostic testing, including duplex ultrasonography, venography, plethysmography, and ambulatory venous pressure measurement. Based upon the results of these examinations, the patient's venous disease can be classified according to standardized classification schemes, which in turn leads to the selection of an appropriate treatment strategy. This article outlines the steps in the clinical assessment and classification of patients with chronic venous insufficiency.
Keywords: Chronic venous insufficiency, diagnosis, patient classification, duplex scanning, venous severity scoring
Historically, the condition known as chronic venous insufficiency has suffered from a lack of detailed, objective investigation into its pathophysiology and treatment. With current technology, however, specific investigations can clearly delineate the cause of chronic venous insufficiency in a particular patient and help guide the clinician to appropriate therapeutic interventions. An orderly and objective diagnostic workup is mandatory. The following steps in diagnosis and clinical assessment of the patient with chronic venous disease have been proposed1:
Determine the nature of the problem.
Determine the severity of the problem.
Perform diagnostic testing.
Determine CEAP classification.
Weigh treatment alternatives.
INITIAL CLINIC EVALUATION
History
Initial evaluation begins in the office setting with a thorough history and physical examination. Typical symptoms of venous insufficiency include aching, pain, tightness, skin irritation, pruritus, heaviness, tingling, muscle cramps, and cosmetically unsatisfying varicose veins.2 Symptoms often worsen during the course of the day and with prolonged standing. Patients with more severe insufficiency can present with complaints of edema, skin changes, or ulceration.3 Several clinical entities can mimic the symptoms of chronic venous insufficiency, including osteoarthritis, sciatica, osteomyelitis, tendonitis, ligamentous injuries, arthritis, peripheral neuropathy, and arterial insufficiency. Specific features of the pain that should be noted include the degree to which the pain interferes with the patient's occupation or lifestyle as well as the amount of time that the patient can stand before the onset of pain or swelling. The age of onset of varicose veins should be recorded, as an early onset may suggest a congenital abnormality such as Klippel-Trenaunay syndrome.4 A family history of varicose veins is present in over one third of patients.4 It is crucial to note whether there has been a personal history of deep vein thrombosis or pulmonary embolism. Some patients may not be able to provide this information directly, and it may be elicited only by asking specific questions regarding a history of leg swelling, previous operations, lower extremity injuries, prolonged bed rest, chest pain, hemoptysis, or anticoagulant use. Finally, a careful history of past treatments for varicose veins, including operative and percutaneous procedures, should be recorded.
Physical Examination
Physical examination of the patient should include a general examination in addition to a detailed examination of the lower extremities. The patient should be examined in the standing position and suitably undressed to permit complete examination of the entire extremity from the groins to the toes. The examination should be performed in a warm and well-lit room that respects the privacy of the patient.4
The examination begins with careful inspection and palpation of the legs. The location and distribution of all major subcutaneous varicosities should be noted and recorded in the chart; this is facilitated by outline drawings of each limb that show both anterior and posterior surfaces. Varicosities of the main saphenous trunk and spider veins should be noted. The limb is also inspected for the presence or absence of edema and angiomatous malformations. Large varicosities over known sites of perforating veins should be identified. Palpation of the limbs may detect additional varicosities that are not readily apparent by inspection. This is especially true of the terminal segments of the greater saphenous vein (GSV) and lesser saphenous vein (LSV) where they join the femoral and popliteal veins, respectively. Careful palpation of the inner thigh and leg as well as the posterior calf may detect saphenous trunk varicosities that may be missed by visual inspection alone. Palpation of the legs should also be performed to detect temperature differences between the legs, areas of induration, and the presence of firm subcutaneous cords, which may be the sequelae of prior episodes of superficial thrombophlebitis.
