ABSTRACT
Local ablation technologies for hepatic malignancy have developed rapidly in the past decade, with advances in several percutaneous or externally delivered treatment methods including radiofrequency ablation, microwave ablation, laser ablation, and high-intensity focused ultrasound. Research has focused on increasing the size of the ablation zone and minimizing heat-sink effects. More recent developments include improvements in treatment planning and navigation with integration of several imaging modalities, as well as automated delivery of the ablation through robotics. These improvements will allow increased consistency in treatment delivery and will facilitate translation to the community setting. Combination therapies with multimodality guidance are on the cutting edge of image-guided, minimally invasive cancer therapies. Local ablation is being combined with regional therapies, such as arterial chemoembolization and local activation of systemically administered drugs, with promising results. Potential combinations with local ablation also include external radiation therapy and antitumor immune modulation. Image-guided oncology is emerging as an important part of the interventional radiology practice, thanks in part to the innovation and imaging background that lies at the roots of our discipline.
Keywords: Local ablation, treatment planning, high-intensity focused ultrasound, microwave, laser
Local ablation of hepatic tumors has evolved tremendously over the course of the past decade, which makes it difficult even for the frequent user to keep up with the latest devices and techniques. Interventional radiology has a long history of rapid device innovation, improvement, and clinical deployment although too often not driven by hypothesis-driven research and validation.
Technological advancements in the tumor ablation arena in the past decade have largely focused upon getting a bigger burn with higher watts in a shorter time (Table 1). The coming decade will focus more on the imaging feedback during ablation; refinement of tumor-specific, organ-specific, and patient-specific algorithms and treatment plans; multimodality guidance; targeting; navigation; robotic needle placement; and needle tracking.
Table 1.
Comparison of Thermal Ablation Modalities
| Modality | RFA | MWA | HIFU | Laser |
|---|---|---|---|---|
| Size ablation | 3–7 cm | 3–7 cm | 2 × 8 mm per focal zone | Small per fiber insertion |
| Time | 15–30 min | 6–10 min | Hours | Longer for insertions |
| Advantages | Standard, available, time-tested | No ground pad fast | Noninvasive | MR compatible |
| Availability | ++++ | ++ | + | + |
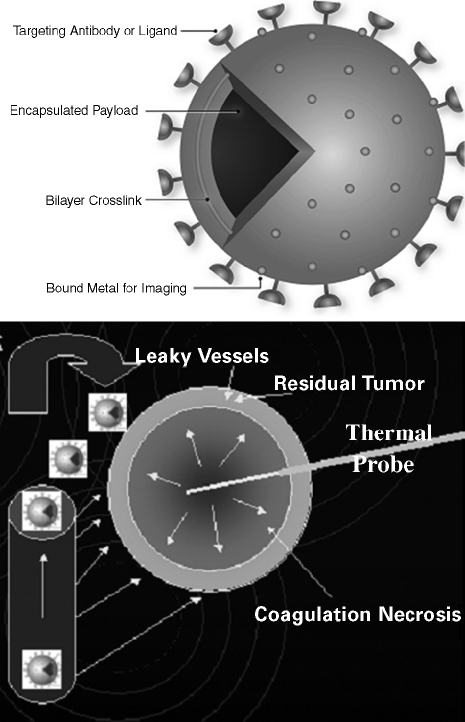
Treatment combinations like radiation and ablation, or drug and device combinations such as heat-activated drug delivery, will become the standard of care for local and regional image-guided oncology therapies. Tumor ablation in the liver will often be accompanied by an intravenous injection of drugs such as heat-activated liposomal chemotherapy like ThermoDox (Celsion Corp., Columbia, MD) (Fig. 1). Ablation will become a method of immune system stimulation that can be accompanied by immunomodulation or vaccination for specific tumors that are sensitive to immunotherapy, like melanoma. Ablation will be performed with a high-intensity, split-beam, spiral, phased-array focused ultrasound or adaptive phased array microwave that will not require skin invasion or needles. Such deposition of electromagnetic energy will activate targeted “smart drugs,” increase permeability, enhance extravasation, facilitate gene transfection, and augment drug delivery. Radiologists will have a competitive edge in this high-technology future that will become more and more dependent upon multimodality imaging feedback, molecular interventions, targeted energy deposition, and novel biomedical engineering and software tools. Ablation tools will become a means to turn on trigger switches for targeted tumor-specific nanoparticles.
Figure 1.
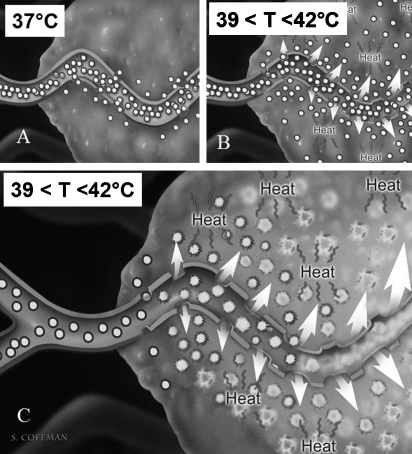
Chemical thermal and anatomic synergy of heat-activated drug delivery showing rationale of this drug-device combination. (A) Leaky tumor vessels. (B) Heat adds permeability and extravasation. (C) Leaky vessels + heat + low-temperature-sensitive liposomes give a synergistic leakage and drug release. (Reprinted with permission from Kong G, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res 2000;60:6950–6957.)
TECHNIQUES
The local ablation of tumors requires careful planning regardless of the method of destruction. The central concept in local ablation is the relationship of the tumor and the burn. The geometry produced by the ablation can be somewhat unpredictable, and its complexity and variability in size and shape is often underemphasized. Neglecting to ensure complete coverage of the tumor by the ablation technique is likely the biggest reason for local failure (along with convective heat loss from vessels leading to viable residual).
Careful planning before treatment and attention to detail during treatment can improve outcomes. It is important to customize the treatment plan and to address potential obstacles particular to each individual patient. Pretreatment imaging allows accurate target identification and access route. Positioning the patient during preablation imaging studies in the orientation most likely to be utilized in the treatment phase can predict internal organ and target mobility. Organ movement during respiration can also complicate the procedure. Respiratory gating solutions during interventions are being developed1,2 but simply having the patient practice breathing and breath holds prior to the treatment can help minimize difficulties and complications associated with respiratory motion. Respiratory bellows belt and split-screen ultrasound (US) with a passive arm transducer holder have been proposed as methods of respiratory gating (Willet Whitmore, verbal communication, Civco, Sarasota, Florida, 2005).3,4,5 Dynamic compensation using electromagnetic tracking or deformable models of breathing pattern analysis has also been proposed. The patient's ability to follow breathing instructions can be enhanced by giving systemic pain medications after the placement of the needle but before ablation begins. Such simple techniques can also improve the accuracy of probe placement.
