ABSTRACT
Yttrium-90 (90Y) radioembolization is a catheter-based therapy that delivers internal radiation to hepatic tumors in the form of microspheres. 90Y can be delivered to the hepatic tumor as either a constituent of a glass microsphere, TheraSphere®, or as a biocompatible resin-based microsphere, SIR-Spheres®. Once embedded within the tumor microcirculation, these microspheres emit β-radiation at therapeutic levels. While the technical aspects of radioembolization are quite complex, the collective clinical experience presented in the literature supports the use of 90Y radioembolization for unresectable hepatic malignancies.
Keywords: TheraSphere®, SIR-Sphere®, radioembolization, yttrium-90, hepatocellular carcinoma, metastatic disease to the liver, liver cancer, liver tumor
Yttrium-90 (90Y) radioembolization is a catheter-based therapy that delivers internal radiation to tumors. 90Y microspheres (TheraSphere®, MDS Nordion, Ottawa, Canada and SIR-Spheres®, SIRTeX Medical, Lake Forest, IL) are administered via percutaneously placed catheters to the hepatic arterial system to treat patients with hepatocellular carcinoma (HCC) and metastatic colon cancer to the liver. Unlike other current therapies for the treatment of unresectable liver tumors, such as hepatic arterial infusion of chemotherapy, transarterial chemoembolization (TACE), and radiofrequency ablation (RFA), 90Y radioembolization is much less often associated with toxicities such as abdominal pain, fever, nausea, and vomiting.1 In fact, there is a significant body of evidence supporting the safety and effectiveness of 90Y radioembolization.
The rationale for intra-arterial delivery of 90Y microspheres for hepatic tumors comes from the anatomic and physiological aspects of these tumors that can be exploited for the delivery of a therapeutic agent. Hepatic tumors derive at least 90% of their blood supply from the hepatic artery, and liver parenchyma obtains 70 to 80% of its blood supply from the portal vein.2,3,4,5,6,7,8,9 This differential pattern of vascular perfusion provides an intrinsic advantage for hepatic arterial regional therapies delivered selectively to liver tumors while minimizing potential compromise to normal liver function. This is further bolstered by the hypervascular nature of many of these tumors. Once delivered to the tumor microcirculation, 90Y is a pure β-emitter with a mean tissue penetration of 2.5 mm. This means that the majority of the radiation from the selectively delivered yttrium affects the tumorous tissue while sparing normal liver parenchyma. Because of this, 90Y internal radiation therapy can provide tumor doses as high as 50 to 150 Gy,10,11,12,13 in contrast to traditional whole liver external beam radiation where the radiation dose has been limited to 30 Gy to prevent adjacent organ injury. The increased radiation dose afforded by this delivery method is significant in light of literature suggesting that median liver radiation doses greater than 95 Gy yield better tumor response to therapy.14
Given the above-described principles, the administration of 90Y represents a true radioembolization, combining the benefits of internal radiation therapy, as well as the embolic effect of the 90Y microspheres. The “radio” portion of radioembolization involves complex dosimetry planning, regions of interest, and isodose curves for target and nontarget tissue. The “embolization” portion involves the injection of permanent particles (the microspheres themselves) as the carrier of the radiation, resulting in occlusion of the microvasculature to the tumors. This portion of radioembolization requires the use of fluoroscopic guidance and immediate live feedback to the authorized user. The end point of radioembolization is the sufficient delivery of radiation and embolic 90Y particles. This end point is usually determined through the use of completion angiography.
90Y can be delivered to the hepatic tumor as either a constituent of a glass microsphere, TheraSphere®, or as a biocompatible resin-based microsphere, SIR-Spheres®. TheraSphere® was approved by the U.S. Food and Drug Administration for unresectable HCC in December 1999 under a Humanitarian Device Exemption; SIR-Spheres® was approved in March 2002 for colorectal cancer metastatic to the liver in conjunction with infusion of intrahepatic continuous infusion floxuridine (FUDR).
DOSIMETRY
TheraSphere® consists of insoluble glass microspheres where 90Y is an integral constituent of the glass. The mean sphere diameter ranges from 20 to 30 μm. Each milligram contains between 22,000 and 73,000 microspheres. TheraSphere® is supplied in 0.05 mL of sterile, pyrogen-free water contained in a 0.3 mL vee-bottom vial secured within a 12-mm clear acrylic vial shield. TheraSphere® is available in six activity sizes: 3 Giga-Becquerel (GBq) (GBq; 81 mCi), 5 GBq (135 mCi), 7 GBq (189 mCi), 10 GBq (270 mCi), 15 GBq (405 mCi), and 20 GBq (540 mCi).15 The corresponding number of microspheres per vial is 1.2, 2, 2.8, 4, 6, and 8 million, respectively. The activity per microsphere is ~2500 Bq.16 Assuming TheraSphere® 90Y microspheres distribute in a uniform manner throughout the liver and 90Y undergoes complete decay in situ, radioactivity required to deliver the desired dose to the liver can be calculated using the following formula:
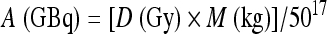 |
where A is activity delivered to the target tissue, D is the absorbed delivered dose to the target liver mass, and M is the target liver mass. Liver volume (mL) is estimated with computed tomography (CT) and then converted to mass using a conversion factor of 1.03 kg/mL.
SIR-Spheres® consist of biocompatible resin-based microspheres containing 90Y with a size between 20 and 40 μm in diameter. SIR-Spheres® is a permanent implant and is provided in a vial with water for injection. Each vial contains 3 GBq of 90Y (at the time of calibration) in 5 mL of sterile water for injection. Each vial contains 40 to 80 million microspheres.18 The corresponding activity per microsphere for SIR-Spheres® is much lower than that of TheraSphere® (50 Bq versus 2500 Bq respectively).16 Just as with TheraSphere®, assuming SIR-Spheres® 90Y microspheres distribute in a uniform manner throughout the liver and undergo complete decay in situ, radioactivity delivered to the liver can be calculated using one of two available methods. The first method incorporates body surface area and estimate of tumor burden as follows:
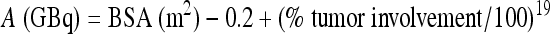 |
where BSA is body surface area.