Several tests can be performed in the outpatient setting during the initial evaluation that often give clues to the source of venous hypertension. The cough impulse test is performed by palpating the thigh at the fossa ovalis over the saphenofemoral junction (SFJ) while the patient is standing. The patient is asked to cough, and a palpable thrill at the SFJ, which is a result of turbulent retrograde flow, indicates reflux at the SFJ. The cough impulse test is difficult to perform in obese patients or in patients who jerk or cough vigorously. The tap test, or percussion test, is also performed while palpating the SFJ of a standing patient. The GSV is tapped at the level of the knee. A palpable transmitted impulse at the SFJ suggests that the GSV is distended with blood. The SFJ is then tapped while the GSV is palpated at the knee. A palpable transmitted pulse at the knee with this maneuver indicates incompetence of GSV valves between the SFJ and the knee.5 These clinical tests are largely of historical interest in the modern era and have been supplanted by more sophisticated diagnostic imaging tests. When compared with Doppler ultrasonography, clinical tests (cough impulse test and percussion test, combined with palpation) were falsely negative in 28% of incompetent SFJs and 36% of incompetent saphenopopliteal junctions (SPJs).6
The Brodie-Trendelenburg test is used to detect venous incompetence and to differentiate between perforator and GSV incompetence. The test is performed by initially draining the superficial lower extremity veins by elevating the lower limbs to 45 degrees and gently stroking the limb from the foot along the course of the major veins. A tourniquet is then placed as close to the groin as possible and applied tightly enough to prevent superficial vein reflux. The patient is asked to stand and the limb is examined. If the distal veins remain collapsed for 15 to 30 seconds after standing, the tourniquet is released. SFJ incompetence is diagnosed if the distal veins fill rapidly upon release of the tourniquet. If the caudal veins fill rapidly when the patient stands with the tourniquet in place, perforator incompetence is suggested. The location of the incompetent perforator can then be determined by varying the position of the tourniquet. Rapid filling of the varices with a tourniquet in the suprapatellar position can identify an incompetent midthigh perforator, and rapid filling with the tourniquet below the knee suggests incompetent lower leg perforators. In the case of combined SFJ incompetence and perforator incompetence, direct palpation of the varix during tourniquet release may detect an increase in venous distention.4 The Brodie-Trendelenburg test is highly sensitive for the identification of superficial and perforator reflux (91%), although poorly specific (15%).5
The Perthes test is performed with the patient in the standing position with a tourniquet positioned below the knee. The patient is asked to activate the calf muscle pump by performing 10 heel raises. Emptying of the varicose veins signifies a site of reflux cranial to the tourniquet, namely the SFJ, SPJ, or thigh perforators. Persistence of distended varicose veins signifies a site of reflux caudal to the tourniquet, that is, calf perforators. Pain associated with heel raising suggests the possibility of deep venous obstruction. As with the Trendelenburg test, the Perthes test is highly sensitive but poorly specific.
Perhaps the most commonly performed maneuver in the office setting is hand-held continuous -wave Doppler ultrasound examination of the saphenous vein. With the patient in the standing position, the SFJ is insonated while an assistant performs compression of the caudal calf. GSV incompetence can be demonstrated by eliciting reflux with the release of calf compression. Similar interrogation of the SPJ can be performed to detect LSV incompetence. Doppler examination of the SFJ to diagnose incompetence has a sensitivity and specificity of 97% and 73%, respectively, when compared with duplex ultrasonography as the “gold standard.”5
ADDITIONAL DIAGNOSTIC STUDIES
Venography
The historical gold standard for the diagnosis of venous insufficiency, in terms of both anatomic localization and hemodynamic quantification, has been venography. Although there are still situations in which venography is necessary for planning treatment, it has several drawbacks that have reduced its once widespread use. Venography is an invasive procedure and carries with it attendant risks. Extravasation of contrast material into the foot can cause a chemical cellulitis and, rarely, this can progress to tissue necrosis, ulceration, or gangrene.7 Other complications include postphlebographic thrombosis (reported in up to 13% of patients8) and the postphlebographic syndrome, characterized by pain, tenderness, and erythema around the ankle joint not associated with thrombosis. There are other limitations of venography besides potential technical complications. Competent valves in the upper leg can obscure valvular reflux in the lower leg, and pressurized contrast injection can create false-positive results. Finally, the venographically observed severity of reflux does not necessarily correlate with the clinical severity of the disease. Contraindications to venography are few and include known contrast allergy and local infection.
