ABSTRACT
The liver depends on a dual blood supply from the hepatic artery and the portal vein. The normal liver receives 70% portal flow and 30% hepatic arterial flow, with most arterial blood feeding the biliary tree. As cirrhosis robs the liver of its regenerative capacity, the portal flow decreases and intrahepatic portosystemic shunting increases with a variable increase in arterial flow across arterioportal shunts. This compensation mechanism attempts to reperfuse remaining sinusoids. Transjugular intrahepatic portosystemic shunts (TIPS) or surgical portosystemic shunts may acutely diminish portal perfusion further, leading to hepatic failure. Small-diameter TIPS or surgical shunts reduce the incidence of complications by preserving nutritive portal flow. Although the inverse relationship of arterial and portal flow is physiologically valid, there is individual variation in the ability to substitute one blood supply for another. This variability may result from anatomic or functional factors influencing the flow across arterioportal shunts. Hepatic perfusion curves derived from enhanced imaging studies can subtype cirrhotic patients into favorable versus unfavorable perfusion patterns. Patients with high arterial flow to the liver or patients with retained portal-type flow curves have better survival and morbidity compared with those patients with unfavorable flow manifest by diminished arterial-type curves on hepatic perfusion analysis.
Keywords: Portal vein flow dynamics, liver blood supply, liver cirrhosis, portosystemic shunts, portal hypertension
The liver and lungs are the only human organs with a dual blood supply. Furthermore, and in contrast to other organs, arterial flow derived from the aorta provides a minor role in nourishment of the biliary and bronchial structures; the bulk of pulmonary and hepatic perfusion arrives as venous blood. The unique vascular anatomy and physiology of the liver creates challenges in both imaging and intervention, whether surgical or percutaneous. From contrast-enhanced imaging to liver transplantation, one must be aware of the patency, flow volume and direction, and relative importance of all three vascular beds (hepatic veins, portal veins, and hepatic artery).
The liver develops from a ventral bud of the foregut endoderm at 1 to 2 weeks of fetal development, a process initiated by signals from the developing heart. The hepatoblasts in this endodermal structure eventually invade the septum transversum mesenchyme, where they differentiate into hepatocytes and bile duct cells. The mesenchyme contributes precursor cells that surround primitive hepatocytes to become the hepatic vascular supply, namely portal veins and hepatic arteries that are connected to centrilobular draining veins via a rich network of hepatic sinusoids. This sinusoidal capillary network bathes the hepatocyte surface across the space of Disse.1 Prior to birth, the fetal liver is entirely fed by the hepatic artery with the portal flow shunted systemically via the ductus venosus off the left portal vein. After birth, when the umbilical vein and ductus venosus occlude, the portal vein supplies ~70% of blood flow to the liver as opposed to 30% hepatic arterial perfusion. Numerous communications exist between the arterial and portal networks, the most important of which is the transplexal route by which the peribiliary arterial plexus can supply blood to the portal venules.2,3,4 Such pathways become important in the setting of compromised portal or hepatic venous flow. In portal vein thrombosis or cirrhotic portal flow reduction, the hepatic artery compensates by increasing flow to sinusoids via such communications. Interestingly, the portal system does not reciprocate with increasing flow after hepatic arterial occlusion. This phenomenon may be due to the different diameter, flow rate, and pressure of hepatic arterioles compared with the portal venous system.5 With occlusion of a hepatic vein, the portal vein can substitute for venous drainage with the hepatic artery taking over nutritive perfusion of the hepatocytes.6 With the advent of dual phase, portal venous and hepatic arterial enhanced cross-sectional imaging, perfusion alterations in hepatic disease are clearly visible and useful from both a diagnostic and therapeutic viewpoint. For example, cavernous transformation or tumorous occlusion of the portal vein and hepatic venous occlusion (Budd-Chiari syndrome) lead to characteristic patterns of parenchymal remodeling and increased regions of hepatic arterial enhancement on cross sectional imaging.7,8