The second method is based on a broad estimate of tumor burden as described in Table 1.18
Table 1.
SIR-Spheres© Dosimetry
| Percent Involvement by the Tumor in the Liver | Recommended SIR-Spheres© Dose (GBq) |
|---|---|
| >50% | 3.0 |
| 25–50% | 2.5 |
| <25% | 2.0 |
Because of the larger number of microspheres and lower activity of SIR-Spheres® compared with TheraSphere®, the delivery of SIR-Spheres® is distinctly different than that for TheraSphere®. Given the greater number of SIR-Spheres® required to deliver the intended dose, it is not uncommon for the entire vascular bed to become saturated with microspheres and an embolic state to be reached. For this reason, fluoroscopic guidance is critical during the infusion. The fluoroscopically guided injection of either 90Y agent represents a true embolization procedure, involving the injection of permanent particles, requiring the monitoring of vascular flow and any alterations in flow characteristics during/after the infusion, as well as the termination of the procedure once a completely embolic state has been reached (radioembolization).
CLINICAL EVALUATION
Regardless of which radioembolization vehicle is chosen, the selection process for patients undergoing radioembolization and their subsequent pretreatment evaluation is similar. Patients' eligibility for repeat radioembolization should be evaluated following every treatment. The components of the selection process are as follows:
History, physical examination, assessment of performance status
Clinical laboratory tests (complete blood count with differential, blood urea nitrogen, serum creatinine, serum electrolytes, liver function, albumin, lactate dehydrogenase, prothrombin time)
Chest X-ray, tumor marker assay (carcinoembryonic antigen [CEA], α-fetoprotein [AFP])
CT/magnetic resonance imaging (MRI) scan of the abdomen and pelvis with assessment of portal vein patency
Arteriography/macroaggregated albumin (MAA) lung shunting study
Patients with hepatic malignancies often have complex medical histories. Many of these patients have undergone one or several rounds of systemic chemotherapy, surgical resection, and/or RFA. Furthermore, they may have had some form of embolization procedure, either bland or in combination with chemotherapy. Relevant chemotherapy history having an impact on the safety and efficacy of radioembolization might include surgically placed intrahepatic chemotherapy pumps (causing chemical vasculitis), the use of radiosensitizers (such as capecitibine or irinotecan), as well as treatment with newer antiangiogenetic chemotherapeutic agents, such as bevacizumab.20 Given the overall lack of controlled phase III combinatorial studies of systemic chemotherapy plus 90Y, a conservative approach should be favored and systemic therapies should be discontinued 2 weeks prior to radioembolization. Chemotherapy may be restarted 2 weeks following radioembolization. Similar to hepatic artery chemoembolization, patients with bilobar disease should be treated in a lobar fashion at staged time intervals, usually 30 to 60 days following the first treatment.
For all patients, one of the most important factors in determining eligibility for radioembolization is Eastern Cooperative Oncology Group (ECOG) performance status. Patients presenting with clearly compromised functional status (ECOG 2 to 4; Table 2) are at high risk for rapid onset of liver failure and associated morbidity with treatment. Notwithstanding this precaution, each patient deserves individual consideration given the favorable toxicity profile of radioembolization; some patients with limited ECOG performance may still benefit from therapy.
Table 2.
ECOG Performance Status and Karnofsky Score
| ECOG Scale | Characteristics | Equivalent Karnofsky Score (%) |
|---|---|---|
| 0 | Asymptomatic and fully active | 100 |
| 1 | Symptomatic; fully ambulatory; restricted in physically strenuous activity | 80–90 |
| 2 | Symptomatic; ambulatory; capable of self-care; more than 50% of waking hours are spent out of bed | 60–70 |
| 3 | Symptomatic; limited self-care; spends more than 50% of time in bed | 40–50 |
| 4 | Completely disabled; no self-care; bedridden | 20–30 |
Patients with liver metastases present with relatively consistent findings on MRI or CT. Triple-phase CT is highly sensitive in detecting hepatic malignancies. Because the majority of liver tumors are angiographically hypervascular, scanning in the early phases results in the maximum likelihood of detection. Later phase imaging is necessary to detect other less vascular lesions and the degree of multifocality, as well as to identify portal vein patency. MRI is also a sensitive modality to identify and characterize lesions, given specific attention to diffusion-weighted imaging sequences. If a mass is identified, pathological confirmation of malignancy metastatic to the liver may be necessary. The status of overall liver function must be assessed when treating liver metastases with radioembolization. In the absence of biliary obstruction, drug toxicity (e.g., capecitibine) or metabolic abnormality (e.g., Gilbert's syndrome), it is extremely unusual for patients with metastatic disease to the liver to exhibit elevated liver functions. In particular, total bilirubin is usually normal in this patient population. In cases where total bilirubin is elevated and all of the above-mentioned factors have been excluded, it is possible that tumor infiltration within the hepatic parenchyma is the causative agent, likely implying a grim prognosis for the patient. The decision to treat such patients should be based on the thorough assessment of the possibility of extending survival or palliating pain.
