ABSTRACT
The lower extremity venous system includes the superficial, deep, and perforating veins. The antegrade flow of blood within these veins is ensured by a system of muscular venous pumps and bicuspid valves. Dysfunction of the system may result from degeneration of the vein wall, post-thrombotic valvular damage, chronic venous obstruction, or dysfunction of the muscular pumps. Although chronic venous disease often receives less attention than arterial disease, it includes an array of manifestations resulting from a complex interaction of anatomy and hemodynamic failure. A thorough understanding of the highly variable venous anatomy is essential to understanding the underlying pathophysiology as well as in directing treatment.
Keywords: Vein, anatomy, thrombosis, valvular incompetence
Although often perceived as less important, many aspects of venous disease are more complex than those associated with arterial occlusive disease. The spectrum of manifestations is greater, ranging from asymptomatic telangiectasias through nonspecific symptoms to edema, skin changes, and overt ulceration. Furthermore, despite a large number of noninvasive tests, there is no single, universally accepted noninvasive measure of venous hemodynamic dysfunction analogous to the ankle-brachial index. Just as in the arterial system, maintenance of appropriate flow depends upon the interaction of an effective pumping mechanism and functional conduits. Unlike those in the arterial system, however, the manifestations of venous disease may result from not only from obstruction but also from directional incompetence of the conduit. Finally, the venous anatomy of the lower extremities is substantially more variable and complicated than the corresponding arterial anatomy. A thorough understanding of this anatomy is essential to an understanding of the underlying pathophysiology of chronic venous disease as well as its diagnosis and treatment.
LOWER EXTREMITY VENOUS ANATOMY
General Anatomy
Although much thinner walled than arteries, veins are also composed of intimal, medial, and adventitial layers. The intimal monolayer rests on the basement membrane and is actively antithrombogenic, producing prostaglandin I2, glycosaminoglycan cofactors of antithrombin, thrombomodulin, and tissue-type plasminogen activator (t-PA).1 However, endothelial perturbation may be accompanied by the induction of procoagulant activity, suppression of anticoagulant, and exposure of neutrophil receptor ligands.2,3 The medial layer consists of three smooth muscle layers interspersed with collagen and elastin, which are adrenergically innervated.4 In comparison with arteries, veins have a weaker muscular layer and less elastic tissue.5 The adventitia is the thickest layer of the veins wall, containing proportionately more collagen and rendering veins stiffer than the arteries. The high capacitance of the venous system is critical to the function of the calf “muscle pump” (described later) and is largely due to the elliptical cross section of the lower extremity veins, which allows volume to increase without an increase in circumference or pressure.4
The superficial, deep, and most perforating veins (as defined later) contain bicuspid valves formed from folds of endothelium supported by a thin layer of connective tissue. Valves are most numerous in the distal leg and decrease toward the hip. In the lower extremities, the valves function to divide the hydrostatic column of blood into segments and to ensure flow from superficial to deep and from caudal to cephalad. As described by van Bemmelen et al,6,7 the lower extremity valve cusps remain open during rest in the supine position. Valve closure is a passive event initiated by reversal of the resting antegrade transvalvular pressure gradient. As the pressure gradient is reversed, there is a short period of retrograde flow, or reflux, until the gradient becomes sufficient to cause valve closure. Thus, valve closure requires first the cessation of antegrade flow, followed by a brief interval of retrograde flow (< 0.5 seconds in the upright position) of sufficient velocity to coapt the cusps completely. In other words, reflux lasting less than 0.5 seconds is a normal and expected finding. In the upright position, retrograde flow persisting > 0.5 seconds is usually defined as pathologic reflux.
The veins of the lower extremity are classified according to their relationship to the muscular fascia and are located in either the superficial or deep compartment. The venous system of the lower extremities includes the deep veins, which lie beneath the muscular fascia and drain the lower extremity muscles; the superficial veins, which are above the deep fascia and drain the cutaneous microcirculation; and the perforating veins that penetrate the muscular fascia and connect the superficial and deep veins. Communicating veins connect veins within the same system (i.e., deep to deep, superficial to superficial). Updates in the nomenclature of the lower extremity veins, used in the following discussion, have clarified many definitions and eliminated many eponyms.8
The Superficial Veins
The superficial venous system includes the reticular veins as well as the great (greater) and small (lesser) saphenous veins and their tributaries. The reticular veins, a network of veins parallel to the skin surface and lying between the saphenous fascia and dermis, drain the lower extremity skin and subcutaneous tissue.9 These veins communicate with either saphenous tributaries or the deep veins through perforators. Direct communication between incompetent reticular veins and the deep venous system through perforating veins has been reported in 60% of patients with extensive thigh telangiectasias.9
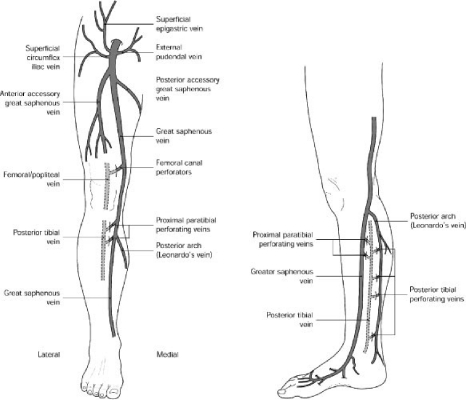
The great saphenous vein arises from the medial aspect of the dorsal pedal venous arch and ascends anterior to the medial malleolus, crossing the tibia at the junction of the distal and middle third of the calf to pass posteromedial to the knee (Fig. 1). The vein then ascends medially in the thigh to perforate the deep fascia and join the common femoral vein 3 to 4 cm (two fingerbreadths) inferior and lateral to the pubic tubercle.5 The saphenous nerve lies anterior to the great saphenous vein in the calf4 and may be injured by procedures extended into the calf.