Cirrhosis, from whatever cause, is a chronic fibrotic process that leads to obliteration of portal venules, sinusoids, central veins, regenerative nodule formation, and parenchymal atrophy. Specific derangements such as collagen deposition in the space of Disse leads to sinusoidal narrowing and impaired nutrient exchange between the blood and the hepatocytes, further increasing portal pressures and functional liver impairment. The overall impact on hepatic hemodynamics is gradual loss of portal flow, hepatic venous outflow obstruction, and hypertrophy of hepatic arteries as a compensatory mechanism for lost portal inflow. Injection/corrosion studies of human cirrhotic livers done by McIndoe in the 1920s as well as animal studies support the above-described hepatic vascular changes leading to portal hypertension, portosystemic venous collateral formation (varices), and, ultimately, hepatic failure9 (Fig. 1). Parallel to this diminution in portal perfusion is the formation of intrahepatic portosystemic shunts, thought to occur between clustered sinusoids and central veins communicating in bands of hepatic fibrosis.10,11 Complicating this picture further is the hyperdynamic circulation in cirrhotic patients who have decreased peripheral vascular resistance and increased cardiac output and total blood volume. Clearly, the hepatic vascular environment in cirrhosis is complex with the diseased liver both obstructing and shunting portal flow as the hepatic artery attempts to compensate by perfusing the abnormal sinusoids via arterioportal communications. The performance of surgical or percutaneous portosystemic shunt procedures invariably disrupts this complex balance of hepatic perfusion. Although these methods usually provide the intended decrease in portal pressure and cessation of variceal hemorrhage or ascites accumulation, portal diversion may also provoke intractable encephalopathy and even hepatic failure and death. Additionally, transjugular intrahepatic portosystemic shunt (TIPS) generally worsens the hyperdynamic circulation in cirrhotic patients, putting them at increased risk for cardiac decompensation.12
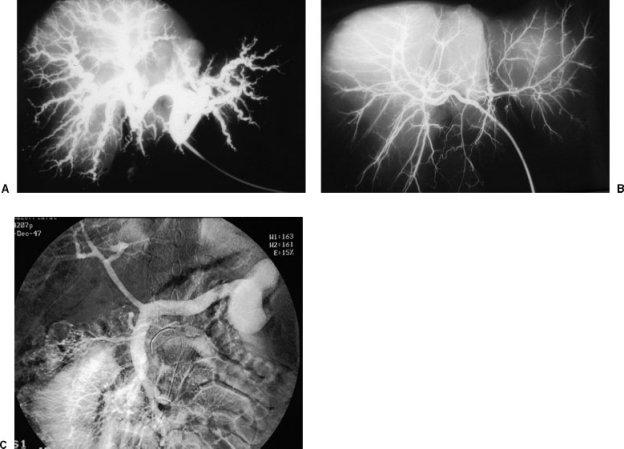
Figure 1.
(A) Barium injection into the hepatic artery in an explant liver from a cirrhotic patient. The hepatic arteries are hypertrophied and tortuous. (B) Normal liver specimen after hepatic artery injection with dilute barium. The caliber of the intrahepatic arterial branches is markedly smaller than that seen in cirrhosis. (C) Venous phase of superior mesenteric arteriogram shows small-caliber portal vein with large splenorenal shunt. The loss of hepatic portal perfusion is evident in this image, with reduced portal flow secondary to both extrahepatic and intrahepatic portosystemic shunts.
Fortunately, there is a large body of research in the surgical literature regarding hepatic perfusion in cirrhosis before and after surgical portosystemic shunt creation. We can broadly apply this body of knowledge to the TIPS procedure in interventional radiology. Ultimately, we hope to better define patients who are at high risk for shunt procedures using a noninvasive or minimally invasive assessment of their hepatic function and perfusion. Although this article is focused on hepatic perfusion as it pertains to cirrhosis and TIPS outcomes, the evaluation of hepatic synthetic function using MELD scoring and other methods, as described by Ferral and Patel,13 is also crucial to the assessment of potential TIPS patients.