The use of 50 mL miniboluses of contrast during computed tomographic (CT)-guided ablations may facilitate target identification, needle positioning, or verification of margin coverage. One example of the utility of this technique is an unpublished case of a Von Hippel Lindau patient with a clandestine kidney tumor visualized only via these injections. The small volume of these contrast injections allows repeated injections to provide near real-time imaging updates during pretreatment planning, ablation treatment, and immediately postablation. Miniboluses can be used at any one or two desired time points and are rarely required for more than this. Even though gas impairs visualization, US can greatly improve lesion localization during treatment, especially with the addition of US contrast agents. When using US, it is important to look through multiple windows to give more three-dimensional spatial feedback about the needle-target geometric relationship. Though freehand placement of the ablation needle under US guidance is acceptable, needle guidance and angle selection tools may improve placement accuracy and minimize complications associated with multiple placements (Fig. 2). Treating from deep to shallow avoids the gas-out effect, where cooked tissue emits gaseous microbubbles that cloud US of any deeper tissue.
Figure 2.
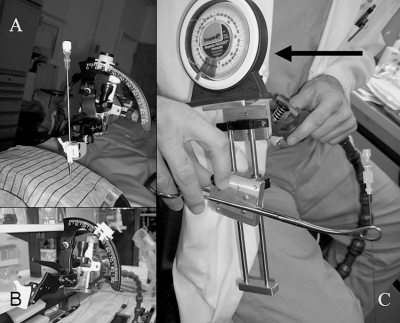
Low-tech needle angle selectors. (A,B) Protractor with bubble leveler connected to passive arm secured to table with disposable clip-on needle guide (Civco, Sarasota, FL. (C) Prototype clamp with plumb bob that selects the angles (arrow) brings carpentry to the interventional radiology suite.
Continuous monitoring of the formation of echogenic microbubbles during ablation can be used as a rough guide during treatment, though they do not accurately correlate with coagulation margins by pathology.5 Temperature of the ablated tissue can be used to verify complete ablation of the lesion. Postablation maximum temperature and cooling rate can be a surrogate marker for adequacy of ablation (at the measurement location). This information is available from Cool-tip (Valleylab Inc., Boulder, CO) (one point) and RITA (RITA Medical Inc., Mountain View, CA) (four to five points) systems. The needle tip with thermistor (Valleylab) can be moved around the treatment zone edges immediately following ablation to test specific points for temperature to try to localize regions of heat-sink or regions at risk for undertreatment. Such “test temperatures” must factor in the cooling expected over time. Independent needle thermometers (22 G SMK with TCA-2 monitor, Valleylab) may also be used to protect heat-sensitive nontarget tissue.6
Liver dome lesions carry increased risk for pleural effusions and many patients experience referred shoulder pain. It is important to aggressively target dome lesions including the diaphragm when appropriate, and they can often be targeted via an intercostal approach, avoiding lung parenchyma. However, avoidance of the lung should not supersede treatment of the lesion, as it is preferable to place a chest tube rather than leave residual tumor. Sterile water may also be infused to displace lung for safe access.
The use of prophylactic antibiotics in local ablation procedures is controversial and varies widely between institutions and practitioners. At this institution, all patients are given prophylactic antibiotics with an added short course of antibiotics postablation if they are at increased risk to develop abscesses; this population includes patients with a history of sphincterotomy, biliary-enteric anastamosis or any prior hepatic artery chemotherapy, chemoembolization, installation of a chemotherapy pump or other liver manipulation, biliary ductal dilation (complete, sequential, or isolated occlusion), and cholangiocarcinoma or other central porta hepatis targets. Rationale for the use of antibiotics is based upon similarity to surgical antibiotic prophylaxis, which is standard care, despite having never been proven with a prospective, randomized controlled trial. Even though ablation should be a sterile procedure, antibiotics make sense in our opinion.
The choice to approach the ablation by percutaneous, laparoscopic, or open technique is variable and often dependent upon practice setting or tumor location (Table 2). Several questions can be examined to arrive at the best approach for each patient. Is the proposed percutaneous route safe and easy? Is the lesion near the surface or deep in the organ? Are adhesions present that would increase difficulty in an open or laparoscopic procedure? Where is the bowel or other neighboring heat-sensitive organs? Will the Pringle maneuver (surgical occlusion of both the hepatic artery and portal vein) or embolization or balloon occlusion be needed or greatly improve the success of the procedure? Can fluid (5% dextrose) be infused to blanket and protect heat-sensitive structures?
Table 2.
Surgical and Interventional Radiology Techniques
| Surgery | IR |
|---|---|
| Laparoscopic | 5% dextrose or CO2 infusion |
| Pringle | TACE, embolization, balloon occlusion |
| US guidance | CT, US, MR guidance |
The efficacy and safety of heat ablation techniques may be improved with specific adjunctive maneuvers that may be easily performed by the interventional radiologist. One limitation with these techniques has been the heat-sink effect created by neighboring blood vessels or overall organ perfusion. Options to minimize heat-sink include Pringle,7 balloon occlusion,8,9,10 or embolization11 procedures of the hepatic vein, hepatic artery, or portal vein, or pharmacological maneuvers such as the use of arsenic.12 One technique to overcome this heat-sink effect is to maximize current density in the region of the tumor closest to the vessels, particularly vessels greater than 3 mm in diameter.13,14 This can be done by ablating as close to the vessel as possible (without being in the vessel) to target tissue at the vessel edge. Occlusion of blood vessels in proximity to the tumor may decrease the heat-sink effect. Occlusion may also allow improved thermal injuries consistent with less variable, more uniform ablation geometry and size distribution.15 Transcatheter arterial embolization with both iodized oil and gelatin sponge may significantly increase the size of the ablation zone as well.11 The added risk of such maneuvers, however, is not yet well defined.
