ABSTRACT
The liver is a common site for primary malignancy and hematogenous metastasis. Although surgical resection of primary or metastatic hepatic tumors is generally regarded as first-line therapy, the majority of patients with hepatic malignancy have disease that is not amenable to surgical resection because of tumor location, poor hepatic reserve, or medical comorbidities. This has led to significant interest in the development of nonsurgical image-guided therapies, including radiofrequency ablation (RFA). RFA is appealing as a minimally invasive therapy that may be performed on an outpatient basis. It enables ablation of an area 3 to 5 cm in diameter, with relatively low morbidity and mortality rates. The results concerning the use of percutaneous RFA in the treatment of hepatocellular carcinoma, colorectal metastases, and other hepatic metastases are reviewed in this article. Clinical and technical considerations and complications are also discussed.
Keywords: Radiofrequency ablation, liver, hepatocellular carcinoma, colorectal metastasis, neuroendocrine metastasis
The liver is a common site for both primary malignancy and hematogenous metastasis.1,2 Although surgical resection of primary or metastatic hepatic tumors is generally regarded as first-line therapy, the majority of patients with hepatic malignancy have disease that is not amenable to surgical resection because of tumor location, poor hepatic reserve, or medical comorbidities.3,4,5,6,7,8,9 As a result, there has been significant interest in the development of nonsurgical image-guided therapies.10 As noted by Rhim et al Gillams, image-guided ablation was first reported in 1989, when the first ultrasound-guided laser treatments were performed for hepatic metastases and the first percutaneous ethanol injections were reported for the treatment of small hepatocellular carcinomas (HCCs).11 Since that time, a wide range of image-guided ablative techniques have developed, including transarterial chemoembolization, percutaneous cryotherapy, microwave coagulation, and radiofrequency ablation (RFA). Percutaneous RFA is a recently introduced alternative to percutaneous ethanol injection; the latter is considered an established technique for percutaneous treatment of small, nodular HCCs in patients with underlying cirrhosis.12 Since its introduction, the use of percutaneous RFA has been gaining favor around the world. It is appealing for being a minimally invasive therapy that may be performed on an outpatient basis, enabling ablation of an area 3 to 5 cm in diameter with relatively low morbidity and mortality rates.12 Percutaneous RFA has the potential to prolong survival by achieving local control while maximizing preservation of normal liver parenchyma.2,10,12
Based upon favorable outcomes reported with RFA from initial clinical series, there has been significant expansion in the types of hepatic tumors treated by this method.11 This review focuses on the role of percutaneous image-guided RFA in the treatment of HCC, hepatic colorectal metastases, and hepatic metastases from other primary sites including neuroendocrine tumors and breast cancer. Issues discussed include patient selection, approaches, survival benefits, comparison with surgical outcomes, and complications.
TECHNICAL CONSIDERATIONS
Radiofrequency thermal ablation is achieved by converting electrical current in the radiofrequency range into heat.13 Heat, generated via an alternating current, results in cell death by coagulative necrosis.14 As described by Rhim et al, RFA is accomplished by creation of a closed-loop circuit with a generator, a grounding pad, a patient, and needle electrode placed in series.10 As a result of the discrepancy between the electrical resistance of the patient's tissue compared with that of the metal electrodes, there is marked agitation of the ions present in the tissue that immediately surrounds the electrode. This ionic agitation causes frictional heat around the electrode, resulting in thermal damage to the surrounding tissues.10 The extent of thermal damage achieved is dependent on the tissue temperature generated and the duration of heating.10 For adequate destruction of tumor tissue, the entire volume of a lesion must be treated with uniform temperatures that are above the threshold for cell death. Thus, an essential objective of ablation is to achieve and maintain a temperature of 50 to 100°C for 4 to 6 minutes. It is important to avoid temperatures above 100°C as excessively high temperatures result in the carbonization, or charring, of surrounding tissues. Carbonization results in the generation of gas, which effectively insulates surrounding tissues from further ablation.10