Rypins et al conducted several important investigations of cirrhotic liver perfusion before and after surgical portocaval shunts.14,15 Their contribution is invaluable for understanding postshunt morbidity and mortality and the concept of “nutritive” portal perfusion. To determine the hepatic artery and portal flow proportions, the investigators used the method of Biersack et al16,17 where the arterial and portal venous components of hepatic perfusion are calculated by generating a time-activity curve over a hepatic region of interest after intravenous injection of a radiopharmaceutical (such as technetium). They modified the Biersack technique and quantified “nutritive” portal flow, defined as prograde portal flow after subtracting intrahepatically shunted blood. Normal patients show an early, sharp upstroke in the curve (arterial phase) with a later and more gradual rise in counts (portal phase). By comparing the slopes of the curve in these two phases, the arterial and portal contribution to hepatic perfusion can be calculated. Cirrhotic patients have a similar curve although the portal phase is blunted and rises to a lower plateau consistent with diminished portal flow. Cirrhotic patients after nonselective or total shunt procedures, however, all show a similar perfusion curve with an intact arterial phase to a plateau without further portal augmentation, reflecting loss of all nutritive prograde portal flow to the liver. In between lie those patients who undergo selective shunts (i.e., distal splenorenal shunt), small-diameter portocaval shunts, or, as we have found, some TIPS shunts. These patients maintain some portal flow, albeit diminished, and have improved clinical outcomes. Rypins et al showed that small-diameter portocaval shunts caused less reduction in portal perfusion and decreased the incidence of postoperative encephalopathy and hepatic failure. In fact, the degree of retained nutritive portal flow was the only factor correlated with good outcome.14,15 In contrast to other investigators, they found no change in the proportion of arterial blood flow to the liver before or after shunt placement, although they showed significant portal flow decreases in cirrhotic patients, particularly after nonselective portocaval shunting. Although this research suggests that if we maintain nutritive portal flow to the liver, we will reduce the complications of TIPS or surgical shunting, this model may be overly simplistic. Hepatic perfusion is not only complex but also its components and its response to disease states may vary among individual patients due to congenital or anatomic reasons. Zimmon and Kessler looked at the relationship of portal pressure to acute shunt procedures by temporarily diverting portal flow in cirrhotic patients to the saphenous vein.18 Two patient populations were apparent in this study. Type A cirrhotic patients showed small decreases in portal pressure with shunting, and type B patients had dramatic reductions in pressure with the same degree of shunting. Accordingly, when the situation was reversed (blood was shunted from the saphenous vein into the portal system), type A patients had more compliant portal systems with only minor increases in pressure as opposed to type B patients who exhibited large increases in portal pressure after this “reverse shunt” (i.e., a noncompliant portal system).18 The authors attribute this discrepancy to the idea that only some patients are able to regulate portal pressure and flow through changes in hepatic arterial flow (hepatic artery buffer response). Those with noncompliant portal systems (type B) are at increased risk of encephalopathy and liver failure after portal diversion surgically.19 Anatomically, the ability to autoregulate portal blood flow with the hepatic artery may relate to the presence and efficiency of the previously described arterioportal shunt networks. The variability of portal flow “compliance” among the population may also account for the disagreement among investigators as to the existence or importance of increased hepatic arterial flow in cirrhosis. This hypothesis may also explain Patel's findings that the post-TIPS hepatic artery blood flow increased to a variable degree and did not correlate with post-TIPS portosystemic pressure gradients.20