Figure 1.
Anatomy of the lower extremity venous system. (From Meissner MH. Pathophysiology of varicose veins and chronic venous insufficiency. In: Hallett JW, Mills JL, Earnshaw JJ, Reekers JA, eds. Comprehensive Vascular and Endovascular Surgery. 1st ed. Edinburgh: Mosby; 2004:571–589. Reprinted with permission.)
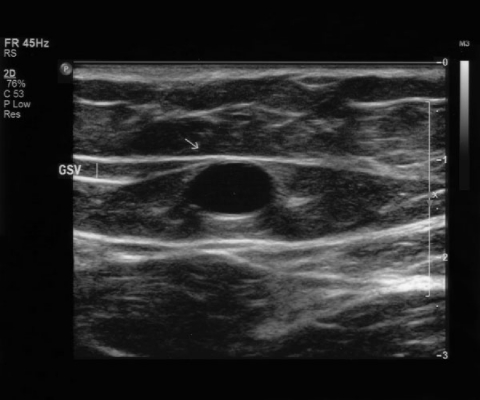
The great saphenous vein usually lies directly on the muscular fascia in the saphenous compartment, a subcompartment of the superficial compartment that is bordered superficially by the hyperechoic saphenous fascia and deeply by the muscular fascia.10 This compartment is readily visualized in the thigh with ultrasound and has been described as having the appearance of an “Egyptian eye” (Fig. 2). Previous designations ascribed to the saphenous fascia including Colles' or Scarpa's fascia and the superficial layer of the deep fascia should be abandoned. The saphenous vein and associated arteries and nerves lie within the saphenous compartment, and the reticular veins, accessory veins, and tributary veins are external to the compartment.8,9,10 True duplication of the great saphenous vein, identified by splitting of the vein into two channels, both lying on the muscular fascia and which later rejoin, is present in the thigh in 8%10 and in the calf in 25% of cases. The great saphenous vein may penetrate the saphenous fascia at the level of the middle or distal thigh and become more superficial.9 Lack of fascia support in these areas has been suggested as a cause of varicose veins,10 which most frequently occur above the level of the superficial fascia.9
Figure 2.
Transverse ultrasound image of the great saphenous vein. The great saphenous vein lies in a subcompartment bordered superficially by the saphenous fascia and deeply by the muscular fascia.
Accessory branches and tributaries of the great saphenous vein may be important in the pathophysiology of chronic venous disease. There are usually two main saphenous tributaries in the calf, an anterior branch and the posterior arch (Leonardo's) vein, which begins behind the medial malleolus and joins the great saphenous vein just distal to the knee. The posterior arch vein drains a network of medial ankle veins11 and is important in that the posterior tibial perforators join this vein rather than the main trunk of the great saphenous vein. Procedures directed toward the great saphenous vein in the calf will not address these perforators. As there may be variable communications with the great saphenous vein, it is sometimes referred to as the posterior arch complex.10 One or more intersaphenous veins also cross the calf obliquely between the great and small saphenous veins. In the thigh, the anterior and posterior accessory saphenous veins ascend parallel to the great saphenous vein, external to the saphenous fascia.8 The venous drainage from the perineum and lower abdominal wall (superficial external pudendal, superficial circumflex iliac, and superficial epigastric veins) commonly joins the great saphenous vein near the saphenofemoral junction9 (Fig. 1).
A valve is present at the saphenofemoral junction in 94% to 100% of individuals, and 81% have at least one valve in the external iliac–common femoral segment above the junction.12 The main trunk of the great saphenous vein usually has at least six valves.13 Varicose great saphenous veins have slightly fewer valves (mean 6.0 ± 1.7) than normal veins (7.3 ± 2.3),14 although the relevance of this observation is unclear.