Ascending venography is typically performed to determine the degree of patency of the deep venous system and to identify the presence of incompetent perforators.9 It is performed with the patient in reverse Trendelenburg position with the limb to be examined in a relaxed non–weight-bearing position. A superficial vein on the lateral aspect of the dorsum of the foot is selected and cannulated with a 21-gauge butterfly needle directed caudally. A tourniquet is inflated above the ankle or the knee to improve deep venous filling (as opposed to filling of the saphenous veins) and assess for perforator incompetence. Then 50 to 100 cm3 of nonionic contrast material is slowly injected by hand and the calf veins are examined under fluoroscopy. The injection site should be visualized intermittently to ensure that there is no extravasation of contrast or immediately if there is any local swelling or pain. If contrast extravasation occurs, the injection should be immediately terminated and the extravasated contrast dispersed by means of saline dilution, massage, and/or warm compresses. After examination of the calf veins, the popliteal, femoral, and iliac veins are sequentially imaged. Opacification of the more cranial veins can be enhanced by raising the calf, having the patient lie supine, and releasing the tourniquet. Distorted veins and valve cusps, excessive collaterals (especially around the thigh, knee, and iliofemoral region), and intraluminal filling defects are pathognomonic for post-thrombotic disease, and their absence suggests primary valvular incompetence.8
Descending venography is used to document the presence and extent of reflux, to define valvular anatomy, and to identify specific incompetent valves. It is also performed with the patient in reverse Trendelenburg position and with the limb to be examined in a relaxed non–weight-bearing position. A catheter is positioned in the common femoral vein, either by direct puncture or by advancement from an arm or the contralateral femoral vein. A slow hand injection of contrast material is performed under fluoroscopy and the patient is instructed to breathe normally. If reflux is identified, individual incompetent valves in the common femoral vein, profunda femoris vein, superficial femoral vein, and GSV are noted. Contrast injection is then repeated while the patient is instructed to perform a sustained Valsalva maneuver. This increases resistance to prograde flow and causes valve closure. Incompetent valves are noted to allow leakage and retrograde flow of contrast material. Individual valve function can be classified as normal (no leakage despite Valsalva), minimal abnormality (wisp of contrast reflux with Valsalva), moderate abnormality (contrast reflux with Valsalva), and severe abnormality (contrast material pours through valve).10 In addition to valve function, the caudal extent of reflux through incompetent valves should be noted.
Ambulatory Venous Pressure Measurement
Along with venography, ambulatory venous pressure (AVP) measurement was a historical gold standard for the diagnosis of chronic venous insufficiency. It is performed by introducing into a dorsal foot vein a 21-gauge needle, which is then connected to a pressure transducer. Baseline measurement is obtained with the patient relaxed and bearing weight on the contralateral limb. In this position, venous pressure approximates the hydrostatic pressure exerted by the column of blood extending from the right atrium to the foot.8 The patient is then asked to perform 10 tiptoe movements at the rate of one per second. The AVP is then measured as the lowest pressure achieved at the end of exercise. The recovery time is defined as the time required for the pressure to rise to 90% of the baseline value after the cessation of exercise. A modification of this technique involves manual calf compression instead of tiptoe movements. This modification has been shown to produce identical results while avoiding the potential difficulties of the patient's cooperation and reactive hyperemia.11
In healthy limbs, calf muscle contraction forces blood up the leg and competent valves prevent retrograde flow.8 During AVP measurement, the venous blood pressure falls rapidly (typically to less than 30 mm Hg) and recovery, which is through capillary inflow, is slow (typically more than 20 seconds). In limbs with incompetent valves, reflux occurs between contractions and the measured pressure remains proportionately high while recovery, which occurs by rapid filling through incompetent valves, is fast. In limbs with deep venous obstruction, the AVP may rise during exercise and produce a bursting pain related to calf vein congestion (akin to a positive Perthes test). AVP has been correlated with the clinical severity of disease. The incidence of ulceration increases in a linear fashion with an increase in the AVP, and ulceration never occurs at AVP less than 30 mm Hg and always occurs at AVP greater than 90 mm Hg.12
Like venography, AVP measurement is an invasive procedure that is not ideally suitable for screening or for repeated examinations to monitor the results of therapy.