Attempts to clarify the impact of hepatic perfusion variations on the morbidity and mortality of therapeutic shunting are now focused on the preshunt status of hepatic blood flow in an attempt to predict high-risk patients before treatment. We have studied blood flow patterns in cirrhotic patients before and after the TIPS procedure using more modern imaging modalities. Several interesting observations arise from these studies. First, quantifying activity over the lungs and liver after direct portal injection of Tc99m-macroaggregated albumin particles reveals that the proportion of portal flow shunted intrahepatically averages 77% in cirrhotic patients and increases to 93% after TIPS.21 Second, the proportion of hepatic flow supplied by the hepatic artery always increases after TIPS but the ratio of arterial/portal perfusion has no correlation with postTIPS survival22—a finding supported by other investigators.15,20 Using a retrograde thermodilutional catheter, Clark et al showed that arterioportal shunting immediately after TIPS averaged 623 mL/min,23 providing quantification of the frequent ultrasound findings of reversed portal venous branch flow after TIPS. Finally, three distinct hepatic perfusion curves are evident in cirrhotic patients, one of which is significantly correlated with poor survival after TIPS (Fig. 2). This last statement derives from studies using nuclear medicine flow studies, contrast-enhanced cine-magnetic resonance angiography of the liver, and, more recently, video ultrasound studies of the liver after injection of ultrasound contrast agents (Fig. 3). Regardless of the enhancement technique used, one can construct hepatic perfusion curves by plotting the intensity of signal over time in a defined area of hepatic parenchyma. Although the shape of these curves discriminates between “arterialized” livers (rapid upstroke to a plateau) and portal-type inflow (slow rise to a peak), the magnitude of blood flow is difficult to quantify. As an internal control, we used renal parenchymal curves to estimate to quantity of hepatic perfusion in addition to its quality (arterial versus portal). Patients with portal-type perfusion curves, as well as patients with arterial-type perfusion curves at or above the peak of renal perfusion, had improved survival over those patients with severely reduced arterial-type hepatic perfusion (mean survival of 2 months for those with unfavorable perfusion compared with 28 months for favorable perfusion profiles)22 (Figs. 4 and 5). Perfusion curves in 23 patients with refractory ascites were also evaluated with cine-magnetic resonance imaging (MRI) prior to TIPS and similar findings were obtained. The 6-month mortality in eight patients with unfavorable perfusion was 100% compared with 20% in the patients with favorable perfusion types.24 Our two perfusion “types” (favorable and unfavorable) may correspond to Zimmon's types A and B cirrhotic perfusion categories with the unfavorable curves representing patients with noncompliant hepatic vasculature—specifically, poorly formed or functioning arterioportal shunt networks unable to provide arterial compensation for lost portal flow. We found unfavorable perfusion patterns primarily in patients with severe cirrhosis and refractory ascites; the subgroup of favorable perfusion patients with very high arterial-type flow were more likely found in the population with bleeding varices. These findings are not surprising given the experience of our group and others that patients receiving TIPS for refractory ascites suffer higher mortality than patients with varices as a primary indication.25 Other studies using hepatic perfusion scintigraphy have suggested a correlation of ascites with perfusion variants of the liver.26 We surmise that the favorable and unfavorable perfusion patterns result from some combination of intrahepatic shunting, perhaps at the sinusoidal level, and the efficiency of the hepatic arterial buffer response to the diminished portal flow of cirrhosis. Further research is needed to clarify the interplay of these perfusion factors. It is possible that patients with an abundant hepatic artery compensation for lost portal flow and relatively little intrahepatic shunting might present with good liver function and bleeding varices, whereas poor hepatic artery compensation and extensive intrahepatic shunting might present later with advanced cirrhosis and refractory ascites, although this remains unproven. Ultimately, we believe that some combination of noninvasive measures of hepatic functional reserve (model for end-stage liver disease, acute physiology and chronic health evaluation, Childs-Pugh scores) and of hepatic perfusion status will optimally stratify risk in patients considered for TIPS and possibly for other procedures such as hepatic artery chemoembolization and liver transplantation.
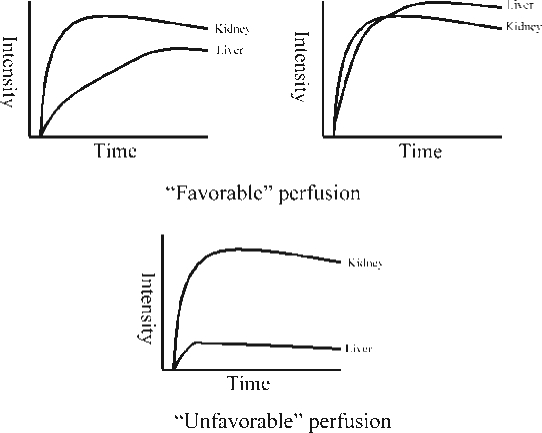
Figure 2.