Evaluation of patients with unresectable HCC is significantly different than those with metastatic liver disease. Ideal candidates for 90Y do not have an infiltrative type HCC or bulk disease (≤ 70% tumor replacement of liver), tumor replacement of liver of at least 50% with an albumin level less than 3.0 g/dL, previous intra-arterial liver-directed treatment, aspartate or alanine aminotransferase levels greater than five times the upper limit of normal, total bilirubin level ≤ 2 mg/dL, or previous external-beam liver radiation therapy.1 Given the risk of bleeding and tract seeding, pathological confirmation of HCC is not always necessary in those patients with classic history, imaging findings, and a serum AFP level 400 ng/mL.21
During the evaluation of the patient for radioembolization, mesenteric angiography and a 99mTc-MAA lung shunting scan must be performed.17,20,22 The angiographic evaluation required has recently been described by Liu et al.20 An abdominal aortogram is performed to facilitate proper visceral catheter selection as well to assess aortic tortuosity and mural atherosclerotic disease. The superior mesenteric artery is studied to assess any variant vessels to the liver (accessory or replaced right hepatic), as well as for visualization and identification of a patent portal vein. The celiac artery is injected to study the hepatic branch anatomy. Subsequently, selective left hepatic (flow to segments 2, 3, 4A, and 4B), right hepatic (flow to segments 1 [caudate lobe may have other blood supply], 5, 6, 7, and 8), and gastroduodenal (flow to the pancreas, stomach, small bowel, and omentum) arteriograms are performed. To visualize small vessels as well as vessels that may demonstrate reversal of flow (e.g., result of flow shunt or sumping secondary to hypervascular tumor), dedicated microcatheter injection with relatively high rates (2 to 3 mL/s for 8 to 12 mL) should be done. Without adequate contrast bolus, many ancillary vessels (which have profound effect on hemodynamics and directed therapy) may go unnoticed. Although it may be argued that high injection rates may represent supraphysiological flow dynamics, the potential changes induced as a result of regional therapy with radioembolization (spasm, ischemia, stasis, and vessel injury) may result in altered physiologic states and thus reflux into these vessels. Ultimately, this is important because unrecognized collateral vessels with consequent infusion of radioactive microspheres are certain to result in clinical toxicities if proper angiographic techniques are not adopted (see TheraSphere®, SIR-Spheres® package inserts). These might include gastrointestinal ulceration, pancreatitis, and skin irritation as well as other nontarget radiation. For this reason, aggressive prophylactic embolization of vessels prior to therapy is recommended such that all hepaticoenteric arterial communications are completely eliminated.23,24,25,26,27,28 These vessels include the falciform, accessory or left phrenic, right or accessory gastric arteries (from the left hepatic artery), supraduodenal, retroduodenal, and accessory right hepatic artery feeding segment 6 (from gastroduodenal artery). At times, it may be necessary to embolize the cystic artery if significant flow (and hence microspheres) are noted. At our institution, where over 600 radioembolizations have been performed, we have found our gastrointestinal toxicity rate to be well below 1%. This is due to our standard practice of: (1) prophylactic embolization of gastroduodenal artery/right gastric and other variant vessels, (2) use of nonembolic TheraSphere® in a lobar and segmental fashion, and (3) use of SIR-Spheres® in a lobar, segmental, and dose-fractionated method (several small doses rather than one larger dose) without reaching a completely embolic state. Authorized users considering whole liver infusion should be using a “bilobar lobar” approach. That is, although the entire liver is treated in one session, infusion is performed with the catheter in one lobar artery, followed by the other. Proper or common hepatic artery infusions are not recommended.
At the conclusion of the initial angiographic evaluation of a patient for 90Y radioembolization, 4 to 5 mCi of 99mTc-MAA is injected in the vessel of interest, followed by imaging for lung shunt fraction in nuclear medicine. This is performed because liver tumors (particularly HCC) often have arteriovenous connections that shunt blood from the liver to the lungs. Given that the likelihood of shunting is low with metastatic disease, we favor whole liver (e.g., proper hepatic) MAA injection to assess the entire liver at one time. Lung shunt fraction is defined as (total lung counts)/(total lung counts + total abdomen counts). Previous preclinical and clinical studies with 90Y microspheres demonstrated that up to 30 Gy to the lungs can be tolerated with a single injection, and up to 50 Gy for multiple injections.29 Therefore, caution is recommended when treating patients who might receive more than 30 Gy cumulative dose to the lungs, as radiation pneumonitis becomes a distinct possibility.
Once a patient has been deemed a candidate for 90Y radioembolization through rigorous review of their medical history, functional status, pertinent imaging and laboratory values, angiographic imaging with aggressive prophylactic embolization, as well as lung shunt analysis, they return to the interventional radiology clinic on a separate date to have their therapy. 90Y radioembolization is performed on an outpatient basis. The selected catheter is advanced into the treatment vessel of choice as determined by pretreatment angiography and either the TheraSphere® or SIR-Spheres® administration device is utilized for microsphere infusion. The dose delivered is calculated as described above.
DISCUSSION
The ideal treatment for hepatic malignancy, either primary or metastatic, is surgical. For HCC, surgical resection or transplantation is favored. For metastatic colorectal cancer to the liver, resection of the primary as well as metastatectomy provides the potential for cure. However, many patients presenting for treatment with these disorders are not operative candidates. Radioembolization has become an option for many of these patients, as described above. The following is a representation from the recent medical literature demonstrating the safety and efficacy of both TheraSphere® and SIR-Spheres® in the treatment of unresectable HCC and metastatic hepatic malignancy.
Hepatocellular Carcinoma
HCC represents one of the most common forms of cancer with more than 1 million new cases estimated annually worldwide. In the United States, the incidence of HCC has steadily increased over the past two decades, with an estimated 18,900 new cases occurring in 2004.30 Traditionally, these patients have had few treatment options for a variety of reasons.31 The therapeutic benefit and safety of TheraSphere®11,13,32,33,34,35 and SIR-Spheres®36,37,38,39 administration in this patient population is well supported in the literature.