Plethysmography
Air plethysmography is a noninvasive test that can quantify venous insufficiency and is reported to correlate with AVP measurements.13 It is performed with a 35-cm-long polyvinyl chloride air chamber that surrounds the lower leg and is connected to a pressure transducer and chart recorder. The pressure transducer is calibrated with 100 mL of air. A baseline reading is obtained with the patient supine and with the leg elevated to 45 degrees to empty the veins. The patient is then asked to stand upright with weight supported on the opposite leg until the veins are full. This change in volume represents the functional venous volume, and the venous filling index (VFI) is calculated by dividing 90% of the venous volume by the time required for filling to 90% of the venous volume. The patient is then asked to perform a heel-raise maneuver and the volume displaced by this maneuver is recorded as the ejection volume. The ejection fraction (EF) is calculated by dividing the ejection volume by the venous volume. Finally, 10 heel-raise maneuvers are performed to reach a residual volume plateau. The residual volume fraction (RVF) is calculated by dividing the residual volume by the venous volume. The VFI is an index of global venous reflux, the EF is a reflection of calf muscle pump function, and the RVF is a reflection of AVP.
The limitations of air plethysmography are that it is imprecise in the localization of segmental reflux and the RVF correlates only loosely with disease severity. A VFI of 2.67 mL/s has been proposed as a cutoff point between normal limbs and limbs with chronic venous insufficiency, with a positive predictive value of 96%.14
Duplex Scanning
Duplex ultrasonography is arguably the most useful initial diagnostic evaluation in the workup of chronic venous disease. Its advantages include that it is noninvasive, can be repeated as often as necessary, gives reproducible results, and allows anatomic, physiologic, and hemodynamic evaluation of the venous system. The study is performed with both B-mode imaging and spectral Doppler analysis. It can identify the underlying pathophysiology (reflux, obstruction, or both) and localize the disease to specific venous segments (deep system, superficial system, perforators). A 5- to 7.5-MHz linear transducer is used to evaluate the limb below the inguinal ligament, and a 2- to 3.5-MHz phased array transducer is used to evaluate the iliac system and the inferior vena cava.
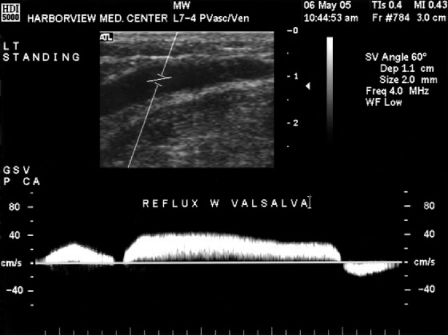
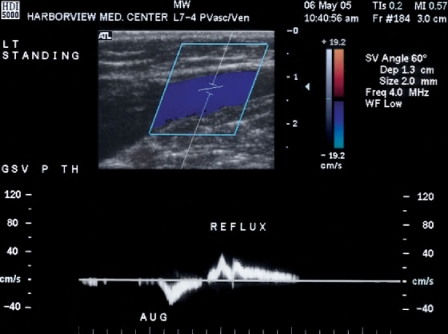
Duplex examination of the veins must be systematic and orderly. The deep venous system is evaluated first, with the patient in the supine position with the hip externally rotated and the knee flexed. The linear transducer is initially placed longitudinally just below the inguinal ligament to visualize the common femoral vein and the confluence of the superficial and deep femoral veins. Proper imaging of the popliteal vein is best performed in the prone position with the calf supported on pillows, although a lateral approach can suffice if the patient is unable to lie prone. Patency of the deep veins is evaluated both by assessing compressibility by the transducer during B-mode imaging and by evaluating flow patterns. Venous flow patterns in patent deep veins are spontaneous and phasic with respiration, although flow augmentation with foot compression may be required to document patency in the crural veins. After assessing patency, venous valvular competence is evaluated with a Valsalva maneuver for upper thigh segments (Fig. 1) and limb compression for lower limb segments (Fig. 2). Normal valve closure time is less than 2 seconds when the patient is in the supine position. For patients in mild reverse Trendelenburg position, valve closure time of greater than 0.5 seconds has a sensitivity of 90% and specificity of 84% for diagnosing valve incompetence when compared with descending venography.15 An alternative to the Valsalva maneuver for assessing valve competence is the rapid cuff deflation technique, which has the benefit of providing a single method for studying all of the veins of the limb without relying on the presence of a more cephalad incompetent valve.16
Figure 1.