Another key component for successful tissue destruction involves the technique of overlapping ablations. Tumors may currently be approached either via use of an expandable needle electrode that deploys multiple hooks into a lesion or via a straight-tip internally cooled electrode. As noted by Rhim et al, inaccurate targeting of a tumor is likely a more common cause of tumor undertreatment than inadequate energy deposition.10 Optimal targeting mandates adequate treatment of the tumor and its margin. As in hepatic resection, physicians performing percutaneous RFA typically strive for a 360-degree, 1-cm-thick tumor-free margin around each tumor. This is crucial to achieve minimal rates of local tumor recurrence that are comparable to those obtained surgically.10 A tumor whose diameter is 2 cm less than the diameter of the zone of ablation can be successfully ablated with a single ablation.10 For example, a 3-cm ablation device can be used to treat a 1-cm diameter tumor. Lesions larger than this require serial, overlapping ablations. To obtain a 3.75-cm diameter area of treatment, for example, six overlapping 3-cm ablations are required.10 As Rhim et al notes, however, this method of treating larger tumors will inevitably result in greater rates of local tumor recurrence given the greater likelihood of missed tumor tissue with needle repositioning. Treatment of even larger lesions may be attempted via creation of zones of ablation that take the form of “thermal cylinders,” created when spherical zones of ablation are overlapped to create a cylinder. These cylinders are then overlapped to systematically ablate a larger tumor.10
The issue of prophylactic antibiotics is controversial, with some operators administering them universally and others only in selected cases. At our institution, all patients undergoing percutaneous liver RFA receive single doses of intravenous ampicillin and gentamycin prior to the procedure. Instances in which patients are at an increased risk of hepatic abscess warrant careful evaluation and prolonged antibiotics. These situations include a history of bilioenteric anastomosis, biliary stent placement, and sphincterotomy, all of which lead to retrograde enteric bacterial communication with the biliary tract. As noted by Choi et al, the mechanism of abscess formation is thought to be due to bacterial colonization and growth in the zone of ablation.15 At our institution, patients with a history of biliary manipulation receive 1 month of oral levofloxacin for prophylaxis. Additional conditions that may warrant consideration of antibiotic prophylaxis include diabetes mellitus, chronic immunosuppression, and severe cirrhosis.16
Tumor Location
The location of a tumor poses its own unique challenges and is an important factor influencing treatment success.10 As noted by Rhim et al, special challenges are posed by subcapsular tumors or those within the hepatic dome, for which no safety margin along the capsule is possible.10 For treatment of subcapsular tumors, ablation of the capsule is recommended if there is evidence for capsular involvement by tumor.10 At our institution, we have utilized a successful transpleural approach for certain dome lesions, although this approach is associated with an increased risk of pneumothorax, hemothorax, symptomatic pleural effusion, and diaphragmatic thermal injury. Tateishi et al describe an intrapleural infusion technique with tumors located adjacent to the diaphragm, in which 500 mL of 5% glucose is infused into the right pleural cavity under ultrasound guidance prior to needle insertion, to visualize the entire tumor.12 For lesions near the inferior tip of the liver that may abut both the anterior and posterior liver capsule, the straight cooled tip may be preferred as it is associated with a lower risk of capsular penetration.10 Additional challenges are posed by those tumors near the large vessels of the hepatic hilum, where the “heat sink” effect of nearby vessels limits the ability to adequately treat the periphery of a tumor. Other challenges are introduced when lesions are located near bowel, the gallbladder, adrenal gland, or when lesions are difficult to visualize with ultrasound or computed tomography (CT).
In the case of hilar tumors, precise needle placement is crucial to maximally treat the desired zone of ablation while avoiding penetration of nearby vessel walls.10 To minimize the risk of vessel trauma and bleeding, Rhim et al recommend the use of multiple overlapping ablations with partial deployment of the multiple array expandable needle electrode, rather than a single ablation with the needle electrode fully deployed.10 In this setting, an alternate method that may be employed involves use of the straight cooled-tip electrode, whose cooling is accomplished with continuous internal infusion of a chilled saline solution. The induced cooling results in a lowered impendence by the tissues nearest the needle electrode, allowing heat to diffuse more efficiently. Greater deposition of radiofrequency energy into the target tissues results, leading to more extensive necrosis.10 Some centers have also advocated the use of a balloon for mechanical occlusion of the portal vein during percutaneous RFA, as the reduction in vascular inflow is associated with more extensive regional necrosis.10 However, this technique is limited in its utility, given its added invasiveness and time.17
Tumors adjacent to or abutting bowel pose a separate challenge, as these viscera, most often the colon and stomach, are at risk for full-thickness burns.18 In some patients, positioning the patient in the decubitus position to separate liver from bowel can avoid injury.18 Another method has been introduced by Tateishi et al, who utilize an intraperitoneal infusion technique, by which 500 to 1000 mL of 5% glucose is injected before and during ablation to maintain a space between tumor and intestine. The use of ionic solutions, such as saline, should be avoided with this technique of “hydrodissection,” given their propensity to conduct rather than insulate an electrical current.12 Techniques utilized to minimize intestinal damage include percutaneous interpositioning of a balloon or injection of carbon dioxide between the tumor and the gastrointestinal tract.19