The small saphenous vein, formerly known as the short or lesser saphenous vein, arises from the dorsal pedal arch and ascends posterolaterally from behind the lateral malleolus to a variable termination in the popliteal vein. The small saphenous vein usually has 7 to 10 closely spaced valves.13 The sural nerve ascends immediately lateral to the vein, which usually lies on and then beneath the muscular fascia prior to its termination.4 Approximately 60% of small saphenous veins join the popliteal vein within 8 cm of the knee joint, 20% join the great saphenous vein via anterior or posterior tributaries, and 20% join the femoral, deep femoral, or internal iliac veins.5 A cranial extension of the small saphenous vein, often referred to as the vein of Giacomini, may ascend posteriorly in the thigh to communicate with the great saphenous vein through the posterior thigh circumflex vein.8 The lateral arch vein is the major tributary of the small saphenous vein and communicates with the peroneal vein through the lateral calf perforators.4 The small saphenous vein may also communicate with the medial ankle perforators through several tributaries.10
Alternative superficial-to-deep drainage systems also exist and are occasionally important in the pathophysiology of chronic venous disease. Lateral superficial veins are remnants of the embryonic vena marginalis lateralis and may be a prominent feature of the Klippel-Trenaunay syndrome. Other pathways include the sciatic drainage system from the posterior thigh to the internal iliac system, the lateral subdermic system draining toward the deep femoral and inferior gluteal veins, drainage through the obturator veins, and alternative venous pathways along the round ligament.9
The Deep Veins
The major deep veins of the lower extremity follow the course of the associated arteries and, with the exception of the femoral vein, are named accordingly. However, there is significant variability and the classic anatomy may be present in as few as 16% of limbs.5 The deep venous system of the calf includes the tibial and peroneal veins as well as the soleal and gastrocnemial veins. The anterior tibial, posterior tibial, and peroneal veins are the venae comitantes of the corresponding arteries, the paired veins communicating in a plexiform arrangement around the artery.5 The muscular venous sinuses are the principal collecting system of the calf muscle pump. There are 1 to 18 soleal sinuses that are numerically of greater importance than those present in the gastrocnemius muscles. The soleal sinuses communicate with the posterior tibial vein in the proximal calf, while the gastrocnemial network coalesces to form the paired gastrocnemial veins draining into the popliteal vein.4 The popliteal vein is formed by the confluence of the calf veins. The deep femoral vein connects directly to the popliteal vein in 38% of limbs and communicates via a tributary in a further 48% of limbs.5 To avoid confusion with the superficial venous system, the phrase “superficial femoral vein” has been abandoned in current nomenclature. The deep vein extending from the popliteal vein to the common femoral vein is now referred to as the femoral vein rather than the superficial femoral vein.8
The anatomy of the iliac veins has been less thoroughly described than that of the infrainguinal veins. A single internal iliac trunk usually drains into the external iliac vein to form the common iliac vein. However, a duplicated internal iliac vein may be present in up to 27% of extremities. The internal iliac vein drains both parietal (superior and inferior gluteal, sacral, sciatic, lumbar, obturator, and internal pudendal) and visceral (hemorrhoidal, vesicoprostatic, uterine, gonadal, and vesicovaginal plexuses) tributaries that have extensive, valveless interconnections.15 These collateral pathways may become important in cases of iliocaval obstruction. Varicosities of the vulva, vagina, and posteromedial thigh as well as symptoms of the pelvic congestion syndrome are also commonly attributed to pelvic venous insufficiency and incompetent tributaries of the internal iliac vein. However, as only 10% of normal internal iliac veins have valves, the role of pelvic venous incompetence is unclear.15 The common iliac veins unite on the right side of the fifth lumbar vertebrae to form the inferior vena cava. The right common iliac vein ascends along a relatively straight course to the inferior vena cava, and the left common iliac vein runs transversely to join the right at an acute angle. Anteroposterior compression of the transversely oriented left common iliac vein between the convexity of the sacroiliac spine and overlying right common iliac artery appears as a filling defect in 50% of venograms.5
The inferior vena cava forms from the development and selective regression of the paired fetal posterior cardinal, subcardinal, and supracardinal veins. The right subcardinal vein ordinarily forms the hepatic and suprarenal inferior vena cava, the right supracardinal vein forms the inferior vena cava, and the caudal aspects of the posterior cardinal veins persist as the common iliac vein.16 Anomalies arising from this complex developmental process are estimated to occur in 1% of subjects.5 Abnormal development of the subcardinal and supracardinal veins may lead to interruption of the inferior vena cava with azygous/hemiazygous continuation or a duplicated or left-sided inferior vena cava, respectively.16
The number of deep venous valves increases from cranial to caudal. Unlike the infrainguinal veins, the iliac veins rarely contain valves. Detailed anatomic dissections have shown valves to be present in only 1.2% of common iliac veins, 27% of external iliac veins (39.6% on the right versus 14.6% on the left), and 10.1% of internal iliac veins.15 There are on average five deep venous valves between the inguinal ligament and popliteal fossa, although the number varies from two to nine.17 Their arrangement is variable, but the external iliac and common femoral vein above the saphenofemoral junction usually have one valve at most; the femoral vein above the adductor canal has three or more valves; the distal superficial femoral and popliteal veins have one or two valves; and the tibial/peroneal veins have numerous valves spaced at ∼2-cm intervals.13,17 Although the muscle sinusoids are valveless, they frequently empty into profusely valved, arborizing veins draining the gastrocnemius and soleus muscles.5,17 Relatively constant stations include a valve in the femoral vein just distal to its confluence with the deep femoral vein and in the popliteal vein just distal to the adductor canal.5 Competence of the popliteal valves is particularly important to calf muscle pump function.
The Perforating Veins
Small anatomic series in cadavers have reported an average of 64 perforating veins between the ankle and the groin.10 They may empty either into the axial deep veins (direct perforators) or into the venous sinuses of the calf (indirect perforators), are invariably accompanied by an artery, and are commonly located in the intramuscular septa. Although numerous and variable overall, perforating veins can be grouped into four groups of clinical significance—those of the foot, the medial and lateral calf, and the thigh. The foot perforators are unique in that they normally direct flow toward the superficial veins, while all others normally direct flow to the deep system.13,18 The major perforators of the medial calf and thigh have one to three valves that direct flow from the superficial to the deep veins.13
The calf contains four groups of perforators—the paratibial perforators connecting the great saphenous and posterior tibial veins, the posterior tibial perforators connecting the posterior accessory great saphenous (posterior arch) and posterior tibial veins, and the lateral and anterior leg perforators. The medial calf perforators, including the paratibial and posterior tibial perforators, are clinically most important. Eponyms associated with the paratibial (Sherman and Boyd perforators) and posterior tibial perforators (Cockett perforators) should no longer be used.8 Most occur along a 3-cm-wide lane ascending the medial calf, surrounding what has been called “Linton's line.” These veins range in number from 7 to 22, averaging ∼14 per leg, with 52% being direct perforators.11 The lower perforators tend to be posterior tibial perforators, and the more superior perforators are paratibial.11 Inferior perforators tend to be short, often only 1 cm in length, whereas toward the middle of the leg they may be 3 to 4 cm long.10 The direct perforators are localized into five groups 7–9 cm, 10–12 cm, 18–22 cm, 23–27 cm, and 28–32 cm proximal to the medial malleolus. The indirect perforators, in contrast, tend to be randomly distributed.11 The paratibial perforators may be of particular importance in that many are not accessible with subfascial endoscopic perforator ligation unless the fascia between the superficial and deep posterior compartments is incised.11 In the lateral calf there are usually four or five paraperoneal perforators along a line from the knee to the ankle.