Venous reflux induced in greater saphenous vein with the Valsalva maneuver. Patient is in the standing position.
Figure 2.
Augmented cephalad flow produced in the greater saphenous vein with calf compression followed by spontaneous reflux. Patient is in the standing position.
After evaluation of the deep venous system is performed, the superficial system is examined. This portion of the examination is ideally performed with the patient in the standing position while bearing weight on the contralateral limb. This position produces maximal venous distention while relaxing the calf pump, which allows provocative maneuvers to identify incompetent segments. The transducer is angled medially in the groin to visualize the SFJ. The GSV is examined for patency and competence throughout its length. Vein diameter is recorded and major tributaries are followed and similarly evaluated for patency and competence. Reflux is identified and documented with pulse wave Doppler imaging during and after an abrupt compression and release of the distal limb. The patient is then turned around, and similar investigation of the LSV is performed. The LSV has a more variable termination in the deep system and significant reflux may exist in the vein of Giacomini.17 Finally, the locations of known perforators should be evaluated for incompetence.
CLASSIFICATION
After obtaining a thorough history, performing a careful physical examination, and obtaining additional diagnostic imaging studies, a final classification of the patient's chronic venous disease can be made. Traditional classification systems were fairly simplistic and categorized disease on the basis of etiology. Examples include classification of disease as primary (idiopathic) or secondary (post-thrombotic) or as reflux due to greater saphenous reflux, lesser saphenous reflux, or perforator incompetence. Other more descriptive systems included Widmer's classification and Porter's classification.18
In the early 1990s, advances in the diagnosis and understanding of the complex pathophysiology of venous disorders led to development of the CEAP classification system. The first CEAP consensus document was developed at the sixth annual meeting of the American Venous Forum in 1994 and was recently revised,2 including the introduction of a basic CEAP version. The CEAP classification (Table 1) consists of four parameters: the clinical manifestation (C), the etiologic factors (E), the anatomic distribution of disease (A), and the underlying pathophysiology (P).
Table 1.
CEAP Classification
| C Class: Clinical Manifestation |
| C0: No visible or palpable signs of venous disease |
| C1: Telangiectasias or reticular veins |
| C2: Varicose veins, distinguished from reticular veins by a diameter ≥ 3 mm |
| C3: Edema |
| C4: Changes in skin and subcutaneous tissue |
| C4a: Pigmentation or eczema |
| C4b: Lipodermatosclerosis or atrophie blanche |
| C5: Healed venous ulcer |
| C6: Active venous ulcer |
| Note: Further subdivided with subscript S (symptomatic) or A (asymptomatic) |
| E Class: Etiologic Factors |
| Ec: Congenital |
| Ep: Primary |
| Es: Secondary (post-thrombotic) |
| En: No venous cause identified |
| A Class: Anatomic Distribution |
| As: Superficial veins |
| Ap: Perforator veins |
| Ad: Deep veins |
| An: No venous location identified |
| P Class: Pathophysiology |
| Pr: Reflux |
| Po: Obstruction |
| Pr,o: Reflux and obstruction |
| Pn: No venous pathophysiology identifiable |
The C class consists of six levels of clinical manifestations. In the basic form of the CEAP, the single highest applicable descriptor is used, whereas in the advanced form of the CEAP, every applicable descriptor is used. Each clinical class is further subdivided by a subscript for the presence of symptoms (S, symptomatic) or absence of symptoms (A, asymptomatic). The E class consists of four levels of etiologic factors, and the A class consists of four levels of anatomic distribution. The P class consists of four levels of underlying pathophysiology. In the basic form of the CEAP, no further subdivision is performed. In the complete (advanced) form of the CEAP, the underlying pathophysiology is further categorized according to 18 named venous segments (Table 2).
Table 2.