In 1998, Lau et al reported on 71 patients with nonresectable HCC treated with SIR-Spheres®. These patients did not have extrahepatic disease. The patients were initially treated with an activity of 0.8 to 5.0 GBq (21.6 to 135.1 mCi; median 3.0 GBq or 81.1 mCi) of 90Y microspheres. There was a 50% reduction in tumor volume in 19 (26.7%) patients after the first treatment. However, the overall objective response in terms of changes in AFP levels was 89% (partial response 67%, complete response 22%) among the 46 patients with elevated pretreatment levels. Treatment was repeated in 15 patients, and the maximum number of treatments any patient received was five. This therapy enabled the residual tumors to be resected in four patients. Two of these resections demonstrated complete histological remission. Occasional viable tumor cells were found in the necrotic centers of the tumors resected from the other two patients. The median survival of the 71 patients was 9.4 months (range 1.8 to 46.4 months). Treatment was well tolerated and there was no bone marrow toxicity or clinical evidence of radiation hepatitis or pneumonitis. The authors concluded that using 90Y microspheres is effective for selected cases of nonresectable HCC and is well tolerated. More importantly, selective internal radiation treatment may convert nonresectable tumors to resectable ones.38
In 2000 Dancey et al reported results of 22 patients to determine response parameters, survival, and toxicity after intra-arterial injection of TheraSphere®. Of the patients who met entry criteria, 20 were evaluated for efficacy including nine patients who were Okuda stage I and II and 11 patients who were Okuda stage III. The median dose delivered was 104 Gy (range 45 to 145 Gy). There were 31 serious adverse events; the most common were liver enzyme elevation and gastrointestinal ulceration. Treatment efficacy was measured by tumor response, duration of response, time to progression, and overall survival. One complete tumor response and three partial responses were reported. The median time to progression was 44 weeks (95% confidence limit), and the median survival was 54 weeks (range 7 to 180 weeks). Multivariate analysis suggested that a total dose > 104 Gy, Okuda stage I, and tumor-to-liver uptake ratio > 2 were the three factors associated with prolonged survival.13
In 2001, Lau et al reported on 82 patients treated with SIR-Spheres®. They divided the patients into two groups: those who lived less than 1 year from date of treatment (51) and those who live for 1 year or longer from the date of first treatment (31). The authors concluded that a lower pretreatment level of AFP and a higher tumor-to-normal uptake ratio of 90Y microspheres favored longer survival. They also offered that repeated treatment of viable residual or recurrent tumors offered further palliation and prolongation of survival.39
Carr in 2004 reported on the safety and efficacy of TheraSphere® for inoperable HCC. Sixty-five patients with biopsy-proven HCC received a median radiation dose of 134 Gy. Toxicities included nine episodes of abdominal pain (which did not fit criteria for postembolization syndrome), two episodes of cholecystitis, and transient elevations in liver functions in 25 patients. A finding previously unreported was lymphopenia in 75% of patients; none was associated with adverse clinical events or opportunistic infections. Median survival was 649 and 302 days for Okuda I (65%) and Okuda II patients (35%), respectively, compared with 244 and 64 days, respectively, for historical controls.35
Geschwind et al in 2004 reported on 80 patients from a relatively large database of 121 patients who were treated with TheraSphere® using segmental, regional, and whole liver approach. Prior to therapy, patients were staged using the Child-Pugh, Okuda, or Cancer of the Liver Italian Program (CLIP) scoring systems. The pretreatment CLIP scores were found to be the best means of stratifying risk. Similar to the Carr data, survival was found to be 628 and 324 days for Okuda I (68%) and II (32%) patients, respectively. These data were instrumental for delineating the essential components to conduct a large randomized control trial comparing TheraSphere® to a standard TACE regimen using potential end points of survival, tumor response, AFP reduction, and quality of life estimates.34
A recent case report by Kulik et al described a patient with HCC who was downstaged to a transplant candidate via treatment with TheraSphere®. At pathology, the explant demonstrated complete necrosis. This patient was initially not a transplant candidate secondary to size criteria. The patient was treated selectively with 202 Gy to segment 3 with TheraSphere®. Follow-up MRI at 1 month revealed a 39% reduction in size, allowing the patient to be downstaged. The patient was transplanted 42 days following TheraSphere® treatment.32
In another recent study, Salem et al reported on 43 consecutive patients treated with TheraSphere® for unresectable HCC. Patients were stratified into three risk groups using method of treatment and risk stratification (group 0: segmental infusion, group 1: lobar infusion/low risk, group 2: lobar infusion/high risk), as well as based on Okuda and Child-Pugh scoring systems. Patients were treated by liver segment or lobe on one or more occasions based on tumor distribution, liver function, and vascular flow dynamics with a volume-weighted average of 138 Gy. Patients were followed for adverse experiences, objective tumor response, and survival. Based on follow-up data, 20 (47%) patients had an objective tumor response based on percent reduction in tumor size (World Health Organization [WHO]), and 34 (79%) patients had a tumor response when percent reduction and/or tumor necrosis were used as a composite measure of tumor response. When patient risk stratification was used, median survival was 46.5 (group 0), 16.9 (group 1), and 11.1 (group 2) months. Median survival in all non-high-risk patients (groups 0 and 1) was 20.8 months. There were no life-threatening adverse events.33
Liver Metastases
The liver is the most frequent site of metastases, primarily due to the spread of cancer cells through the portal circulation. Similar to HCC, surgical resection of metastatic hepatic disease is the treatment of choice. However, as in those with hepatoma, surgical resection is often not feasible, being possible in less than 20% of patients.40 The benefits of radioembolization with TheraSphere®41,42,43 and SIR-Spheres®19,36,37,44,45,46,47,48,49,50,51,52 in these patients have been reported in many studies.