Schematic of kidney/liver perfusion curves. The upper two graphs illustrate patients with favorable perfusion curves, which are either retained portal-type perfusion (left) or arterially compensated flow with intensity values equal or higher to the kidney (right). The lower graph shows unfavorable perfusion with weak arterial flow patterns far below renal values.
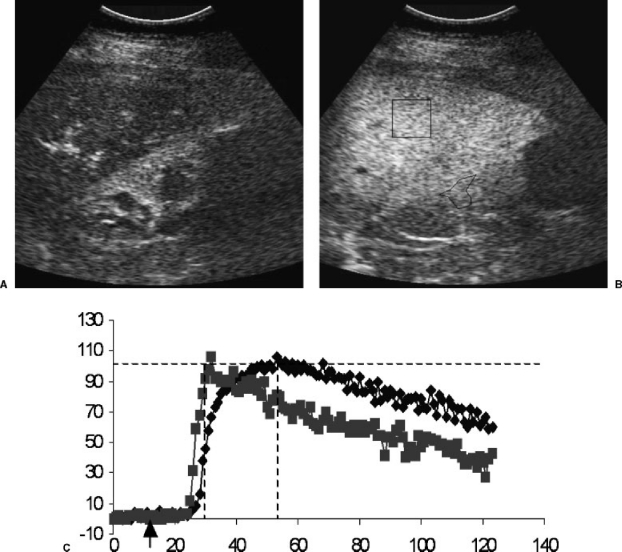
Figure 3.
(A) Longitudinal contrast-enhanced ultrasound image of the liver (top) and kidney (bottom) during the arterial phase. The renal parenchyma and hepatic peribiliary tissues are enhancing. (B) portal phase ultrasound images with regions of interest drawn for time-intensity curve generation. (C) Resulting perfusion curves for kidney (square points) and liver (diamond points) in a patient with a normal liver. Notice the steep arterial slope for the kidney and the more gradual slope and delayed plateau for the liver, which relies primarily on portal flow. x-axis, time in seconds; y-axis, signal intensity; arrow on x-axis, time of bolus intravenous injection of ultrasound contrast agent.
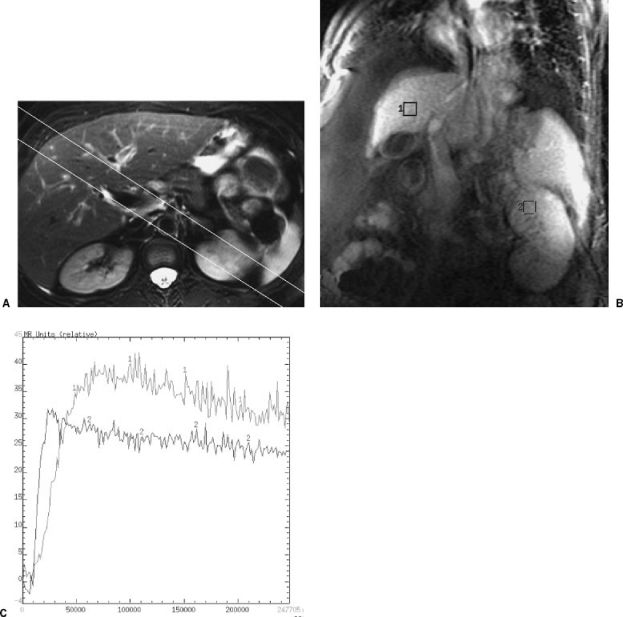
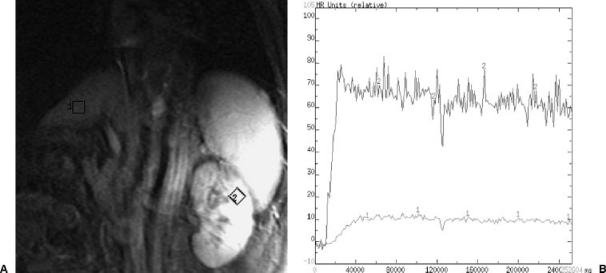
Figure 4.