Venous Segments in the Complete CEAP P Class
| Superficial Veins |
| 1. Telangiectasias or reticular veins |
| 2. Greater saphenous vein above the knee |
| 3. Greater saphenous vein below the knee |
| 4. Lesser saphenous vein |
| 5. Nonsaphenous vein |
| Deep Veins |
| 6. Inferior vena cava |
| 7. Common iliac vein |
| 8. Internal iliac vein |
| 9. External iliac vein |
| 10. Pelvic (gonadal veins, broad ligament veins, other) |
| 11. Common femoral vein |
| 12. Deep femoral vein |
| 13. Femoral vein |
| 14. Popliteal vein |
| 15. Crural (paired peroneal, anterior tibial, posterior tibial veins) |
| 16. Muscular (gastrocnemial veins, soleal veins, other) |
| Perforating Veins |
| 17. Thigh |
| 18. Calf |
In addition to the actual classification, it is recommended that the level of investigation that led to the classification be added, according to the following levels:
Level I: Office visit with history and physical examination, with or without hand-held Doppler scanning
Level II: Noninvasive vascular laboratory testing, including duplex color scanning and plethysmography, as indicated
Level III: Invasive or complex imaging studies, including ascending and descending venography, venous pressure measurements, computed tomography, or magnetic resonance imaging.
The CEAP system has been widely published in several languages in journals around the world, making it a truly universal document. It is a complete classification system that accounts for all etiologies, pathophysiologies, and anatomic segments and, as such, has become the standard system used in most published clinical papers on chronic venous disease. Despite this, there are certainly limitations to the CEAP classification. It is rather complex and somewhat daunting for the new user and is difficult to use in everyday routine. This may be mitigated by the development of software packages to facilitate classification. Other limitations include the absence of strict hierarchy between the clinical classes, omission from the clinical classification of corona phlebectasia (fan-shaped intradermal telangiectasias on the medial or lateral aspect of the foot, felt by many to be an early sign of chronic venous insufficiency18), and lack of an accounting of the degree of reflux in the physiologic classification.19
VENOUS SEVERITY SCORING
Although the CEAP system is an excellent scheme for standardized classification of chronic venous disease, it is a relatively static system. The C (clinical) class of disease does represent a spectrum of disease severity, but it does not allow a practical assessment of change in response to treatment or adverse events. In response to this, the American Venous Forum's Ad Hoc Committee on Venous Outcomes proposed three different severity scoring systems to assess objectively an individual patient's response to treatment as well as to allow improved outcome assessment.20
The Venous Clinical Severity Score (VCSS) consists of nine clinical descriptors, each graded on a scale of 0 to 3 representing the spectrum of absent, mild, moderate, and severe features. The nine descriptors are pain, varicose veins, edema, pigmentation, induration, inflammation, number of active ulcers, duration of active ulceration, and size of the largest current ulcer (Table 3). This scoring system is designed to be complementary to, rather than a replacement of, the C class of the CEAP. As such, VCSS takes advantage of the progressive order of severity intrinsic to the C class but also gives additional weight to some of the upper level attributes. The VCSS also includes only elements that are dynamic over a relatively short period of time. The VCSS has been shown to be valid in relation to the C class and also reliable, with acceptable intraobserver variability.21
Table 3.