In 2001, Gray et al44 published a phase III randomized clinical trial of 74 patients conducted to assess whether a single injection of 90Y SIR-Spheres® in combination with intrahepatic FUDR could increase the tumor response rate, time to disease progression in the liver, and survival when compared with FUDR alone. All patients had undergone complete surgical resection of a primary adenocarcinoma of the large bowel, and only those with nonresectable metastases limited to the liver and lymph nodes in the porta hepatis were included in the study. In addition, patients were required to have a WHO performance status of 0 to 2 and adequate hematological and hepatic function and not have evidence of cirrhosis or ascites. Both treatment arms received 12-day cycles of FUDR at 0.3 mg/kg of body weight per day that were repeated at 4-week intervals and continued for 18 cycles (or until evidence of tumor progression, extrahepatic metastases requiring a systemic chemotherapy change, unacceptable toxicity, port failure, or at the patient's request). The SIR-Spheres® treatment arm also received a predetermined quantity of 90Y that varied (2 GBq, 2.5 GBq, or 3 GBq) depending on the size of the tumor. 90Y microspheres were administered one time only, within 4 weeks of insertion of the hepatic artery access port. The mean 90Y dose administered was 2.156 ± 0.32 GBq. Six of 34 patients (18%) in the hepatic artery chemotherapy arm had at least a partial response, and 16 of 36 patients (44%) in the hepatic artery chemotherapy + selective internal radiation therapy arm had at least a partial response.
Also in 2001, Stubbs et al45 published a clinical trial of 50 patients with extensive colorectal liver metastases not suitable for either resection or cryotherapy. The study compared experience with 90Y SIR-Spheres® alone (n = 7) and in combination (n = 43) with fluorouracil (5-FU). For all patients, 90Y microspheres were administered as a single treatment within 10 days of hepatic artery port placement. The dose was titrated to the estimated extent of disease (<25% liver replacement: 2 GBq, 25 to 50% liver replacement: 2.5 GBq, and >50% liver replacement: 3 GBq). Forty-three of the 50 enrolled patients also received 5-FU given at the time of 90Y continuously over 4 days (1 g/d), every 4 weeks. Acute pain and/or nausea was experienced in 14 patients (28%) at the time of administration of 90Y and was managed with narcotics and antiemetics. Six patients (12%) developed an acute duodenal ulcer within 2 months after 90Y therapy and the initial cycle of 5-FU, which was due to misperfusion of the duodenum by either 90Y, 5-FU, or both. Antitumor effect was assessed by tumor marker (CEA) and CT response. Median CEA levels were reduced to 25% of baseline values at 1 month posttreatment with 90Y and remained <30% of baseline when followed for 6 months. Median survival for all liver metastases patients from the time of diagnosis was 14.5 months (range 1.9 to 91.4) and from the time of treatment was 9.8 months (range 1.0 to 30.3).
Subsequently, Stubbs et al46 published on 38 patients with extensive colorectal liver metastases who received SIR-Spheres®. Liver involvement was <25% in 19 patients, 25 to 50% in nine patients, and >50% in 10 patients. Patients received 90Y in the hepatic artery via an arterial port and subsequent 4-week cycles of hepatic artery chemotherapy with 5-FU. The treatments were well tolerated, and no treatment-related mortality was observed. Response to SIR-Spheres® therapy, as indicated by decreasing tumor markers and serial 3-month CT scans, were seen in over 90% of patients. Estimated survival at 6, 12, and 18 months was 70, 46, and 46%, respectively, and was principally determined by the development of extrahepatic metastases. The authors concluded that SIR-Spheres® was well tolerated in patients with extensive colorectal liver metastases and achieved encouraging liver tumor responses, which are well maintained by hepatic artery chemotherapy.
In 2002, Wong et al reported on eight patients with unresectable colorectal liver metastases treated with TheraSphere® (13 hepatic lobes treated). At 3 months posttreatment, positron-emission tomography (PET) assessment demonstrated metabolic response in 12 treated lobes, compared with CT/MRI, which showed an anatomic response in only two lobes. Serum CEA levels decreased, correlating with PET findings. They concluded that PET is an accurate indicator of treatment response.43
In 2004, Wong et al concluded that it is feasible to utilize F-18 fluoro-deoxyglucose (18FDG)-PET for quantifying metabolic response following TheraSphere® administration. They reported on 27 patients with metastatic colorectal cancer to the liver demonstrating tumor progression despite polychemotherapy. The average administered dose of radiation was 2.5 GBq. Patients were followed at 3 months with both PET scanning and CEA levels. Following treatment with TheraSphere®, 20 patients demonstrated improvement on PET scanning (both decreased standard uptake values and visual estimate grading) and seven patients demonstrated no response. Serum CEA levels showed a decreasing trend in all 23 patients who had an elevated CEA prior to TheraSphere®.42
In 2004, Van Hazel at al reported a randomized study of 21 patients (11 patients received combination treatment with SIR-Spheres® along with systemic 5-FU/leucovorin chemotherapy and 10 patients received the chemotherapy regimen alone). The mean administered radiation dose in those receiving SIR-Spheres® was 2.25 GBq. The authors concluded that the administration of SIR-Spheres® along with a standard chemotherapeutic regimen significantly increased treatment-related response (10 versus 0 patients demonstrated a partial response on follow-up CT), time to disease progression (18.6 versus 3.6 months), and survival (29.4 versus 12.8 months) when compared with chemotherapy alone. Although there were more toxicities associated with the combination therapy, there was no difference in quality of life over a 3-month period.19