The hepatic vein nearest the target may be the technically easiest vessel to access and occlude. This is done in angiography via a jugular approach, after which the patient is taken to CT with the balloon down (Fig. 3). Intermittent occlusion (3 minutes up, 1 minute down) may improve vessel patency.16 As multimodality CT-angiography suites become more prevalent, this combination procedure will become easier without patient transfer. This transient occlusion is an alternative to the more permanent embolization of the portal vein or hepatic artery; it is also a considerably less invasive alternative to the surgical Pringle maneuver.
Figure 3.
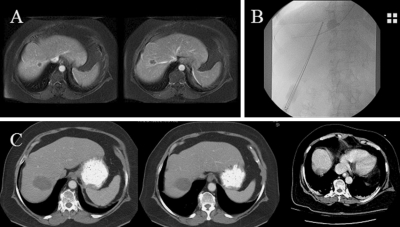
Balloon occlusion. (A) Two colorectal metastases touching the hepatic vein preablation. (B) Hepatic vein balloon occlusion with cool-tip cluster RFA needle, to decrease convective heat-sink (Valleylab). (C) Six months post-RFA shows successful ablation.
Infusion of 5% dextrose (Fig. 4) or CO2 can provide thermal and electrical insulation during the ablation and move neighboring organs (such as the large bowel) out of harm's way.17 Normal saline use is not recommended due to its ability to conduct electric current.18 Anecdotally, these techniques may also decrease pain associated with the ablation, especially when fluid is adjacent to liver capsule and diaphragm. This technique should be used with caution if the tumor is touching or invading the diaphragm, as fluid may keep the diaphragm from reaching lethal temperatures.
Figure 4.
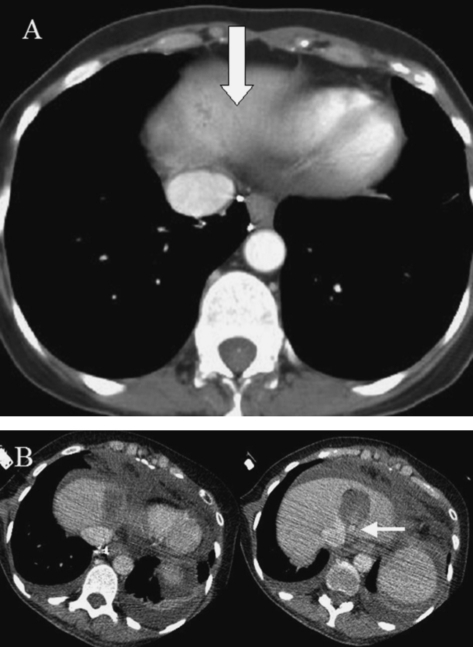
D5 infusion. (A) Pre-RFA CT image showing solitary sarcoma liver metastasis touching heart and left hepatic vein. (B) Immediately following ablation and following instillation of 5% dextrose water to insulate and protect the heart. The image on the right demonstrates a hepatic vein occlusion balloon (arrow).
Five percent dextrose infusion is the more commonly attempted approach and easier than CO2, which necessitates manometry or repeated instillation and imaging. Five percent dextrose is infused through a Chiba 22-G needle under US guidance near the sensitive structure (generally colon) with a target volume of 20 to 30 mL. Next a 4-Fr or 5-Fr side-hole sheath needle is deployed in the space created by the small injection (Yueh Sheath Needle, Cook Inc., Bloomington, IN or One-Step Centesis Catheter, Merit Medical, South Jordan, Utah—both produce the appropriate equipment), again under US guidance. If 5% dextrose hung to gravity runs freely, then the needle should be in the correct space and up to 3 L can be infused to achieve needs, although 500 mL is usually adequate; larger volumes may actually increase post–radiofrequency ablation (RFA) bloating pain. US or CT confirms the insulation effect. In an alternative approach, 5% dextrose can be injected through a 22 G Chiba directly into the organ. Five percent dextrose is again hung to gravity and the needle slowly withdrawn from the organ, with correct needle location again indicated by free-flowing 5% dextrose.
CO2 serves as an alternative to 5% dextrose infusion. Though less commonly used, CO2 has the advantage of better insulation versus 5% dextrose. Another difference is more rapid resorption by the body versus 5% dextrose. A laparoscopic tower can be used in conjunction with a manometry set and laparoscopic miniport, allowing real-time monitoring and consistent maintenance of the pressure. However, this requires extra equipment and may not be readily available in all practices.
NAVIGATION, TREATMENT PLANNING, ROBOTICS, AND MAGNETIC RESONANCE THERMOMETRY
The future decade will see the proliferation of CT-integrated robotics, needle navigation with electromagnetic tracking, and complex treatment plans similar to radiation therapy. Energy deposition will be planned and adjusted according to where it is desired, with built-in feedback loops to minimize risk to collateral normal tissue.19,20 CT scanners will merge with rotational flat detector angiography into one machine for both modalities. US will be available on passive arms on the CT scanner that can display layers of imaging data, including preprocedural CT, magnetic resonance (MR), or positron-emission tomography (PET).21 Ablation needles and US transducers may also have the position and orientation tracked electromagnetically22,23 (Fig. 5). Image fusion will combine many different modalities for use before, during, and after ablation, combining functional, metabolic, anatomic, and morphological imaging. Fusion of preprocedural images to post-RFA images can better define treatment effect with the patient still on the table. These methods allow the use of preprocedural CT for targeting arterial phase-enhancing tumors, such as hepatoma, and may facilitate repositioning during the ablation process. PET data may also be used in the procedure room for automated targeting. MR thermometry can provide valuable real-time feedback but requires special equipment that has been developing slowly, and the technique has been slow to be accepted into routine clinical use. CT thermometry is feasible as well.
Figure 5.
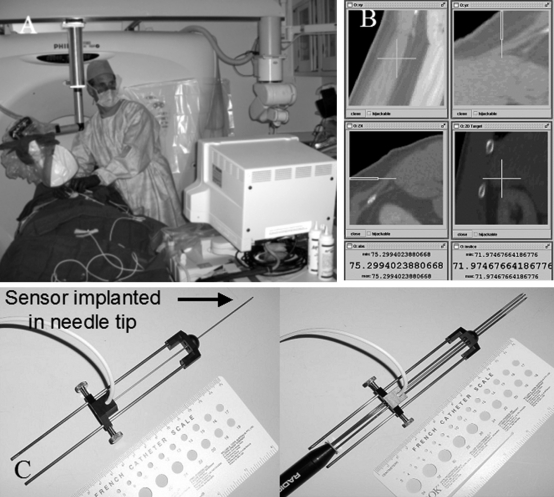
Electromagnetic tracking inside the needle allows use of preprocedural CT imaging during the ablation with real-time feedback of position. (A) Electromagnetic field generator mounted on a stereotactic CT frame in proximity to the patient. (B) Real-time multiplaner graphical user interface allows targeting and guidance of the needle tip using preprocedural imaging. (C) Magnetically trackable ablation needle (left) and magnetically trackable ablation needle with cool-tip cluster needle inserted (right).
