Abstract
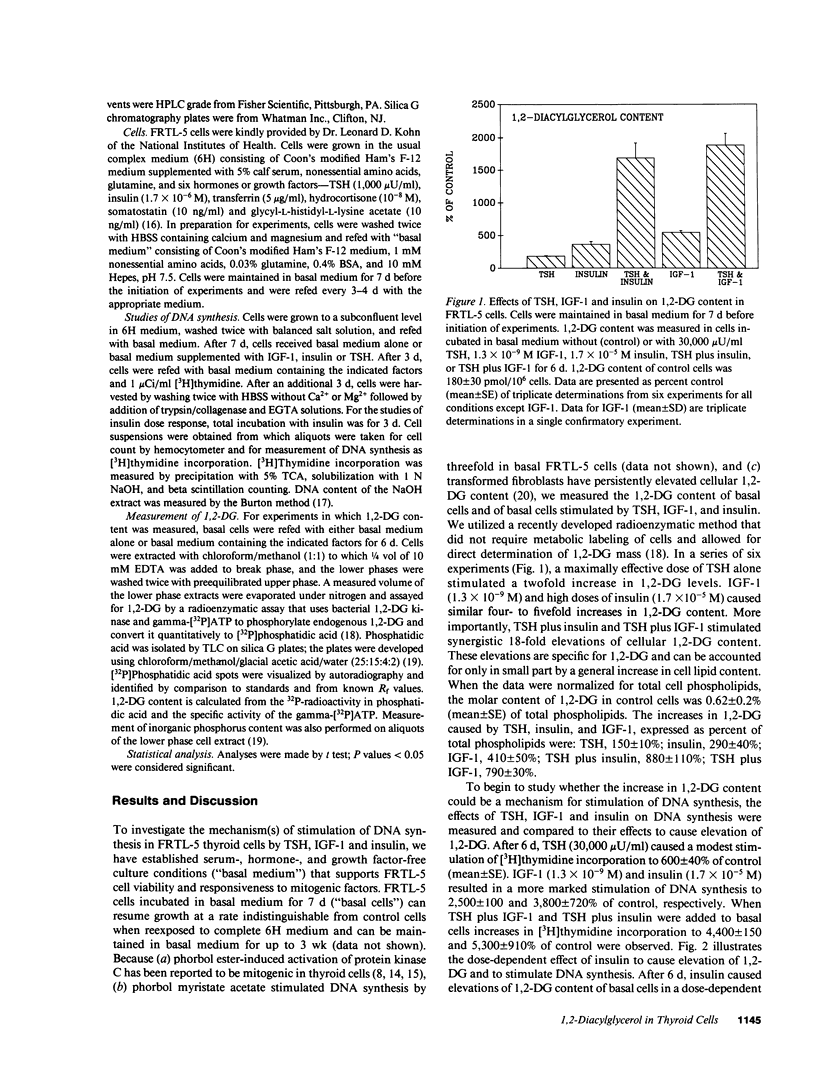
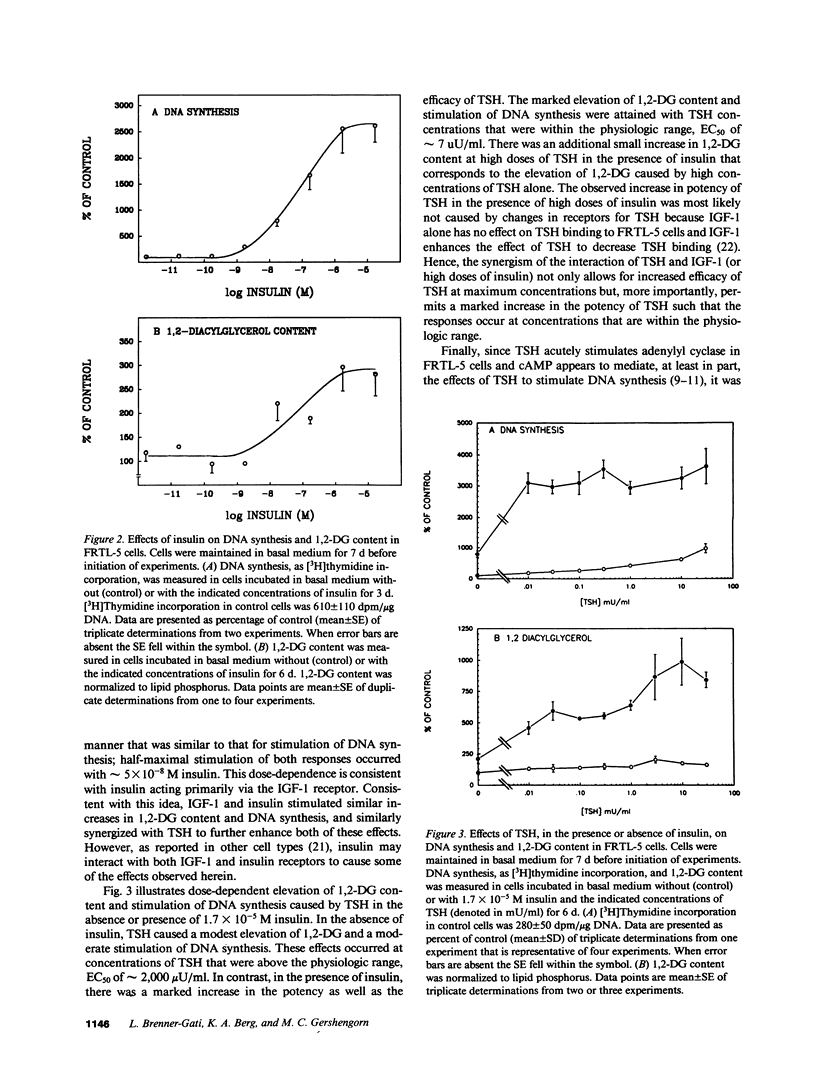
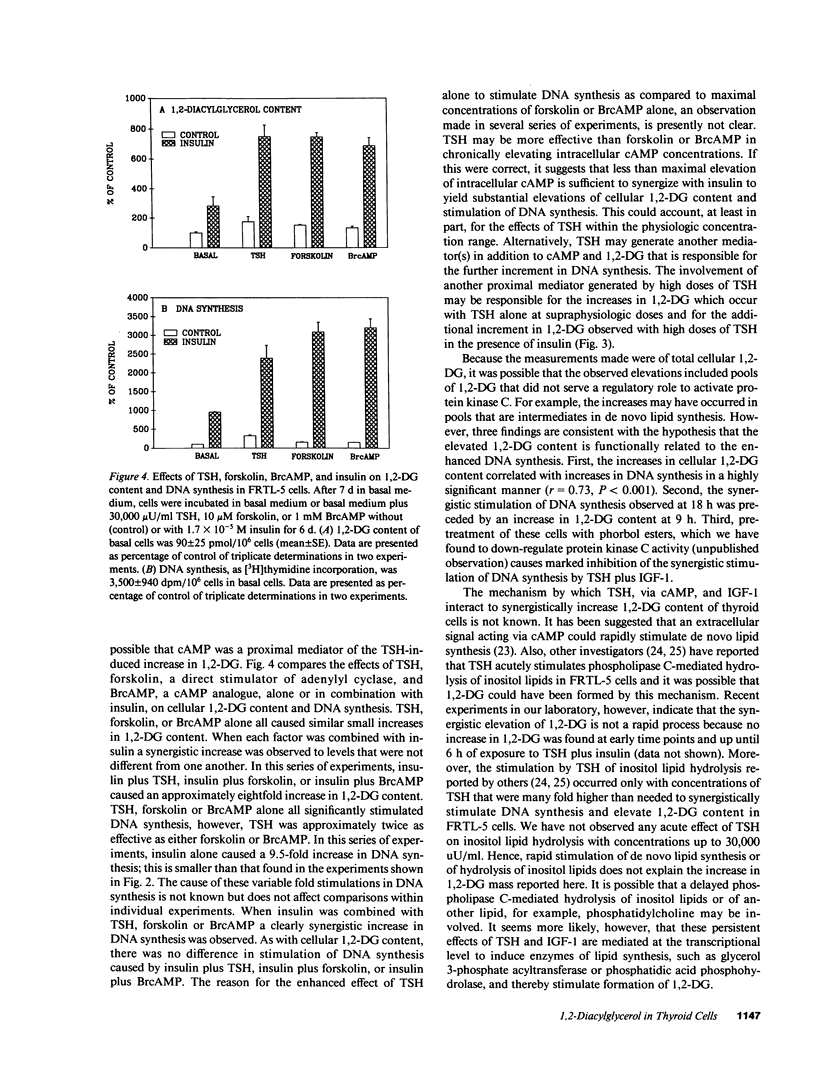
Thyroid-stimulating hormone (TSH) and insulin-like growth factor-1 (IGF-1) synergistically stimulate DNA synthesis in thyroid cells. In this report, a novel mechanism for mediation of this synergistic interaction is described in rat thyroid (FRTL-5) cells. Because phorbol myristate acetate stimulates DNA synthesis, the effects of TSH, IGF-1 and insulin on FRTL-5 cell content of 1,2-diacylglycerol (1,2-DG), the endogenous activator of protein kinase C, were measured. After 6 d, TSH, IGF-1 and insulin caused increases in cellular 1,2-DG (mean +/- SE) to 180 +/- 10%, 540 +/- 50%, and 360 +/- 40% of control, respectively, whereas TSH plus IGF-1 and TSH plus insulin synergistically increased 1,2-DG to 1,890 +/- 310% and 1,690 +/- 230%, respectively. In the absence of insulin, the effect of TSH to elevate 1,2-DG exhibited an EC50 of approximately 2,000 microU/ml. The synergistic interaction of insulin and TSH was found to increase the potency of TSH by 300-fold (EC50 was approximately 7 microU/ml) in addition to increasing the efficacy of TSH. The effect of TSH appeared to be mediated by TSH-stimulated increases in cyclic AMP (cAMP). Forskolin and 8-bromo-cAMP, like TSH, caused modest increases in 1,2-DG and DNA synthesis, whereas forskolin plus insulin and 8-bromo-cAMP plus insulin markedly elevated 1,2-DG content and stimulated DNA synthesis. Under all conditions, increases in 1,2-DG content correlated with stimulation of DNA synthesis. These findings suggest that the synergistic stimulation of DNA