(A) Angled coronal MRI section used for cine-MRI sequences to generate hepatic and left renal perfusion curves. (B) Resulting magnetic resonance image with hepatic and renal regions of interest. (C) Perfusion curves for liver (labeled 1) and kidney (labeled 2) showing the favorable perfusion curve of hepatic arterialization with enhancement intensity above that of the kidney.
Figure 5.
(A) Image from an angled coronal cine MRI as in Fig. 3 in a patient with refractory ascites prior to TIPS. (B) Unfavorable perfusion curve (labeled 1) with arterial-type rise to plateau far below the renal intensity (labeled 2). This patient expired 60 days after TIPS procedure.
REFERENCES
- Zhao R, Duncan S A. Embryonic development of the liver. Hepatology. 2005;41:956–967. doi: 10.1002/hep.20691. [DOI] [PubMed] [Google Scholar]
- Bookstein J J, Cho K J, Davis G B, Dait D. Arterio-portal communications: observations and hypotheses concerning transsinusoidal and transvasal types. Radiology. 1982;142:581–590. doi: 10.1148/radiology.142.3.7063671. [DOI] [PubMed] [Google Scholar]
- Kan Z, Ivancev K, Hagerstrand I, et al. In vivo microscopy of the liver after injection of Lipiodol into the hepatic artery and portal vein in the rat. Acta Radiol. 1989;30:419–425. [PubMed] [Google Scholar]
- Demachi H, Matsui O, Takashima T. Scanning electron microscopy of intrahepatic microvasculature casts following experimental hepatic arterial embolization. Cardiovasc Intervent Radiol. 1991;14:158–162. doi: 10.1007/BF02577719. [DOI] [PubMed] [Google Scholar]
- Ternberg J L, Butcher H R., Jr Blood-flow relation between hepatic artery and portal vein. Science. 1965;150:1030–1031. doi: 10.1126/science.150.3699.1030. [DOI] [PubMed] [Google Scholar]
- Kanazawa S, Wright K C, Kasi L P, et al. Preliminary experimental evaluation of temporary segmental hepatic venous occlusion: angiographic, pathologic, and scintigraphic findings. J Vasc Interv Radiol. 1993;4:759–766. doi: 10.1016/s1051-0443(93)71969-7. [DOI] [PubMed] [Google Scholar]
- Itai Y, Matsui O. Blood flow and liver imaging. Radiology. 1997;202:306–314. doi: 10.1148/radiology.202.2.9015047. [DOI] [PubMed] [Google Scholar]
- Gryspeerdt S, Van Hoe L, Marchal G, Baert A L. Evaluation of hepatic perfusion disorders with double-phase spiral CT. Radiographics. 1997;17:337–348. doi: 10.1148/radiographics.17.2.9084076. [DOI] [PubMed] [Google Scholar]
- Keller A Z. Cirrhosis of the liver, alcoholism and heavy smoking associated with cancer of the mouth and pharynx. Cancer. 1967;20:1015–1022. doi: 10.1002/1097-0142(196706)20:6<1015::aid-cncr2820200612>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Popper H, Elias H, Petty D E. Vascular pattern of the cirrhotic liver. Am J Clin Pathol. 1952;22:717–729. doi: 10.1093/ajcp/22.8.717. [DOI] [PubMed] [Google Scholar]
- Hales M R, Allan J S, Hall E M. Injection-corrosion studies of normal and cirrhotic livers. Am J Pathol. 1959;35:909–941. [PMC free article] [PubMed] [Google Scholar]
- Azoulay D, Castaing D, Dennison A, et al. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology. 1994;19:129–132. [PubMed] [Google Scholar]