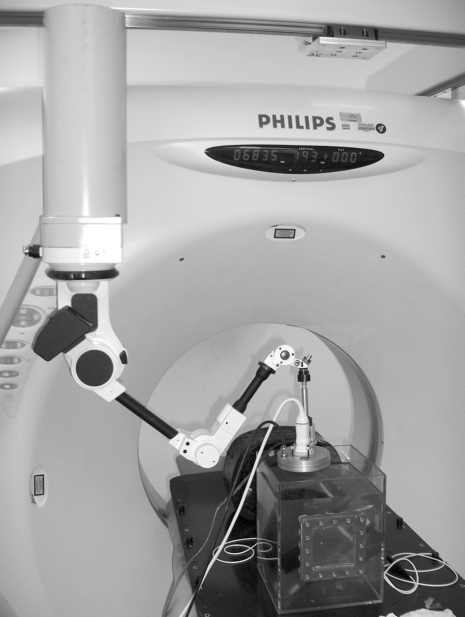
Robotic arms will facilitate CT-guided ablation with point-and-click, see-and-treat platforms. Highly accurate, precise, and inexpensive robots that are directly integrated to CT are being developed that do not need additional software or manpower. Treatment planning software will automatically perform segmentation of tumor, organ, and blood vessels to better plan for automatic angle selection or needle insertion24,25 (Fig. 6). Navigation, visualization, and automation will vastly simplify, improve, and normalize variability in the delivery of local ablation (Fig. 7).
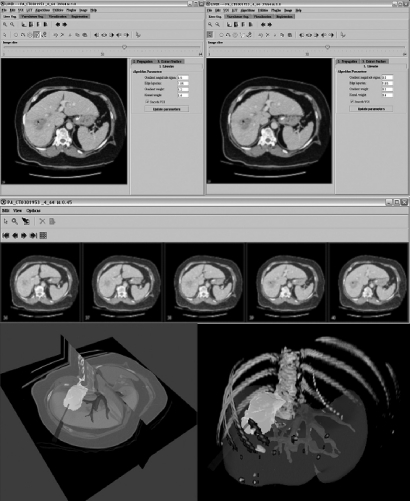
Figure 6.
Custom NIH software (MIPAV: Medical Image Processing and Visualization, Center for Information Technology, NIH Clinical Center, Bethesda, MD) for RFA treatment planning performs semiautomatic segmentation, propagation and thresholding with virtual needles, and virtual ablation zone.
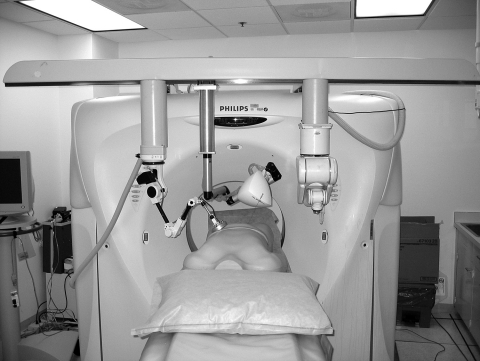
Figure 7.
CT portion of the multimodality interventional suite with rotational angiography also on the same table. Tools attached to CT stereotactic frame include diagnostic and therapeutic US (left) electromagnetic field generator (middle) and 6-degree-of-freedom robot for needle insertions (right).
REVIEW OF LITERATURE
Microwave
Percutaneous microwave tumor ablation (MWA) deposits thermal energy produced by the vibration and rotation of water molecules in tissue induced by microwaves emitted from an antenna on the ablation needle (Figs. 8 and 9). Thus, one advantage of MWA versus RFA is lack of need for a grounding pad. Microwave may be less susceptible to convective heat loss versus RFA, and thus may be less prone to the heat-sink effect. Although this could potentially translate into improved local control, this is currently somewhat speculative. MWA is faster and less dependent on thermal conduction than RFA, as a larger volume of tissue is heated to higher temperatures in a shorter time frame. RFA relies on more of a linear or curvilinear heat source, which then conducts from the center outward. MWA is more broadly heated simultaneously, similar to a microwave oven. This may also have the advantage of improved uniformity of the thermal lesion, although this too is speculative at this time.
Figure 8.
Triple straight (A) and curled (B) needle/antennas in Vivant microwave prototype (ValleyLab).
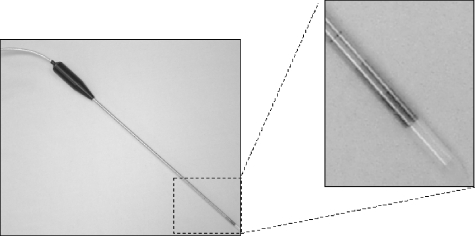
Figure 9.
Microwave ablation needle/antenna using dielectric materials for insulation (Microsulis Americas, Inc., Cooper City, FL).
Although RFA has become prevalent and has a proven track record with small needle percutaneous ablation, one disadvantage in RFA has been the need to burn each needle one at a time. The development of a new switch box26 allows rapid switching between multiple RFA needles, making simultaneous treatment zones possible for the first time, similar to the simultaneous treatment zone stacking utilized in cryoablation and MWA. MWA can use multiple needles simultaneously, which can result in constructive interference of the microwaves between antennae if needles are in close proximity. Multiple applicator techniques are being investigated for both MWA and RFA to reduce treatment time.27
Many emerging technologies hold promise for future clinical ablation improvements, including needle-based systems with mature technology (bipolar RFA [Fig. 10], simultaneous RFA, rapid switch RFA, coherent MWA, electroporation, sonoporation), needle-based systems with immature technology (incoherent MWA, phase-modulated MWA, tumor electrolysis), mature needleless technology (high-intensity focused ultrasound [HIFU]), as well as immature needleless technology (adaptive phased array microwave28) (Fig. 11).