The perforators of the femoral canal (hunterian perforators) connect the great saphenous vein ∼15 cm proximal to the knee with the distal superficial femoral or proximal popliteal vein. This perforator may give rise to medial thigh varicosities in the presence of a competent proximal great saphenous vein.4
CEAP AND VENOUS ANATOMY
The CEAP classification system, developed under the auspices of the American Venous Forum, provides a mechanism for the uniform diagnosis of venous disease and comparison of populations of patients. The four components of the CEAP classification are a description of the clinical disease class (C) based upon objective signs, the etiology (E), the anatomic (A) distribution of reflux and obstruction, and the underlying pathophysiology (P), whether related to reflux or obstruction.19
Briefly, the CEAP system recognizes seven clinical disease categories including asymptomatic limbs (class 0) and those with telangiectasias (class 1), varicose veins (class 2), edema (class 3), skin changes without ulceration (class 4), healed ulcers (class 5), and active ulcers (class 6). The underlying etiology can be classified as congenital, primary, or secondary. Primary venous disorders are not associated with an identifiable mechanism of venous dysfunction. In contrast, secondary venous disorders result from an antecedent event, usually an episode of acute deep venous thrombosis (DVT).
In its most detailed form, the anatomic distribution of reflux and obstruction is classified into 18 venous segments as illustrated in Table 1. Each segment is further characterized with respect to the underlying pathophysiology, whether reflux or obstruction. In a more simplified form, the anatomic sites of venous disease are classified as superficial (As), deep (Ad), or perforating (Ap).
Table 1.
Anatomic Classification of Chronic Venous Disease
| Segment No. | |
|---|---|
| Adapted from the International Consensus Committee on Chronic Venous Disease. Reporting standards in venous disease: an update. J Vasc Surg 1995;21:635–645. Reprinted with permission. | |
| Superficial veins (As1–5) | |
| 1 | Telangiectasias/reticular veins |
| Great (long) saphenous veins | |
| 2 | Above knee |
| 3 | Below knee |
| 4 | Small (short or lesser) saphenous vein |
| 5 | Nonsaphenous |
| Deep veins (AD6–16) | |
| 6 | Inferior vena cava |
| Iliac | |
| 7 | Common |
| 8 | Internal |
| 9 | External |
| 10 | Pelvic, gonadal, broad ligament |
| Femoral | |
| 11 | Common |
| 12 | Deep |
| 13 | Superficial |
| 14 | Popliteal |
| 15 | Tibial (anterior, posterior, or peroneal) |
| 16 | Muscular (gastrocnemial, soleal, other) |
| Perforating veins (AP17,18) | |
| 17 | Thigh |
| 18 | Calf |
THE CALF MUSCLE PUMP
The accumulation of blood in the lower extremity veins while upright is limited by the physical properties of the venous wall, the function of the venous valves, and the action of the calf muscle pump. Approximately 90% of the venous return in the lower extremities is through the deep veins through the action of the foot, calf, and thigh muscle pumps.20 The action of these valved pumps is dependent on the deep fascia of the leg, which constrains the muscles during contraction and allows high pressures to be generated within the muscular compartments. Among the three pumps, the calf pump has the largest capacitance, generates the highest pressures, and is of greatest importance.18,21 The ejection fraction of the calf muscle pump is ∼65%, in comparison with only 15% for the thigh pump. With contraction of the calf, pressure in the posterior compartment rises to as high as 250 mm Hg,21 the veins are emptied of blood, and resting venous pressure is lowered as the valves prevent retrograde flow. Pressure in the posterior tibial vein accordingly decreases from 80 to 100 to less than 30 mm Hg. A reduction in deep venous pressure during the postcontraction relaxation phase favors flow from the superficial to the deep system through the perforating veins.