synthesis in thyroid cells by TSH, via cAMP, and IGF-1 is mediated by 1,2-DG. Moreover, they implicate a novel interaction between the lipid and adenylyl cyclase signaling systems for the regulation of cell proliferation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrach L. K., Eggo M. C., Mak W. W., Burrow G. N. Phorbol esters stimulate growth and inhibit differentiation in cultured thyroid cells. Endocrinology. 1985 Apr;116(4):1603–1609. doi: 10.1210/endo-116-4-1603. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol lipids and cell proliferation. Biochim Biophys Acta. 1987 Apr 20;907(1):33–45. doi: 10.1016/0304-419x(87)90017-5. [DOI] [PubMed] [Google Scholar]
- Bone E. A., Alling D. W., Grollman E. F. Norepinephrine and thyroid-stimulating hormone induce inositol phosphate accumulation in FRTL-5 cells. Endocrinology. 1986 Nov;119(5):2193–2200. doi: 10.1210/endo-119-5-2193. [DOI] [PubMed] [Google Scholar]
- Dere W. H., Rapoport B. Control of growth in cultured rat thyroid cells. Mol Cell Endocrinol. 1986 Mar;44(3):195–199. doi: 10.1016/0303-7207(86)90124-3. [DOI] [PubMed] [Google Scholar]
- Farese R. V. Phosphoinositide metabolism and hormone action. Endocr Rev. 1983 Winter;4(1):78–95. doi: 10.1210/edrv-4-1-78. [DOI] [PubMed] [Google Scholar]
- Field J. B., Ealey P. A., Marshall N. J., Cockcroft S. Thyroid-stimulating hormone stimulates increases in inositol phosphates as well as cyclic AMP in the FRTL-5 rat thyroid cell line. Biochem J. 1987 Nov 1;247(3):519–524. doi: 10.1042/bj2470519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J. S., Usher P., Moses A. C. Monoclonal antibody to the type I insulin-like growth factor (IGF-I) receptor blocks IGF-I receptor-mediated DNA synthesis: clarification of the mitogenic mechanisms of IGF-I and insulin in human skin fibroblasts. Proc Natl Acad Sci U S A. 1986 Feb;83(3):664–668. doi: 10.1073/pnas.83.