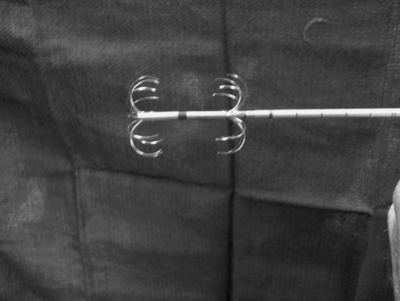
Figure 10.
Coaxial bipolar RFA needle (Boston Scientific, Marlborough, MA).
Figure 11.
Celsion Corporation adaptive phased array microwave device prototype for enhancing drug delivery in deep-seated cancer treatment (courtsey of Celsion Corporation, Columbia, MD). The adaptive phased array microwave device prototype is based on an MIT patent. (From Fenn AJ. The Celsion adaptive thermodynamic therapy (TDT) drug delivery system for treating deep-seated cancer. Drug Delivery Technology 2002;2:74–79. Reprinted by permission.)
Much of the current literature uses outdated microwave and various evolving RFA technologies, so one must read and interpret with caution. The summary that follows is thus not reflective of modern MWA versus RFA technologies, with those studies in early or design phases at present. Comparison of RFA and MWA in small liver tumors in one of the first randomized prospective studies of percutaneous ablation techniques demonstrated the ability to treat equal tumor sizes, though microwave required more treatment sessions with early generation systems.29 The trial did not demonstrate statistically significant differences in complication rates or residual viable tumor between the two modalities; however, this may be due to the small numbers. Early generation MWA and RFA systems also have demonstrated similar local recurrence rates, particularly in large tumors.30
Some reports exist of microwave burn tracking along the portal vein.31 The cause of this has not been fully examined, but it may involve heating of the periportal fat. The safety issues for recent generation MWA systems are likely similar to RFA, except that larger, faster burns may mean more risk of collateral damage, regardless of the technology used for the larger burn. More prospective randomized trials with larger patient populations and new equipment are warranted for further comparison of RFA and MWA. However, as is the problem with many long-term prospective studies of ablation technologies, the technology is so rapidly advancing and evolving that it makes meaningful comparison and control groups difficult as the standard is a moving target.
HIFU
HIFU functions as a tumor therapy by thermal ablation or by augmentation of drug delivery or gene transfection. In HIFU, the intensity of the US beams is substantially increased by converging the beams within a tight focal zone (Fig. 12). The size and depth of the focal zone is dependent on the geometry of the HIFU transducer. HIFU is a truly noninvasive approach as the HIFU transducer transmits the beams from outside the body. The intensity of the beams is below the threshold to cause damage as they pass through the tissue toward the focal zone.
Figure 12.
Integrated diagnostic US, CT, and HIFU with shared spatial coordinates, which allows HIFU ablation for drug delivery to be targeted with US or CT.
HIFU shows early clinical promise for local ablation of liver tumors, although there are time constraints that limit the practicality of current technologies for thermal ablation in many oncology settings. In one trial with a Chinese HIFU system from Chongqing, 474 primary or metastatic liver tumors were ablated with HIFU with a margin of 1.5 to 2.2 cm around the tumor site. The mean size of treated hepatic tumors was just over 8 cm with a range of 4 to 14 cm in diameter. Changes on contrast-enhanced MR imaging were demonstrated at 1 to 2 weeks post-HIFU. Surgically removed specimens from several patients demonstrated coagulation necrosis and severe damage or destruction of small blood vessels in the treated areas, with a sharp boundary between ablated and untreated tissue.32
A significant survival benefit was reported for hepatocellular carcinoma with combined HIFU and transcatheter arterial chemoembolization (TACE) versus TACE alone at 6-month (80.4 to 85.4% versus 13.2% survival) and 1-year follow-up (42.9% and 0% survival, respectively).33 A median HIFU treatment time of 4.9 hours was observed with a range of 2 to 8 hours.
The length of treatment time needed is one major limitation for HIFU. The use of split-beam and spiraling transducers may help decrease the long treatment times.34,35 Microbubble contrast agents that have been developed for diagnostic US may increase ablation volume in animal experiments36 and may provide another means of decreasing the long treatment time required in HIFU ablation, although the bubbles may lead to ablation zone distortion.37 Aside from the thermal mechanism of HIFU ablation, cavitation bubbles also may play a role.37 Cavitation refers to the rapid development and collapse of microbubbles that result in highly concentrated energy and pressure. But cavitation is a violent implosion of a gaseous microbubble causing uncontrolled release of energy, which may be difficult to target or predict. The role of cavitation has also caused concern for increased metastatic potential. Wu et al examined the risk of HIFU-induced metastases by examining blood samples before and after HIFU ablation of solid tumors with reverse transcriptase polymerase chain reaction. Findings indicated no increased risk and showed possible elimination of preexisting circulating tumor-specific marker RNA.38 Such a mechanism could also be a potent immunostimulator for immunotherapy. HIFU ablation can enhance systemic antitumor immune response as indicated by a statistically significant increase in absolute CD4+ cells and CD4+/CD8+ ratio following HIFU treatment.39 This effect may be tumor-specific and could augment tumoricidal effects or immunotherapies.
Though HIFU can reach various locations in the body with depths dependent on the focal length of the transducer, the ability of HIFU to reach a lesion can be limited by air-filled organs such as the lung and the bowel. Focusing the HIFU beam through bone is another obstacle; however, attenuation correction and prediction based upon reference CT or MR has been performed.40 Bone also presents a problem for tumor localization, as many devices utilize a coaxial diagnostic probe to guide the ablation treatment. However, MR-guided systems can help eliminate this problem. One MR-integrated HIFU system was recently approved for uterine fibroid ablation but should find utility in oncological interventions (Insightec, Dallas, TX).
Continuous HIFU causes substantial temperature increases to yield local ablation. However, pulsed HIFU does not produce the ablative effects in tissue and can be designed to have reversible effects on vessel leakage of targeted nanoparticles and intravenous injectable drugs. This is possible by implementing low-duty cycles that allow for dissipation of heat between pulses. Compared with continuous HIFU for thermal ablation, in pulsed HIFU, acoustic radiation forces displace tissue, reorganize collagen, and allow increased delivery of liposomal doxorubicin, nanoparticles, fluorophores, and naked DNA injected systemically to locally sonicated tumor tissue in vivo.41,42 This technique provides a potential alternative method to treating tumors by incorporating chemotherapy or gene therapy locally where it is needed while theoretically avoiding systemic side effects of systemic drug or gene therapy administration. Integration of such a system into the multimodality clinical interventional radiology suite (CT- and MR-based varieties) is underway and is one of the most exciting frontiers in interventional image-guided molecular oncology.