In 2005, Popperl et al reported on 23 patients with unresectable hepatic malignancies (21 with metastatic disease and two with HCC) treated with SIR-Spheres®. The mean activity of treatment was 2.27 GBq. Three-month follow-up investigations were available in 13 of 23 patients. These results showed a marked decrease of FDG uptake, a drop of tumor markers, and unchanged or slightly decreasing lesion size (CT) in 10 of 13 patients (one of whom had HCC). Two patients showed stable findings, and another patient showed progressive disease. Long-term follow-up investigations were available in 2 of 23 patients, showing hepatic and extrahepatic progression 6 and 9 months after selective internal radiation therapy. Common minor side effects included abdominal pain and fever. Mild pancreatitis and gastric ulceration were each observed once.37
In April 2005, Lim et al reported on 46 patients with unresectable hepatic malignancies (32 with colorectal cancer, five with HCC, and nine with other disease) treated with SIR-Spheres®. These selected patients had an ECOG score of 2 or less, were expected to live at least 3 months, and did not have brain metastases at the time of treatment. Follow-up data was available for 43 of the patients. Of these, 12 patients (27%) demonstrated partial response to therapy (10 with metastatic colorectal cancer and one with hepatoma) and another 12 patients (27%) demonstrated stable disease. The median duration of response for all patients was 8.6 months. Although early toxicities were minimal, four patients developed severe gastric ulceration later.36
At the 2005 Society of Interventional Radiology annual meeting, Coldwell and Nutting reported on 34 women with unresectable breast cancer metastatic to the liver treated with SIR-Spheres®. Inclusion criteria included only those patients with an ECOG performance score of 0 or 1 with an expected survival of at least 3 months. The average dose of radiation administered was 1.75 GBq. Although 100% of patients demonstrated response to treatment with a reduction of the number and size of the hepatic lesions on PET scans, all patients also experienced mild to moderate postembolization syndrome.52
In August 2005, Murthy et al reported on 12 patients with advanced unresectable colorectal hepatic metastases treated with SIR-Spheres® (17 infusions). The average median prescribed dose was 39.6 mCi. The delivered dose in six (35%) infusions was less than the prescribed dose as a result of embolic arterial occlusion. Radiological response was stable in five of nine patients (56%) and carcinoembryonic antigen levels decreased in four of seven patients (57%). Median survival times from diagnosis and treatment were 24.6 and 4.5 months, respectively. In 7 of the 17 infusions (41%), the patient developed transient abdominal pain and nausea. Gastric ulceration was observed in one patient and was managed nonoperatively.51
In a recent article by Wong et al, 19 patients with unresectable, chemotherapy-refractive hepatic metastatic disease of various origins were treated with SIR-Spheres®. The median absorbed dose for the tumor was 76 Gy. Each patient was monitored at 3 months by using PET. By described PET criteria, 15 (79%) of the patients demonstrated response to therapy, and four demonstrated no response. They concluded that there is a significant reduction of hepatic metastatic load as evaluated by PET following radioembolization.50
In another recent article, Lewandowski et al reported on 27 patients with unresectable colorectal cancer treated with TheraSphere®. Included patients had an ECOG performance score less than 3. The targeted absorbed radiation dose was 135 to 150 Gy. They found that TheraSphere® provided stabilization of liver disease in those failing chemotherapy. Tumor response measured by 18FDG-PET imaging exceeded that of CT imaging for first (88% versus 35%) and second treated (73% versus 36%) lobes, respectively. Tumor replacement < 25% (compared with >25%) was associated with a statistically significant increase in median survival (339 days versus 162 days). Treatment-related toxicities included mild fatigue (48%), nausea (15%), and vague abdominal pain (19%). There was one case of radiation-induced gastritis from inadvertent deposition of microspheres to the gastrointestinal tract (4%).41
CONCLUSION
Although the technical aspects of radioembolization are quite complex and should not be undertaken lightly, the collective clinical experience presented in the literature supports the therapeutic benefits and safety of both TheraSphere® and SIR-Spheres® in the setting of unresectable hepatic malignancy. This statement is further strengthened by a recently published article. Rhee et al53 describe using CT angiography to delineate the volume and blood supply to a targeted hepatic segment. This information allows superselective radioembolization, significantly increasing the effective 90Y tumor radiation dose without clinically altering liver function. This technique represents a technical advancement and is applicable to all 90Y-based therapies.
Further clinical investigation of radioembolization is warranted and should be directed toward a more rigorous approach to investigating patient selection criteria, as well as optimal dosimetry to obtain the desired therapeutic effect. Future studies should be initiated to compare radioembolization to more traditional therapies (such as TACE, RFA, bland embolization) as well as to combine radioembolization with other therapeutic agents/radiosensitizers. This technology may also prove to be applicable to extrahepatic malignancies that are readily accessible angiographically, such as renal cell carcinomas, head and neck tumors (e.g., meningiomas), bone, soft tissue, and possibly even lung tumors.