Reflux or pathologic retrograde flow occurs when the valves are absent or rendered incompetent either by degenerative processes (primary venous disease) or by an episode of DVT (secondary venous disease). Under these circumstances, retrograde flow during calf muscle relaxation prevents the usual reduction in pressure and rapid venous refilling occurs from the retrograde flow of blood as well as slow capillary inflow. High venous pressure may also be transmitted from the deep to the superficial veins through incompetent perforators. The function of the calf muscle pump may also be impaired in patients with chronic venous disease, an observation that is at least partially related to a reduced ankle range of motion.22
The severe clinical manifestations of chronic venous insufficiency are primarily due to ambulatory venous hypertension or failure to lower venous pressure adequately with exercise. The severity of chronic venous disease is closely related to the magnitude of venous hypertension as measured through a 21-gauge dorsal foot vein needle after 10 tiptoe maneuvers. Ulceration usually does not occur at ambulatory venous pressures less than 30 mm Hg, but the incidence is 100% at pressures greater than 90 mm Hg.23 However, the determinants of ambulatory venous pressure are complex and include venous reflux as well as obstruction and calf muscle pump dysfunction.24,25 For any degree of reflux, the ambulatory venous pressure is worsened by associated venous obstruction. Similarly, abnormal calf muscle pump function is associated with a higher incidence of ulceration and noninvasive indices of venous pressure.22 Although the relationship with disease severity has not been consistent,26,27,28 the calf muscle pump ejection fraction is lowest in limbs with active ulceration (35%), followed by limbs with healed ulcers (49%) and those without ulceration but with duplex evidence of reflux (53%).29 This observation may be related to the progressive decrease in ankle range of motion with increasing severity of disease.22
ABNORMAL VENOUS ANATOMY
Primary Venous Disease
Varicose veins are usually defined as dilated, palpable tortuous veins greater than 4 mm in diameter that do not discolor the overlying skin. Reticular veins are dilated but nonpalpable, blue dermal veins less than 4 mm in diameter and are distinguished from smaller red to purple telangiectasias. The major saphenous trunks are not usually involved in patients with telangiectasias and reticular veins but are often incompetent with varicose veins. In contrast, reticular vein incompetence is present in most patients with telangiectasias.9
The histologic features associated with varicose veins are diverse and vary in different regions of the vein. However, characteristic changes include irregular thickening of the intima, fibrosis between the intima and adventitia, atrophy and disruption of elastic fibers, thickening of individual collagen fibers, and disorganization of the muscular layers.20,30,31,32,33,34 These abnormalities are heterogeneously distributed through the great saphenous vein and its tributaries,31 with some areas appearing hypertrophic while others appear atrophic or normal. The saccular dilations constituting the varices are consistently located just to the distal (upstream) side of valve cusps.14 The configuration of the valve sinus itself is preserved in most varicose segments.
Although reflux is the primary hemodynamic abnormality in primary venous disease, there is no evidence to support either the descending development of varicose veins from sequential valve failure or a primary valvular abnormality as the etiology of varicose veins. Among 839 extremities with large varicose veins, great saphenous reflux was identified in only 51% of legs and only 66% of these extremities had reflux at the saphenofemoral junction.9 Studies of surgical specimens suggest that varices, rather than being initiated at the saphenofemoral junction, can occur anywhere along the course of the great saphenous vein. These observations are supported by ultrasound studies showing primary valvular incompetence to be a multicentric disease that develops simultaneously in discontinuous venous segments.35 It further appears that varicose changes precede the development of overt valvular incompetence14,36,37 and that valvular dysfunction is a secondary phenomenon. Theories have focused on intrinsic structural and biochemical abnormalities of the vein wall. Such “weak wall” theories hypothesize that varicose veins develop because of underlying connective tissue defects and altered venous tone.12,38,39
Histologic studies have suggested that varicose veins have reduced contractility and compliance. Saphenous smooth muscle content, as well as total protein content, is reduced in patients with varicose veins, and effective contraction may be further compromised by fragmentation of the muscle layers.31,40 The smooth muscle cells are also transformed from a contractile to a secretory phenotype,41 and there are corresponding changes in the extracellular matrix of both involved and uninvolved venous segments. Varicose saphenous veins show an increased collagen and reduced elastin content,36 and decreased venous elasticity has been demonstrated both in limbs with overt varices and in those without varices but at high risk for their development.42 Similar connective tissue defects have also been identified in the forearm veins of patients with varicosities.38 These findings suggest that abnormalities in the vein wall architecture precede the development of both overt varicosities and valvular incompetence. Reflux is presumed to occur when the weakened vein wall dilates, causing stretching of the commissure between the valve cusps and separation of the valve leaflets.43
The etiology of the functional, biochemical, and structural changes associated with varicose veins remains unclear. Proposed mechanisms have included hypoxia-mediated endothelial changes,41 cell cycle dysfunction with inhibition of programmed cell death,34 changes in enzyme activity,44 and underlying defects in venous tone.31,45
Secondary Venous Disease