Laser
Laser has been investigated as a successful local ablation technique. Known by a plethora of names—laser-induced thermotherapy, interstitial laser thermotherapy, laser-induced thermotherapy, or laser thermal ablation—laser light has been harnessed to achieve local thermal ablation of tumors. Several studies of laser ablation have demonstrated its utility as an ablation technique in primary and metastatic liver tumors with complications similar to those of other local ablation techniques.43,44 Ablation volumes from laser can be similar to RFA but require many more fiber insertions.
One main difference between laser and other percutaneous local ablation techniques is that the fiber optics that delivers the laser light are fully MR compatible. When combined with an open MR imaging system, this technique allows MR guidance to the desired ablation site. Also, unlike RFA, laser can be monitored during continuous ablation with real-time MR thermometry, whereas RFA requires sophisticated switch box technology to intermittently turn off the RFA during MR scans. Although this compatibility with MR provides several advantages, including absence of radiation exposure to the patient and operator, other limitations exist, including MR reimbursement factors, the lack of large bore, interventional radiology–friendly MR system, and constraints on MR scanner time.
Laser can be employed in conjunction with photosensitizers for ablation along with photodynamic therapy (PDT). A local-regional technique, PDT uses laser energy to locally activate systemically injected photosensitizer agents that destroy the targeted tumor tissue through cytotoxic pathways, often via a toxic oxygen species.
Injectables and Transcatheter
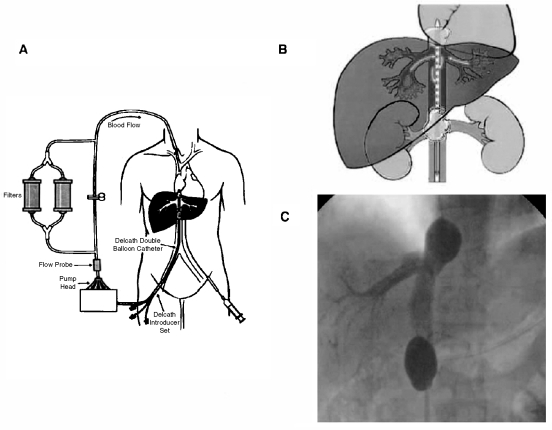
Injectable therapies and regional therapies offer alternatives to thermal ablation in some scenarios (such as when ablation technologies are not available or when disease is too diffuse for local therapies). Even the best imaging often does not depict micrometastases on the cellular level: ablated histologies often recur elsewhere in the liver. Percutaneous isolated liver perfusion can deliver high-dose chemotherapy to the liver only via the hepatic vein. High response rates in select patient populations have been demonstrated45 (Delcath, Stamford, CT) (Fig. 13). Iron beads laced with doxorubicin have been targeted with external magnets for hepatocellular carcinoma (FeRx, San Diego, CA). Intra-arterial doxorubicin-eluting beads have been shown to increase delivery and concentration of the chemotherapeutic drug versus intra-arterial doxorubicin alone in an animal model.46 Yttrium glass beads can be locally injected for selective destruction of liver tumors (TheraSphere, MDS Nordion, Ottawa, Ontario, Canada and SIR-spheres, Sirtex Medical Inc., Lake Forest, IL). Alcohol or acetic acid injections may be inexpensive alternatives to thermal ablation for smaller liver tumors, although they are unlikely to be as effective.
Figure 13.
Percutaneous isolated liver perfusion allows high-dose chemotherapy to be regionally delivered where it is needed (which may decrease systemic side effects) and then filtered outside the body with the return of clean blood. (From Pingpank JF, et al. Phase I study of hepatic arterial melphalan infusion and hepatic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J Clin Oncol 2005;23:3465–3474. Reprinted with permission from the American Society of Clinical Oncology.)
COMBINATION THERAPIES
Various complementary treatment combinations have been investigated and myriad more are possible. Drug-device combination therapies will likely become the standard for local and regional image-guided oncology therapies (Fig. 14). A phase I clinical trial on heat-activated liposomal doxorubicin delivered intravenously and combined with RFA for liver tumors is underway (NIH and Celsion Corp., Columbia, MD). Other doxorubicin-laden beads are also available (Dox-Spheres, Sirtex Medical Inc., Lake Forest, IL). HIFU and RFA can both assist in the deposition of doxorubicin. Magnetic microspheres can selectively produce localized heating when combined with an external electromagnetic device (Thermo-Spheres, Sirtex Medical Inc., Lake Forest, IL). Radiation or brachytherapy may be potent adjuvants for thermal ablation, and trials are underway to study radiation and RFA for lung cancer. RFA also induces an antitumor immune response that is both systemic and tumor-specific.47,48,49 Combining ablation technologies with existing immunotherapy regimens could be high-yield research.
Figure 14.
Targeted nanoparticles may travel selectively to tumors by binding to surface proteins, then locally releasing a lethal payload, which may be partly activated by electromagnetic energies such as used for ablation.
There is no consensus on the “perfect” local ablation technique. Survival data must be fully elucidated for the different techniques, and the growing implementation of combination treatments adds more complexity to the choice of techniques. Survival data should be expected to be similar for similar methods of local tissue destruction. If differences materialize, it may be due to differing technical aspects such as operator variability, device reliability and precision, and specific needle/probe/antennae geometries. Techniques producing smaller treatment volumes per needle or catheter insertion (laser and cryoablation), or treatment time (HIFU), may be more susceptible to treatment gaps and treatment failure. Because of this, radiofrequency and microwave may have an advantage over laser, cryoablation, and HIFU, especially in liver tumor ablation where larger volumes of tumor burden are present. Steep learning curves and lack of validated training mechanism and treatment planning software could also limit or delay translation to the community setting. Image-guided deposition of electromagnetic energy for killing tumor, drug delivery, gene transfection, or enhancement of radiation or immunotherapy will play an emerging role in targeted local and regional oncology, guided by multimodality navigation, automation, and visualization.