REFERENCES
- Goin J E, Salem R, Carr B I, et al. Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: factors associated with liver toxicities. J Vasc Interv Radiol. 2005;16:205–213. doi: 10.1097/01.rvi.00001142592.89564.f9. [DOI] [PubMed] [Google Scholar]
- Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- Schenk W G, Jr, McDonald J C, McDonald K, Drapanas T. Direct measurement of hepatic blood flow in surgical patients. Ann Surg. 1962;156:463–471. doi: 10.1097/00000658-196209000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Lunderquist A, Hägerstrand I, Boijsen E. Postmortem examination of the blood supply and vascular pattern of small liver metastases in man. Surgery. 1984;96:517–526. [PubMed] [Google Scholar]
- Grindlay J H, Mann F C. Measurement of the blood flow of the liver. Am J Physiol. 1941;132:489–496. [Google Scholar]
- Tygstrup N, Winkler K, Mellemgaard K, Andreassen M. Determination of the hepatic arterial blood flow and oxygen supply in man by clamping the hepatic artery during surgery. J Clin Invest. 1962;41:447–454. doi: 10.1172/JCI104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almersjo O, Bengmark S, Engevik L, Hafstrom L O, Nilsson L A. Hepatic artery ligation as pretreatment for liver resection of metastatic cancer. Rev Surg. 1966;23:377–380. [PubMed] [Google Scholar]
- Bierman H R, Byron R L, Jr, Kelley K H, Grady A. Studies on the blood supply of tumors in man. III: vascular patterns of the liver by hepatic arteriography in vivo. J Natl Cancer Inst. 1951;12:107–131. [PubMed] [Google Scholar]
- Healey J E., Jr Vascular patterns in human metastatic liver tumors. Surg Gynecol Obstet. 1965;120:1187–1193. [PubMed] [Google Scholar]
- Lewin K, Millis R R. Human radiation hepatitis: a morphologic study with emphasis on the late changes. Arch Pathol. 1973;96:21–26. [PubMed] [Google Scholar]
- Andrews J C, Walker S C, Ackermann R J, Cotton L A, Ensminger W D, Shapiro B. Hepatic radioembolization with yttrium-90 containing glass microspheres: preliminary results and clinical follow-up. J Nucl Med. 1994;35:1637–1644. [PubMed] [Google Scholar]
- Sarfaraz M, Kennedy A S, Cao Z J, et al. Physical aspects of yttrium-90 microsphere therapy for nonresectable hepatic tumors. Med Phys. 2003;30:199–203. doi: 10.1118/1.1538235. [DOI] [PubMed] [Google Scholar]
- Dancey J E, Shepherd F A, Paul K, et al. Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. J Nucl Med. 2000;41:1673–1681. [PubMed] [Google Scholar]
- Goin J E, Dancey J E, Hermamm G A, et al. Treatment of unresectable metastatic colorectal carcinoma to the liver with intrahepatic Y-90 microspheres: a dose-ranging study. World J Nucl Med. 2003;2:216–225. [Google Scholar]
- Yttrium-90 microspheres (TheraSphere®) [package insert] Kanata, Canada: MDS Nordion; 2004.
- Kennedy A S, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60:1552–1563. doi: 10.1016/j.ijrobp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Salem R, Thurston K G, Carr B I, Goin J E, Geschwind J F. Yttrium-90 microspheres: radiation therapy for unresectable liver cancer. J Vasc Interv Radiol. 2002;13:S223–S229. doi: 10.1016/s1051-0443(07)61790-4. [DOI] [PubMed] [Google Scholar]
- Yttrium-90 microspheres (SIR-Spheres®) [package insert] Lane Cove, Australia: Sirtex Medical; 2004.
- Hazel G Van, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88:78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- Liu D M, Salem R, Bui J T, et al. Angiographic considerations in patients undergoing liver-directed therapy. J Vasc Interv Radiol. 2005;16:911–935. doi: 10.1097/01.RVI.0000164324.79242.B2. [DOI] [PubMed] [Google Scholar]
- Soresi M, Magliarisi C, Campagna P, et al. Usefulness of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma. Anticancer Res. 2003;23:1747–1753. [PubMed] [Google Scholar]
- Salem R, Lewandowski R, Roberts C, et al. Use of Yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma in patients with portal vein thrombosis. J Vasc Interv Radiol. 2004;15:335–345. doi: 10.1097/01.rvi.0000123319.20705.92. [DOI] [PubMed] [Google Scholar]
- Carr B I. Hepatic artery chemoembolization for advanced stage HCC: experience of 650 patients. Hepatogastroenterology. 2002;49:79–86. [PubMed] [Google Scholar]
- Chun H J, Byun J Y, Yoo S S, Choi B G. Added benefit of thoracic aortography after transarterial embolization in patients with hemoptysis. AJR Am J Roentgenol. 2003;180:1577–1581. doi: 10.2214/ajr.180.6.1801577. [DOI] [PubMed] [Google Scholar]
- Chung J W, Park J H, Han J K, et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996;198:33–40. doi: 10.1148/radiology.198.1.8539401. [DOI] [PubMed] [Google Scholar]
- Inaba Y, Arai Y, Matsueda K, Takeuchi Y, Aramaki T. Right gastric artery embolization to prevent acute gastric mucosal lesions in patients undergoing repeat hepatic arterial infusion chemotherapy. J Vasc Interv Radiol. 2001;12:957–963. doi: 10.1016/s1051-0443(07)61576-0. [DOI] [PubMed] [Google Scholar]
- Ueno K, Miyazono N, Inoue H, Miyake S, Nishida H, Nakajo M. Embolization of the hepatic falciform artery to prevent supraumbilical skin rash during transcatheter arterial chemoembolization for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1995;18:183–185. doi: 10.1007/BF00204147. [DOI] [PubMed] [Google Scholar]
- Arora R, Soulen M C, Haskal Z J. Cutaneous complications of hepatic chemoembolization via extrahepatic collaterals. J Vasc Interv Radiol. 1999;10:1351–1356. doi: 10.1016/s1051-0443(99)70242-3. [DOI] [PubMed] [Google Scholar]
- Leung T W, Lau W Y, Ho S K, et al. Radiation pneumonitis after selective internal radiation treatment with intra-arterial 90-yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys. 1995;33:919–924. doi: 10.1016/0360-3016(95)00039-3. [DOI] [PubMed] [Google Scholar]
- American Cancer Society Cancer Facts and Figures 2004. Atlanta: A.P.; 2004.