- Ferral H, Patel N H. Selection criteria for patients undergoing transjugular intrahepatic portosystemic shunt procedures: current status. J Vasc Interv Radiol. 2005;16:449–455. doi: 10.1097/01.RVI.0000149508.64029.02. [DOI] [PubMed] [Google Scholar]
- Rypins E B, Henderson J M, Fajman W, et al. Portal venous-total hepatic flow ratio by radionuclide angiography. J Surg Res. 1981;31:463–468. doi: 10.1016/0022-4804(81)90183-9. [DOI] [PubMed] [Google Scholar]
- Rypins E B, Milne N, Sarfeh I J, et al. Quantitation and fractionation of nutrient hepatic blood flow in normal persons, in persons with portal hypertensive cirrhosis, and after small-diameter portacaval H grafts. Surgery. 1988;104:335–342. [PubMed] [Google Scholar]
- Biersack H J. Quantitative liver perfusion scintigraphy: experimental and clinical investigation in normal and pathologic liver perfusion. Langenbecks Arch Chir. 1980;351:23–37. doi: 10.1007/BF01241928. [DOI] [PubMed] [Google Scholar]
- Biersack H, Thelen M, Schulz S, et al. Die sequentielle hepatosplenoszintigraphie zur quantitiven beurteilung der leberdurchblutung. Rofo. 1977;126:47–52. doi: 10.1055/s-0029-1230532. [DOI] [PubMed] [Google Scholar]
- Zimmon D S, Kessler R E. Effect of portal venous blood flow diversion on portal pressure. J Clin Invest. 1980;65:1388–1397. doi: 10.1172/JCI109803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmon D S. Pumping portal blood for therapy and knowledge. J Hepatol. 1996;25:106–108. doi: 10.1016/s0168-8278(96)80335-7. [DOI] [PubMed] [Google Scholar]
- Patel N H, Sasadeusz K J, Seshadri R, et al. Increase in hepatic arterial blood flow after transjugular intrahepatic portosystemic shunt creation and its potential predictive value of postprocedural encephalopathy and mortality. J Vasc Interv Radiol. 2001;12:1279–1284. doi: 10.1016/s1051-0443(07)61552-8. [DOI] [PubMed] [Google Scholar]
- Walser E M, Harris V M, Harman J T, et al. Quantification of intrahepatic portosystemic shunting after placement of a transjugular intrahepatic portosystemic shunt. J Vasc Interv Radiol. 1996;7:263–267. doi: 10.1016/s1051-0443(96)70775-3. [DOI] [PubMed] [Google Scholar]
- Walser E M, DeLa Pena R, Villanueva-Meyer J, et al. Hepatic perfusion before and after the transjugular intrahepatic portosystemic shunt procedure: impact on survival. J Vasc Interv Radiol. 2000;11:913–918. doi: 10.1016/s1051-0443(07)61811-9. [DOI] [PubMed] [Google Scholar]
- Clark T WI, Itkin M, Stavropoulos S W, et al. Portal blood flow in patients with transjugular portosystemic shunts (TIPS): new insights into shunt physiology. Paper presented at the Annual Meeting of the Society of Interventional Radiology; April 3. New Orleans, LA: 2005.
- Walser E M, Ozkan O S, Raza S A, et al. Hepatic perfusion as a predictor of mortality after transjugular intrahepatic portosystemic shunt creation in patients with ascites. J Vasc Interv Radiol. 2003;14:1251–1257. doi: 10.1097/01.rvi.0000092665.72261.b0. [DOI] [PubMed] [Google Scholar]
- Malinchoc M, Kamath P S, Gordon F D, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- El-Khalily H, Hoeffken H, von Wichert P, Joseph K. Hepatic perfusion scintigraphy: relationship of liver perfusion and ascites in patients with liver cirrhosis. Clin Nucl Med. 1996;21:132–135. doi: 10.1097/00003072-199602000-00012. [DOI] [PubMed] [Google Scholar]