ACKNOWLEDGMENTS
This research was supported in part by the Intramural Research Program of the NIH, Clinical Center.
REFERENCES
- Clifford M A, Banovac F, Levy E, Cleary K. Assessment of hepatic motion secondary to respiration for computer assisted interventions. Comput Aided Surg. 2002;7:291–299. doi: 10.1002/igs.10049. [DOI] [PubMed] [Google Scholar]
- Borgert J, Kruger S, Timinger H, et al. Respiratory motion compensation with tracked internal and external sensors during CT guided procedures. Paper presented at Annual Meeting of the International Society for Computer Aided Surgery. Berlin, Germany: June 23, 2005. [DOI] [PMC free article] [PubMed]
- Frohlich H, Dohring W. A simple device for breath-level monitoring during CT. Radiology. 1985;156:235. doi: 10.1148/radiology.156.1.4001413. [DOI] [PubMed] [Google Scholar]
- Carlson S K, Felmlee J P, Bender C E. Intermittent-mode CT fluoroscopy-guided biopsy of the lung or upper abdomen with breath-hold monitoring and feedback: system development and feasibility. Radiology. 2003;229:906–912. doi: 10.1148/radiol.2293021496. [DOI] [PubMed] [Google Scholar]
- Leyendecker J R, Dodd G D, Halff G A, et al. Sonographically observed echogenic response during intraoperative radiofrequency ablation of cirrhotic livers: pathologic correlation. AJR Am J Roentgenol. 2002;178:1147–1151. doi: 10.2214/ajr.178.5.1781147. [DOI] [PubMed] [Google Scholar]
- Diehn F E, Neeman Z, Hvizda J L, Wood B J. Remote thermometry to avoid complications in radiofrequency ablation. J Vasc Interv Radiol. 2003;14:1569–1576. doi: 10.1097/01.rvi.0000096769.74047.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn W K, Dodd G D, III, Kohlmeier R E, et al. Radiofrequency tissue ablation: effect of hepatic blood flow occlusion on thermal injuries produced in cirrhotic livers. Ann Surg Oncol. 2003;10:773–777. doi: 10.1245/aso.2003.09.013. [DOI] [PubMed] [Google Scholar]
- de Baere T, Bessoud B, Dromain C, et al. Percutaneous radiofrequency ablation of hepatic tumors during temporary venous occlusion. AJR Am J Roentgenol. 2002;178:53–59. doi: 10.2214/ajr.178.1.1780053. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Tsuji K, Sakurai Y, et al. Percutaneous radiofrequency ablation for unresectable large hepatic tumours during hepatic blood flow occlusion in four patients. Clin Radiol. 2004;59:812–818. doi: 10.1016/j.crad.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Yamasaki T, Kurokawa F, Shirahashi H, et al. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow: comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2002;95:2353–2360. doi: 10.1002/cncr.10966. [DOI] [PubMed] [Google Scholar]
- Sugimori K, Nozawa A, Morimoto M, et al. Extension of radiofrequency ablation of the liver by transcatheter arterial embolization with iodized oil and gelatin sponge: results in a pig model. J Vasc Interv Radiol. 2005;16:849–856. doi: 10.1097/01.RVI.0000157780.44868.78. [DOI] [PubMed] [Google Scholar]
- Horkan C, Ahmed M, Liu Z, et al. Radiofrequency ablation: effect of pharmacologic modulation of hepatic and renal blood flow on coagulation diameter in a VX2 tumor model. J Vasc Interv Radiol. 2004;15:269–274. doi: 10.1097/01.rvi.0000109396.74740.c4. [DOI] [PubMed] [Google Scholar]
- Lu D SK, Raman S S, Vodopich D J, et al. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol. 2002;178:47–51. doi: 10.2214/ajr.178.1.1780047. [DOI] [PubMed] [Google Scholar]
- Lu D S, Raman S S, Limanond P, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267–1274. doi: 10.1097/01.rvi.0000092666.72261.6b. [DOI] [PubMed] [Google Scholar]
- Chang I, Mikityansky I, Wray-Cahen D, Pritchard W F, Karanian J W, Wood B J. Effects of perfusion on radiofrequency ablation in swine kidneys. Radiology. 2004;231:500–505. doi: 10.1148/radiol.2312021248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudheendra D, Neeman Z, Kam A, Locklin J, Libutti S K, Wood B J. Intermittent hepatic vein balloon occlusion during radiofrequency ablation in the liver. doi: 10.1007/s00270-006-0040-9. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam A W, Littrup P J, Walther M M, Hvisda J, Wood B J. Thermal protection during percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol. 2004;15:753–758. doi: 10.1097/01.rvi.0000133535.16753.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeseke P F, Sampson L A, Winter T C, III, Lee F T., Jr Use of dextrose 5% in water instead of saline to protect against inadvertent radiofrequency injuries. AJR Am J Roentgenol. 2005;184:1026–1027. doi: 10.2214/ajr.184.3.01841026. [DOI] [PubMed] [Google Scholar]
- Yanof J, Wood B J, Seip R, et al. Dual-modality navigation and display system for targeting high-intensity focused ultrasound (HIFU) therapy for drug delivery or thermal ablation: utilization of multislice-CT (MS-CT) and real-time ultrasound (US) Chicago, IL: Info Rad Exibit presented at Radiological Society of North America Annual Meeting; December 2, 2004.
- Yanof J, Bauer C, Renisch S, et al. CT-integrated treatment planning for robot-assisted laser-guided radiofrequency ablation. Abstract presented at Computer Assisted Radiology and Surgery 19th International Congress and Exhibition. Berlin, Germany: June 22–25, 2005.
- Wood B J, Hvizda J, Yanof J H, Renisch S, Bitter I, Mcauliffe M. Intra-procedural real-time registration and fusion of ultrasound and CT for image-guided percutaneous liver procedures. Info Rad Exibit presented at Radiological Society of North America Annual Meeting. Chicago, IL: November 30–December 5, 2003.
- Viswanathan A, Wood B, Glossop N, Banovac F, Cleary K, Kruecker J. Multimodality navigation for radiofrequency ablation (RFA) with tracked needles. Paper presented at Scientific Assembly and Annual Meeting of The Radiological Society of North America. Chicago, IL: November 29, 2004.