The Venous Clinical Severity Score
| Attribute | Absent = 0 | Mild = 1 | Moderate = 2 | Severe = 3 |
|---|---|---|---|---|
| GS, greater saphenous; LS, lesser saphenous. | ||||
| Pain | None | Occasional, not restricting activity or requiring analgesics | Daily, moderate activity limitation, occasional analgesics | Daily, severe, limiting activities or requiring regular analgesic use |
| Varicose veins | None | Few, scattered: branch VVs | Multiple: GS varicose veins confined to calf or thigh | Extensive: thigh and calf, or GS and LS distribution |
| Venous edema | None | Evening ankle edema only | Afternoon edema, above ankle | Morning edema above ankle and requiring activity change, elevation |
| Skin pigmentation | None or focal low intensity (tan) | Diffuse but limited in area and old (brown) | Diffuse over most of gaiter distribution (lower third) or recent pigmentation (purple) | Wider distribution (above lower third) and recent pigmentation |
| Inflammation | None | Mild cellulitis, limited to marginal area around ulcer | Moderate cellulitis, involves most of gaiter area (lower third) | Severe cellulitis (lower third and above) or significant venous eczema |
| Induration | None | Focal, circummalleolar (< 5 cm) | Medial or lateral, less than lower third of leg | Entire lower third of leg or more |
| No. of active ulcers | 0 | 1 | 2 | > 2 |
| Active ulceration, duration | None | < 3 months | > 3 months, < 1 year | Not healed > 1 year |
| Active ulcer, size | None | < 2 cm diameter | 2–6 cm diameter | > 6 cm diameter |
| Compressive therapy | Not used or not compliant | Intermittent use of stockings | Wears elastic stockings most days | Full compliance: stockings and elevation |
A second scoring system is the Venous Segmental Disease Score (Table 4). This system was designed to correlate pathophysiologic designations with specific venous segments. A major motivation for the development of this score was the ability to gather the necessary information for accurate scoring by duplex scanning. The third scoring system is the Venous Disability Score (Table 5), which is a simpler system that classifies patients on the ability carry out usual activities with or without compressive therapy or limb elevation.
Table 4.
The Venous Segmental Disease Score
| Reflux | Obstruction |
|---|---|
| ½ Lesser saphenous thrombosed | 1 Greater saphenous (only if from groin to below knee) |
| 1 Greater saphenous | 1 Calf veins, multiple |
| ½ Perforators, thigh | 2 Popliteal vein |
| 1 Perforators, calf | 1 Superficial femoral vein |
| 2 Calf veins, multiple (posterior tibial vein alone = 1) | 1 Profunda femoris vein |
| 2 Popliteal vein | 2 Common femoral vein |
| 1 Superficial femoral vein | 1 Iliac vein |
| 1 Profunda femoris vein | 1 Inferior vena cava |
| 1 Common femoral vein and above | |
| 10 Maximum reflux score | 10 Maximum obstruction score |
Table 5.
The Venous Disability Score
| 0 | Asymptomatic |
| 1 | Symptomatic but able to carry out usual activities without compressive therapy |
| 2 | Can carry out usual activities only with compression and/or limb elevation |
| 3 | Unable to carry out usual activities even with compression and/or limb elevation |
Severity scoring can be used to reflect the degree of change in disease severity associated with treatment as well as to provide a background against which to compare outcomes among various treatment groups. Thus, it can be very useful for studies of outcome assessment in patients with chronic venous insufficiency.20
CONCLUSION
Current evaluation of patients with chronic venous insufficiency benefits from improved understanding of the etiology and pathophysiology of the disease as well as precise diagnostic tests. Evaluation of these patients begins in the office with a careful history and physical examination. Bedside diagnostic tests can be performed, although these have essentially been replaced by more accurate imaging modalities. All patients who are likely to require intervention should first undergo duplex scanning, with additional tests such as venography, plethysmography, and venous pressure measurement reserved for equivocal findings. On the basis of these studies, the patient's disease can be classified according to the CEAP system and venous severity scoring systems, which then serves as the basis for appropriate selection of patients for treatment or interventions, or both.
REFERENCES
- Kistner R L, Masuda E M. In: Rutherford RB, editor. Vascular Surgery. Philadelphia: WB Saunders; 2000. A practical approach to the diagnosis and classification of chronic venous disease. pp. 1990–1999.
- Eklof B, Rutherford R B, Bergan J J, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248–1252. doi: 10.1016/j.jvs.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Meissner M H. In: Hallett JW, Mills JL, Earnshaw JJ, Reekers JA, editor. Comprehensive Vascular and Endovascular Surgery. Edinburgh: Mosby; 2004. Pathophysiology of varicose veins and chronic venous insufficiency. pp. 571–590.
- Browse N L, Burnand K G, Irvine A T, et al. Diseases of the Veins. 2nd ed. London: Arnold Publishers; 1999. pp. 169–189.