Limbs developing post-thrombotic skin changes and ulceration are more likely to have a combination of reflux and obstruction than either abnormality alone.46,47 However, symptoms are more closely correlated with a reduction in venous refilling time than with persistent abnormalities of venous outflow, such that valvular incompetence is often regarded as the more important of these factors.48 The anatomy of reflux and venous obstruction are also important determinants of severe post-thrombotic manifestations. Whereas reflux in asymptomatic and mildly symptomatic patients is usually isolated and segmental,26 that in patients with skin changes and ulceration is usually multisegmental and frequently involves the deep, superficial, and perforating veins. Approximately two thirds of patients with ulcers have multisystem disease (that is, disease involving more than one anatomic vein system).49 Reflux in the popliteal and posterior tibial veins, particularly when combined with superficial venous reflux, is most significantly associated with post-thrombotic skin changes.50,51 Similarly, persistent popliteal obstruction also appears to be an important factor determining the severity of chronic venous manifestations.52
The mechanism by which valvular insufficiency develops following venous recanalization remains an important question. Among patients with established chronic venous insufficiency, thrombus is seen fusing the leaflet to the valve sinus in 50% of cases, and endothelial erosion with thickening of the basement membrane is present in the remainder.53 However, valvular destruction does not appear to be a universal consequence of acute DVT. Only 33% to 59% of initially thrombosed segments show evidence of reflux on duplex ultrasonography 1 year after the acute event.54 Such clinical observations are supported by histologic evidence that thrombus organization rarely involves the valve cusps. In most cases, the thrombus is separated from the valve cusp by a clear zone that is postulated to arise from the local fibrinolytic activity of the valvular endothelium.55,56,57 The valve cusps may thus be protected from the destructive processes accompanying recanalization, although this mechanism appears to fail in ∼10% of cases in which thrombus adherence to the valve cusp is noted.55,56 Initial thrombus adherence to the valve presumably accounts for the histologic findings in established post-thrombotic syndrome.53
SUMMARY
The anatomy of the lower extremity venous system is complex and highly variable. Effective communication and an understanding of the treatment options require a common nomenclature as updated by an international consensus committee.8 The veins of the leg include the superficial and deep veins, which are defined by their relationship to the muscular fascia; the perforating veins that traverse the fascia and connect the superficial and deep veins; and the communicating veins that connect veins within the same venous system. A system of muscular venous pumps and bicuspid valves ensure flow from superficial to deep and from caudal to cephalad within the lower extremity. Dysfunction of the system may result from valvular incompetence or reflux, chronic venous obstruction, or dysfunction of the muscular pumps. Primary varicose veins are associated with several changes in vein wall architecture that may precede the development of reflux, giving rise to several weak wall hypotheses. In contrast, post-thrombotic valvular damage may result from initial thrombus adherence to the valve cusp. The manifestations of chronic venous disease result from a complex interaction of anatomy and hemodynamic failure. Primary or secondary dysfunction of specific anatomic areas, such as the popliteal vein, posterior tibial-peroneal veins, or the superficial veins, may be associated with more severe manifestations of disease. A thorough understanding of the mechanisms of hemodynamic failure and the underlying anatomy is essential in directing treatment of the patient with chronic venous disease.
REFERENCES
- Carter C J, Anderson F A, Wheeler H B. In: Hull RD, Raskob GE, Pineo GF, editor. Venous Thromboembolism: An Evidence-Based Atlas. Armonk, NY: Futura Publishing Company; 1996. Epidemiology and pathophysiology of venous thromboembolism. pp. 3–20.
- Nawroth P P, Handley D A, Esmon C T, Stern D M. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci USA. 1986;83:3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G J. Neutrophils and deep venous thrombosis. Haemostasis. 1993;23(suppl 1):127–140. doi: 10.1159/000216922. [DOI] [PubMed] [Google Scholar]
- Moneta G L, Nehler M R. In: Gloviczki P, Yao JST, editor. Handbook of Venous Disorders: Guidelines of the American Venous Forum. 1st ed. London: Chapman and Hall Medical; 1996. The lower extremity venous system: anatomy and physiology of normal venous function and chronic venous insufficiency. pp. 3–26.
- Browse N, Burnand K, Thomas M. Diseases of the Veins: Pathology, Diagnosis, and Treatment. London: Edward Arnold; 1988.
- Bemmelen P S van, Beach K, Bedford G, Strandness D E. The mechanism of venous valve closure. Arch Surg. 1990;125:617–619. doi: 10.1001/archsurg.1990.01410170063013. [DOI] [PubMed] [Google Scholar]
- Bemmelen P S van, Bedford G, Beach K, Strandness D E. Quantitative segmental evaluation of venous valvular reflux with duplex ultrasound scanning. J Vasc Surg. 1989;10:425–431. doi: 10.1067/mva.1989.14123. [DOI] [PubMed] [Google Scholar]
- Caggiati A, Bergan J J, Gloviczki P, Jantet G, Wendell-Smith C P, Partsch H. Nomenclature of the veins of the lower limbs: an international interdisciplinary consensus statement. J Vasc Surg. 2002;36:416–422. doi: 10.1067/mva.2002.125847. [DOI] [PubMed] [Google Scholar]
- Somjen G M. Anatomy of the superficial venous system. Dermatol Surg. 1995;21:35–45. doi: 10.1111/j.1524-4725.1995.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Thomson H. The surgical anatomy of the superficial and perforating veins of the lower limb. Ann R Coll Surg Engl. 1979;61:198–205. [PMC free article] [PubMed] [Google Scholar]
- Mozes G, Gloviczki P, Menawat S S, Fisher D R, Carmichael S W, Kadar A. Surgical anatomy for endoscopic subfascial division of perforating veins. J Vasc Surg. 1996;24:800–808. doi: 10.1016/s0741-5214(96)70016-2. [DOI] [PubMed] [Google Scholar]
- Leu H J, Vogt M, Pfrunder H. Morphological alterations of non-varicose and varicose veins. (A morphological contribution to the discussion on pathogenesis of varicose veins) Basic Res Cardiol. 1979;74:435–444. doi: 10.1007/BF01908395. [DOI] [PubMed] [Google Scholar]
- Mozes G, Carmichael S W, Gloviczki P. In: Gloviczki P, Yao JST, editor. Handbook of Venous Disorders: Guidelines of the American Venous Forum. 2nd ed. London: Arnold; 2001. Development and anatomy of the venous system. pp. 11–24.