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Measurement of lipid turnover in response to thyrotropin-releasing hormone. Methods Enzymol. 1987;141:100–101. doi: 10.1016/0076-6879(87)41059-8. [DOI] [PubMed] [Google Scholar]
- Isozaki O., Kohn L. D. Control of c-fos and c-myc proto-oncogene induction in rat thyroid cells in culture. Mol Endocrinol. 1987 Nov;1(11):839–848. doi: 10.1210/mend-1-11-839. [DOI] [PubMed] [Google Scholar]
- Jin S., Hornicek F. J., Neylan D., Zakarija M., McKenzie J. M. Evidence that adenosine 3',5'-monophosphate mediates stimulation of thyroid growth in FRTL5 cells. Endocrinology. 1986 Aug;119(2):802–810. doi: 10.1210/endo-119-2-802. [DOI] [PubMed] [Google Scholar]
- Metcalfe J. C., Moore J. P., Smith G. A., Hesketh T. R. Calcium and cell proliferation. Br Med Bull. 1986 Oct;42(4):405–412. doi: 10.1093/oxfordjournals.bmb.a072159. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47:425–442. doi: 10.1146/annurev.ph.47.030185.002233. [DOI] [PubMed] [Google Scholar]
- Roger P. P., Reuse S., Servais P., Van Heuverswyn B., Dumont J. E. Stimulation of cell proliferation and inhibition of differentiation expression by tumor-promoting phorbol esters in dog thyroid cells in primary culture. Cancer Res. 1986 Feb;46(2):898–906. [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Smith P., Wynford-Thomas D., Stringer B. M., Williams E. D. Growth factor control of rat thyroid follicular cell proliferation. Endocrinology. 1986 Oct;119(4):1439–1445. doi: 10.1210/endo-119-4-1439. [DOI] [PubMed] [Google Scholar]