- Kim J, Richards S, Kent P J. Clinical examination of varicose veins: a validation study. Ann R Coll Surg Engl. 2000;82:171–175. [PMC free article] [PubMed] [Google Scholar]
- Hoare M C, Royle J P. Doppler ultrasound detection of saphenofemoral and saphenopopliteal incompetence and operative venography to ensure precise saphenopopliteal ligation. Aust N Z J Surg. 1984;54:49–52. doi: 10.1111/j.1445-2197.1984.tb06684.x. [DOI] [PubMed] [Google Scholar]
- Jackson J E, Allison D J, Hemingway A P. In: Grainger RG, Allison D, Adam A, Dixon AK, editor. Diagnostic Radiology: A Textbook of Medical Imaging. London: Churchill Livingstone; 2001. Principles, techniques, and complications of angiography. pp. 158–162.
- Karch L A, Sumner D S. In: Ernst CB, Stanley JC, editor. Current Therapy in Vascular Surgery. St. Louis: Mosby; 2001. Invasive methods of diagnosing venous disease. pp. 825–828.
- Kistner R L. Diagnosis of chronic venous insufficiency. J Vasc Surg. 1986;3:185–188. [PubMed] [Google Scholar]
- Kistner R L, Ferris E B, Randhawa G, et al. A method of performing descending venography. J Vasc Surg. 1986;4:464–468. doi: 10.1067/mva.1986.avs0040464. [DOI] [PubMed] [Google Scholar]
- Raju S, Fredericks R. Evaluation of methods for detecting venous reflux. Arch Surg. 1990;125:1463–1467. doi: 10.1001/archsurg.1990.01410230057010. [DOI] [PubMed] [Google Scholar]
- Nicolaides A N, Hussein M K, Szendro G, et al. The relation of venous ulceration with ambulatory venous pressure measurements. J Vasc Surg. 1993;17:414–419. doi: 10.1067/mva.1993.37694. [DOI] [PubMed] [Google Scholar]
- Payne S PK, Thrush A J, London N JM, et al. Venous assessment using air plethysmography: a comparison with clinical examination, ambulatory venous pressure measurement, and duplex scanning. Br J Surg. 1993;80:967–970. doi: 10.1002/bjs.1800800808. [DOI] [PubMed] [Google Scholar]
- Ting A CW, Cheng S WK, Wu L LH, et al. Air plethysmography in chronic venous insufficiency: clinical diagnosis and quantitative assessment. Angiology. 1999;50:831–836. doi: 10.1177/000331979905001007. [DOI] [PubMed] [Google Scholar]
- Masuda E M, Kistner R L. Prospective comparison of duplex scanning and descending venography in the assessment of venous insufficiency. Am J Surg. 1992;164:254–259. doi: 10.1016/s0002-9610(05)81081-5. [DOI] [PubMed] [Google Scholar]
- Markel A, Meissner M H, Manzo R A, et al. A comparison of the cuff deflation method with Valsalva's maneuver and limb compression in detecting venous valvular reflux. Arch Surg. 1994;129:701–705. doi: 10.1001/archsurg.1994.01420310033005. [DOI] [PubMed] [Google Scholar]
- Min R J, Khilnani N M, Golia P. Duplex ultrasound evaluation of lower extremity venous insufficiency. J Vasc Interv Radiol. 2003;14:1233–1241. doi: 10.1097/01.rvi.0000092663.72261.37. [DOI] [PubMed] [Google Scholar]
- Allegra C, Antignani P L, Bergan J J, et al. The “C” of CEAP: suggested definitions and refinements—an International Union of Phlebology conference of experts. J Vasc Surg. 2003;37:129–131. doi: 10.1067/mva.2003.47. [DOI] [PubMed] [Google Scholar]
- Antignani P L. Classification of chronic venous insufficiency: a review. Angiology. 2001;52(suppl 1):S17–S26. doi: 10.1177/0003319701052001S03. [DOI] [PubMed] [Google Scholar]
- Rutherford R B, Padberg F T, Jr, Comerota A J, et al. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307–1312. doi: 10.1067/mva.2000.107094. [DOI] [PubMed] [Google Scholar]
- Meissner M H, Natiello C, Nicholls S C. Performance characteristics of the venous clinical severity score. J Vasc Surg. 2002;36:889–895. doi: 10.1067/mva.2002.128637. [DOI] [PubMed] [Google Scholar]