- Salem R, Hunter R. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma: a review. Int J Radiat Oncol Biol Phys. 2005 doi: 10.1016/j.ijrobp.2006.02.061. In press. [DOI] [PubMed] [Google Scholar]
- Kulik L M, Mulcahy M F, Hunter R D, Nemcek A A, Jr,Abecassis M M, Salem R. Use of yttrium-90 microspheres (TheraSphere) in a patient with unresectable hepatocellular carcinoma leading to liver transplantation: a case report. Liver Transpl. 2005;11:1127–1131. doi: 10.1002/lt.20514. [DOI] [PubMed] [Google Scholar]
- Salem R, Lewandowski R J, Atassi B, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol. 2005;16:1627–1639. doi: 10.1097/01.RVI.0000184594.01661.81. [DOI] [PubMed] [Google Scholar]
- Geschwind J F, Salem R, Carr B I, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127(suppl 1):S194–S205. doi: 10.1053/j.gastro.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Carr B I. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl. 2004;10(suppl 1):S107–S110. doi: 10.1002/lt.20036. [DOI] [PubMed] [Google Scholar]
- Lim L, Gibbs P, Yip D, et al. Prospective study of treatment with selective internal radiation therapy spheres in patients with unresectable primary or secondary hepatic malignancies. Intern Med J. 2005;35:222–227. doi: 10.1111/j.1445-5994.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- Popperl G, Helmberger T, Munzing W, Schmid R, Jacobs T F, Tatsch K. Selective internal radiation therapy with SIR-Spheres in patients with nonresectable liver tumors. Cancer Biother Radiopharm. 2005;20:200–208. doi: 10.1089/cbr.2005.20.200. [DOI] [PubMed] [Google Scholar]
- Lau W Y, Ho S, Leung T W, et al. Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90Y yttrium microspheres. Int J Radiat Oncol Biol Phys. 1998;40:583–592. doi: 10.1016/s0360-3016(97)00818-3. [DOI] [PubMed] [Google Scholar]
- Lau W Y, Ho S, Leung W T, Chan M, Lee W Y, Johnson P J. What determines survival duration in hepatocellular carcinoma treated with intraarterial Yttrium-90 microspheres? Hepatogastroenterology. 2001;48:338–340. [PubMed] [Google Scholar]
- Sasson A R, Sigurdson E R. Surgical treatment of liver metastases. Semin Oncol. 2002;29:107–118. doi: 10.1053/sonc.2002.31676. [DOI] [PubMed] [Google Scholar]
- Lewandowski R J, Thurston K G, Goin J E, et al. Yttrium-90 microsphere (TheraSphere) treatment for unresectable metastatic colorectal cancer to the liver: treatment response at targeted doses of 135–150 Gy as measured by 18FDG-PET and CT imaging. J Vasc Interv Radiol. 2005;16:1641–1651. doi: 10.1097/01.RVI.0000179815.44868.66. [DOI] [PubMed] [Google Scholar]
- Wong C Y, Salem R, Qing F, et al. Metabolic response after intraarterial 90Y-glass microsphere treatment for colorectal liver metastases: comparison of quantitative and visual analyses by 18F-FDG PET. J Nucl Med. 2004;45:1892–1897. [PubMed] [Google Scholar]
- Wong C Y, Salem R, Raman S, Gates V L, Dworkin H J. Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG PET: a comparison with CT or MRI. Eur J Nucl Med Mol Imaging. 2002;29:815–820. doi: 10.1007/s00259-002-0787-4. [DOI] [PubMed] [Google Scholar]
- Gray B, Hazel G Van, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12:1711–1720. doi: 10.1023/a:1013569329846. [DOI] [PubMed] [Google Scholar]
- Stubbs R S, Cannan R J, Mitchell A W. Selective internal radiation therapy with 90yttrium microspheres for extensive colorectal liver metastases. J Gastrointest Surg. 2001;5:294–302. doi: 10.1016/s1091-255x(01)80051-2. [DOI] [PubMed] [Google Scholar]
- Stubbs R S, Cannan R J, Mitchell A W. Selective internal radiation therapy (SIRT) with 90yttrium microspheres for extensive colorectal liver metastases. Hepatogastroenterology. 2001;48:333–337. [PubMed] [Google Scholar]
- Rubin D, Nutting C, Jones B. Metastatic breast cancer in a 54-year-old woman: integrative treatment with yttrium-90 radioembolization. Integr Cancer Ther. 2004;3:262–267. doi: 10.1177/1534735404268444. [DOI] [PubMed] [Google Scholar]
- Boan J F, Martinez A, Sangro B, Rodriguez J, Penuelas I, Richter J A. European Journal of Nuclear Medicine and Molecular Imaging. 2004;31(suppl 2):P954. Abstract. [Google Scholar]
- Coldwell D, Nutting C, Kennedy A S. Initial clinical results in the treatment of unresectable hepatic tumors with resin-based yttrium-90 radioembolization. In: Cardiovascular and Interventional Radiology Society of Europe (CIRSE), 2004.
- Wong C Y, Qing F, Savin M, et al. Reduction of metastatic load to liver after intraarterial hepatic yttrium-90 radioembolization as evaluated by [18F]fluorodeoxyglucose positron emission tomographic imaging. J Vasc Interv Radiol. 2005;16:1101–1106. doi: 10.1097/01.RVI.0000168104.32849.07. [DOI] [PubMed] [Google Scholar]
- Murthy R, Xiong H, Nunez R, et al. Yttrium 90 resin microspheres for the treatment of unresectable colorectal hepatic metastases after failure of multiple chemotherapy regimens: preliminary results. J Vasc Interv Radiol. 2005;16:937–945. doi: 10.1097/01.RVI.0000161142.12822.66. [DOI] [PubMed] [Google Scholar]
- Coldwell D, Nutting C. Treatment of hepatic metastases from breast cancer with yttrium-90 SIR-Spheres radioembolization. In: Society of Interventional Radiology Annual Meeting. New Orleans, LA: 2005.
- Rhee T K, Omary R A, Gates V, et al. The effect of catheter-directed CT angiography on yttrium-90 radioembolization treatment of hepatocellular carcinoma. J Vasc Interv Radiol. 2005;16:1085–1091. doi: 10.1097/01.RVI.0000177063.92678.21. [DOI] [PubMed] [Google Scholar]