- Tramontano D., Cushing G. W., Moses A. C., Ingbar S. H. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves'-IgG. Endocrinology. 1986 Aug;119(2):940–942. doi: 10.1210/endo-119-2-940. [DOI] [PubMed] [Google Scholar]
- Tramontano D., Moses A. C., Ingbar S. H. The role of adenosine 3',5'-monophosphate in the regulation of receptors for thyrotropin and insulin-like growth factor I in the FRTL5 rat thyroid follicular cell. Endocrinology. 1988 Jan;122(1):133–136. doi: 10.1210/endo-122-1-133. [DOI] [PubMed] [Google Scholar]
- Tramontano D., Moses A. C., Picone R., Ingbar S. H. Characterization and regulations of the receptor for insulin-like growth factor-I in the FRTL-5 rat thyroid follicular cell line. Endocrinology. 1987 Feb;120(2):785–790. doi: 10.1210/endo-120-2-785. [DOI] [PubMed] [Google Scholar]
- Tramontano D., Moses A. C., Veneziani B. M., Ingbar S. H. Adenosine 3',5'-monophosphate mediates both the mitogenic effect of thyrotropin and its ability to amplify the response to insulin-like growth factor I in FRTL5 cells. Endocrinology. 1988 Jan;122(1):127–132. doi: 10.1210/endo-122-1-127. [DOI] [PubMed] [Google Scholar]
- Valente W. A., Vitti P., Kohn L. D., Brandi M. L., Rotella C. M., Toccafondi R., Tramontano D., Aloj S. M., Ambesi-Impiombato F. S. The relationship of growth and adenylate cyclase activity in cultured thyroid cells: separate bioeffects of thyrotropin. Endocrinology. 1983 Jan;112(1):71–79. doi: 10.1210/endo-112-1-71. [DOI] [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]