- Cotton L T. Varicose veins: gross anatomy and development. Br J Surg. 1961;48:589–597. doi: 10.1002/bjs.18004821203. [DOI] [PubMed] [Google Scholar]
- LePage P A, Villavicencio J L, Gomez E R, Sheridan M N, Rich N M. The valvular anatomy of the iliac venous system and its clinical implications. J Vasc Surg. 1991;14:678–683. doi: 10.1067/mva.1991.31717. [DOI] [PubMed] [Google Scholar]
- Mathews R, Smith P A, Fishman E K, Marshall F F. Anomalies of the inferior vena cava and renal veins: embryologic and surgical considerations. Urology. 1999;53:873–880. doi: 10.1016/s0090-4295(99)00007-2. [DOI] [PubMed] [Google Scholar]
- Negus D. In: Dodd H, Cockett FB, editor. The Pathology and Surgery of the Veins of the Lower Limb. 2nd ed. Edinburgh: Churchill Livingstone; 1976. The surgical anatomy of the veins of the lower limb. pp. 18–49.
- Burnand K G. In: Gloviczki P, Yao JST, editor. Handbook of Venous Disorders: Guidelines of the American Venous Forum. 2nd ed. London: Arnold; 2001. The physiology and hemodynamics of chronic venous insufficiency of the lower limb. pp. 49–57.
- Porter J, Moneta G. Reporting standards in venous disease: an update. J Vasc Surg. 1995;21:635–645. doi: 10.1016/s0741-5214(95)70195-8. [DOI] [PubMed] [Google Scholar]
- Goldman M P, Fronek A. Anatomy and pathophysiology of varicose veins. J Dermatol Surg Oncol. 1989;15:138–145. doi: 10.1111/j.1524-4725.1989.tb03020.x. [DOI] [PubMed] [Google Scholar]
- Ludbrook J. The musculovenous pumps of the human lower limb. Am Heart J. 1966;71:635–641. doi: 10.1016/0002-8703(66)90313-9. [DOI] [PubMed] [Google Scholar]
- Back T L, Padberg F T, Jr, Araki C T, Thompson P N, Hobson R W. Limited range of motion is a significant factor in venous ulceration. J Vasc Surg. 1995;22:519–523. doi: 10.1016/s0741-5214(95)70030-7. [DOI] [PubMed] [Google Scholar]
- Nicolaides A N, Hussein M K, Szendro G, Christopoulos D, Vasdekis S, Clarke H. The relationship of venous ulceration with ambulatory venous pressure measurements. J Vasc Surg. 1993;17:414–419. doi: 10.1067/mva.1993.37694. [DOI] [PubMed] [Google Scholar]
- Nicolaides A N. Investigation of chronic venous insufficiency: a consensus statement (France, March 5–9, 1997) Circulation. 2000;102:E126–E163. doi: 10.1161/01.cir.102.20.e126. [DOI] [PubMed] [Google Scholar]
- Hosoi Y, Zukowski A, Kakkos S K, Nicolaides A N. Ambulatory venous pressure measurements: new parameters derived from a mathematic hemodynamic model. J Vasc Surg. 2002;36:137–142. doi: 10.1067/mva.2002.124622. [DOI] [PubMed] [Google Scholar]
- Labropoulos N, Giannoukas A D, Nicolaides A N, Veller M, Leon M, Volteas N. The role of venous reflux and calf muscle pump function in nonthrombotic chronic venous insufficiency: correlation with severity of signs and symptoms. Arch Surg. 1996;131:403–406. doi: 10.1001/archsurg.1996.01430160061011. [DOI] [PubMed] [Google Scholar]
- Cordts P R, Hartono C, LaMorte W W, Menzoian J O. Physiologic similarities between extremities with varicose veins and with chronic venous insufficiency utilizing air plethysmography. Am J Surg. 1992;164:260–264. doi: 10.1016/s0002-9610(05)81082-7. [DOI] [PubMed] [Google Scholar]
- Bemmelen P S van, Mattos M A, Hodgson K J, et al. Does air plethysmography correlate with duplex scanning in patients with chronic venous insufficiency? J Vasc Surg. 1993;18:796–807. [PubMed] [Google Scholar]
- Araki C T, Back T L, Padberg F T, et al. The significance of calf muscle pump function in venous ulceration. J Vasc Surg. 1994;20:872–877. doi: 10.1016/0741-5214(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Bouissou H, Julian M, Pieraggi M T, Louge L. Vein morphology. Phlebology. 1988;3(suppl 1):1–11. [Google Scholar]
- Lowell R C, Gloviczki P, Miller V M. In vitro evaluation of endothelial and smooth muscle function of primary varicose veins. J Vasc Surg. 1992;16:679–686. [PubMed] [Google Scholar]
- Porto L C, Azizi M A, Pelajo-Machado M, Matos da S P, Lenzi H L. Elastic fibers in saphenous varicose veins. Angiology. 2002;53:131–140. doi: 10.1177/000331970205300202. [DOI] [PubMed] [Google Scholar]
- Jones G T, Solomon C, Moaveni A, Rij A M van, Thomson I A, Galvin I. Venous morphology predicts class of chronic venous insufficiency. Eur J Vasc Endovasc Surg. 1999;18:349–354. doi: 10.1053/ejvs.1999.0902. [DOI] [PubMed] [Google Scholar]
- Ascher E, Jacob T, Hingorani A, Tsemekhin B, Gunduz Y. Expression of molecular mediators of apoptosis and their role in the pathogenesis of lower-extremity varicose veins. J Vasc Surg. 2001;33:1080–1086. doi: 10.1067/mva.2001.113976. [DOI] [PubMed] [Google Scholar]
- Labropoulos N, Giannoukas A D, Delis K, et al. Where does venous reflux start? J Vasc Surg. 1997;26:736–742. doi: 10.1016/s0741-5214(97)70084-3. [DOI] [PubMed] [Google Scholar]