- Wood B J, Zhang H, Durrani A, et al. Navigation with electromagnetic tracking for interventional radiology procedures: a feasibility study. J Vasc Interv Radiol. 2005;16:493–505. doi: 10.1097/01.RVI.0000148827.62296.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreedy E, Cheng R, Hemler P, et al. Radio frequency ablation registration, segmentation, and fusion tool. Paper presented at The 18th IEEE International Symposium on Computer Based Medical Systems. Trinity College, Dublin, Ireland: June 23–24, 2005.
- Wood B J, Sofer A, Uecker D, Klahr P, Bauer C, Yanof J. Integration of pre-procedural treatment planning and intra-procedural temperature visualization for CT-guided, robot-assisted RF ablation (RFA) of liver tumors. Education Exhibit Presented at Radiological Society of North America Annual Meeting. Chicago, IL: November 28–December 3, 2004.
- Lee F T, Jr, Haemmerich D, Wright A S, Mahvi D M, Sampson L A, Webster J G. Multiple probe radiofrequency ablation: pilot study in an animal model. J Vasc Interv Radiol. 2003;14:1437–1442. doi: 10.1097/01.rvi.0000096771.74047.c8. [DOI] [PubMed] [Google Scholar]
- Haemmerich D, Lee F T., Jr Multiple applicator approaches for radiofrequency and microwave ablation. Int J Hyperthermia. 2005;21:93–106. doi: 10.1080/02656730412331286894. [DOI] [PubMed] [Google Scholar]
- Vargas H I, Dooley W C, Gardner R A, et al. Focused microwave phased array thermometry for ablation of early-stage breast cancer: results of thermal dose escalation. Ann Surg Oncol. 2004;11:139–146. doi: 10.1245/aso.2004.03.059. [DOI] [PubMed] [Google Scholar]
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–337. doi: 10.1148/radiol.2232010775. [DOI] [PubMed] [Google Scholar]
- Xu H X, Xie X Y, Lu M D, et al. Ultrasound-guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol. 2004;59:53–61. doi: 10.1016/j.crad.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Wright A S, Lee F T, Jr, Mahvi D M. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol. 2003;10:275–283. doi: 10.1245/aso.2003.03.045. [DOI] [PubMed] [Google Scholar]
- Wu F, Wang Z B, Chen W Z, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11:149–154. doi: 10.1016/j.ultsonch.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Wu F, Wang Z B, Chen W Z, et al. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology. 2005;235:659–667. doi: 10.1148/radiol.2352030916. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Azuma T, Kawabata K I, et al. Effect of split-focus approach on producing larger coagulation in swine liver. Ultrasound Med Biol. 2003;29:591–599. doi: 10.1016/s0301-5629(02)00792-5. [DOI] [PubMed] [Google Scholar]
- Palussiere J, Salomir R, Le Bail B, et al. Feasibility of MR-guided focused ultrasound with real-time temperature mapping and continuous sonication for ablation of VX2 carcinoma in rabbit thigh. Magn Reson Med. 2003;49:89–98. doi: 10.1002/mrm.10328. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Maruyama T, Takegami K, et al. Use of microbubble agent to increase the effects of high intensity focused ultrasound on liver tissue. Eur Radiol. 2005;15:1415–1420. doi: 10.1007/s00330-005-2663-7. [DOI] [PubMed] [Google Scholar]
- Bailey M R, Couret L N, Sapozhnikov O A, et al. Use of overpressure to assess the role of bubbles in focused ultrasound lesion shape in vitro. Ultrasound Med Biol. 2001;27:695–708. doi: 10.1016/s0301-5629(01)00342-8. [DOI] [PubMed] [Google Scholar]
- Wu F, Wang Z B, Jin C B, et al. Circulating tumor cells in patients with solid malignancy treated by high-intensity focused ultrasound. Ultrasound Med Biol. 2004;30:511–517. doi: 10.1016/j.ultrasmedbio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Wu F, Wang Z B, Lu P, et al. Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med Biol. 2004;30:1217–1222. doi: 10.1016/j.ultrasmedbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Clement G T, White P J, King R L, McDannold N, Hynynen K. A magnetic resonance imaging-compatible, large-scale array for trans-skull ultrasound surgery and therapy. J Ultrasound Med. 2005;24:1117–1125. doi: 10.7863/jum.2005.24.8.1117. [DOI] [PubMed] [Google Scholar]
- Dittmar K M, Xie J, Hunter F, et al. Pulsed high-intensity focused ultrasound enhances systemic administration of naked DNA in squamous cell carcinoma model: initial experience. Radiology. 2005;235:541–546. doi: 10.1148/radiol.2352040254. [DOI] [PubMed] [Google Scholar]
- Yuh E L, Shulman S G, Mehta S A, et al. Delivery of systemic chemotherapeutic agent to tumors by using focused ultrasound: study in a murine model. Radiology. 2005;234:431–437. doi: 10.1148/radiol.2342030889. [DOI] [PubMed] [Google Scholar]
- Vogl T J, Straub R, Woitaschek D, Mack M R. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: experience with complications in 899 patients (2,520 lesions) Radiology. 2002;225:367–377. doi: 10.1148/radiol.2252011171. [DOI] [PubMed] [Google Scholar]
- Dick E A, Joarder R, De Jode M, et al. MR-guided laser thermal ablation of primary and secondary liver tumors. Clin Radiol. 2003;58:112–120. doi: 10.1053/crad.2002.1129. [DOI] [PubMed] [Google Scholar]
- Pingpank J F, Libutti S K, Chang R, et al. Phase I study of hepatic arterial melphalan infusion and hepatic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J Clin Oncol. 2005;23:3465–3474. doi: 10.1200/JCO.2005.00.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind J F, Khwaja A, Liapi E, Torbenson M. New intraarterial drug delivery system: pharmacokinetics and tumor response in an animal model of liver cancer. Abstract presented at Annual Scientific Meeting of the Society of Interventional Radiology. New Orleans, LA: April 2, 2005.
- den Brok M H, Sutmuller R P, der Voort R van, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–4029. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- Hansler J, Neureiter D, Wasserburger M, et al. Percutaneous US-guided radiofrequency ablation with perfused needle applicators: improved survival with the VX2 tumor model in rabbits. Radiology. 2004;230:169–174. doi: 10.1148/radiol.2301021136. [DOI] [PubMed] [Google Scholar]
- Wissniowski T T, Hansler J, Neureiter D, et al. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003;63:6496–6500. [PubMed] [Google Scholar]