- Gandhi R H, Irizarry E, Nackman G B, Halpern V J, Mulcare R J, Tilson M D. Analysis of the connective tissue matrix and proteolytic activity of primary varicose veins. J Vasc Surg. 1993;18:814–820. [PubMed] [Google Scholar]
- Rose S S, Ahmed A. Some thoughts on the aetiology of varicose veins. J Cardiovasc Surg (Torino) 1986;27:534–543. [PubMed] [Google Scholar]
- Vanhoutte P M, Corcaud S, de Montrion C. Venous disease: from pathophysiology to quality of life. Angiology. 1997;48:559–567. doi: 10.1177/000331979704800702. [DOI] [PubMed] [Google Scholar]
- Clarke G H, Vasdekis S N, Hobbs J T, Nicolaides A N. Venous wall function in the pathogenesis of varicose veins. Surgery. 1992;111:402–408. [PubMed] [Google Scholar]
- Travers J P, Brookes C E, Evans J, et al. Assessment of wall structure and composition of varicose veins with reference to collagen, elastin and smooth muscle content. Eur J Vasc Endovasc Surg. 1996;11:230–237. doi: 10.1016/s1078-5884(96)80058-x. [DOI] [PubMed] [Google Scholar]
- Michiels C, Arnould T, Thibaut-Vercruyssen R, Bouaziz N, Janssens D, Remacle J. Perfused human saphenous veins for the study of the origin of varicose veins: role of the endothelium and of hypoxia. Int Angiol. 1997;16:134–141. [PubMed] [Google Scholar]
- Clarke G H, Vasdekis S N, Hobbs J T, Nicolaides A N. Venous wall function in the pathogenesis of varicose veins. Surgery. 1992;111:402–408. [PubMed] [Google Scholar]
- Alexander C J. The theoretical basis of varicose vein formation. Med J Aust. 1972;1:258–261. doi: 10.5694/j.1326-5377.1972.tb50912.x. [DOI] [PubMed] [Google Scholar]
- Haardt B. A comparison of the histochemical enzyme pattern in normal and varicose veins. Phlebology. 1987;2:135–158. [Google Scholar]
- Barber D A, Wang X, Gloviczki P, Miller V M. Characterization of endothelin receptors in human varicose veins. J Vasc Surg. 1997;26:61–69. doi: 10.1016/s0741-5214(97)70148-4. [DOI] [PubMed] [Google Scholar]
- Johnson B F, Manzo R A, Bergelin R O, Strandness D E. Relationship between changes in the deep venous system and the development of the postthrombotic syndrome after an acute episode of lower limb deep vein thrombosis: a one- to six-year follow-up. J Vasc Surg. 1995;21:307–313. doi: 10.1016/s0741-5214(95)70271-7. [DOI] [PubMed] [Google Scholar]
- Johnson B F, Manzo R A, Bergelin R O, Strandness D E. The site of residual abnormalities in the leg veins in long-term follow-up after deep venous thrombosis and their relationship to the development of the post-thrombotic syndrome. Int Angiol. 1996;15:14–19. [PubMed] [Google Scholar]
- Killewich L A, Martin R, Cramer M, Beach K W, Strandness D E. An objective assessment of the physiological changes in the postthrombotic syndrome. Arch Surg. 1985;120:424–426. doi: 10.1001/archsurg.1985.01390280018003. [DOI] [PubMed] [Google Scholar]
- Hanrahan L M, Araki C T, Rodriguez A A, Kechejian G J, LaMorte W W, Menzoian J O. Distribution of valvular incompetence in patients with venous stasis ulceration. J Vasc Surg. 1991;13:805–811. [PubMed] [Google Scholar]
- Gooley N A, Sumner D S. Relationship of venous reflux to the site of venous valvular incompetence: implications for venous reconstructive surgery. J Vasc Surg. 1988;7:50–59. [PubMed] [Google Scholar]
- Bemmelen P S van, Bedford G, Beach K, Strandness D E., Jr Status of the valves in the superficial and deep venous system in chronic venous disease. Surgery. 1991;109:730–734. [PubMed] [Google Scholar]
- Meissner M H, Caps M T, Zierler B K, et al. Determinants of chronic venous disease after acute deep venous thrombosis. J Vasc Surg. 1998;28:826–833. doi: 10.1016/s0741-5214(98)70057-6. [DOI] [PubMed] [Google Scholar]
- Budd T W, Meenaghan M A, Wirth J, Taheri S A. Histopathology of veins and venous valves of patients with venous insufficiency syndrome: ultrastructure. J Med. 1990;21:181–199. [PubMed] [Google Scholar]
- Markel A, Manzo R A, Bergelin R O, Strandness D E. Valvular reflux after deep vein thrombosis: incidence and time of occurrence. J Vasc Surg. 1992;15:377–384. [PubMed] [Google Scholar]
- Sevitt S. The mechanisms of canalisation in deep vein thrombosis. J Pathol. 1973;110:153–165. doi: 10.1002/path.1711100207. [DOI] [PubMed] [Google Scholar]
- Sevitt S. Organization of valve pocket thrombi and the anomalies of double thrombi and valve cusp involvement. Br J Surg. 1974;61:641–649. doi: 10.1002/bjs.1800610812. [DOI] [PubMed] [Google Scholar]
- Glas-Greenwalt P, Dalton B C, Astrup T. Localization of tissue plasminogen activator in relation to morphological changes in human saphenous veins used as coronary artery bypass autografts. Ann Surg. 1975;181:431–441. doi: 10.1097/00000658-197504000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]