Lesions near the gallbladder, as with those near the intestine, are challenging because their proximity to tumor has the potential to cause damage to the gallbladder, a suboptimal ablation, or both. As noted by Chopra et al, many patients with tumor near the gallbladder experience right upper-quadrant pain postprocedure with or without symptoms of nausea, vomiting, and fever; these symptoms are similar in quality to those of acute cholecystitis.20 In their review of 14 tumors ≤ 1 cm from the gallbladder, Chopra et al postulate a probable focal thermal injury resulting in mild chemical cholecystitis and cite imaging findings in their patients including focal gallbladder wall edema.20 To minimize the likelihood of complications, these authors advocate careful choice and placement of needles. Special care must be taken so that the gallbladder wall is not traversed by the needle probe and perforated, and straight needles are favored over multiple array probes for their ease and accuracy in placement.20 Although serious complications have been reported in this setting, including a bile duct stricture requiring endoscopic stent placement, the procedure is considered largely feasible and safe.21
Lesions located near the adrenal gland in the posterior aspect of the right lobe of liver pose a separate problem. Heating of the adrenal gland has been known to precipitate hypertensive crisis during RFA, secondary to massive catecholamine release from the gland.22 If adrenal heating is highly probable, patient sedation and monitoring by an anesthesiologist and premedication with antihypertensives may be of benefit.
Tumors that are difficult to see pose the most fundamental problem. For lesions that are well seen on magnetic resonance imaging (MRI) but poorly seen on noncontrast CT, we utilize the surrounding intrahepatic landmarks, including the hepatic and portal veins, to localize the desired zone of ablation on noncontrast CT. In some cases, contrast injection facilitates visualization of a lesion that is poorly seen on noncontrast CT. In these cases, we utilize 120 mL of nonionic contrast (Isovue 300; Bracco Diagnostics, Princeton, NJ), administered at a rate of 3 mL/s via power injector, just prior to needle electrode placement. This may result in only transient visualization of the tumor during contrast opacification and washout; however, anatomic landmarks can be used thereafter to guide ablation.
Patient Selection
In general, all patients who are candidates for percutaneous hepatic RFA meet with a member of our staff in our Interventional Radiology Clinic. The risks, benefits, and alternatives to the procedure are discussed with the patient, and questions are answered. Patients who are felt to be appropriate candidates must have preprocedural imaging including multiphase contrast-enhanced CT or MRI with gadolinium for tumor staging. At a minimum, routine laboratory values are obtained on all patients including a complete blood count, prothrombin time, partial thromboplastin time, and tumor markers. Our patients are often seen in consultation with the Hematology/Oncology Service or Transplant Service, where liver function studies and serum tumor marker levels are also obtained. Pretreatment assessment of candidates for percutaneous RFA of HCC also include either a histopathological diagnosis of HCC or imaging findings and serum tumor markers characteristic of the same.
Percutaneous RFA is suitable in patients who are not surgical candidates on the basis of liver function impairment, number and distribution of tumors, cardiopulmonary dysfunction, or in those who voluntarily prefer ablation despite surgery also being a treatment option.12 Patients with end-stage cirrhosis (Child-Pugh C) are generally not appropriate candidates for RFA as their life expectancy is usually limited by their hepatic dysfunction and not by their cancer. Patients with uncorrectable coagulopathies, active alcohol abuse, or those unwilling or unable to comply with follow-up requirements are also not ideal RFA candidates.12
HEPATOCELLULAR CARCINOMA
Cancer estimates from the year 2000 indicate that HCC remains the fifth and eighth most common malignancy in men and women, respectively.23 This incidence of HCC is increasing in several developed countries including the United States; this trend is expected to continue for several decades, largely secondary to chronic infection with hepatitis C virus.23 It is estimated that 8500 to 11,500 new cases of HCC occur annually in the United States.24 In high-risk countries in East Asia and Central and Western Africa, HCC can occur before the age of 20, secondary to the effects of viral exposures early in life.24
Surgical resection and orthotopic transplantation are considered the mainstays of curative therapy in patients with HCC confined to the liver. However, these therapies are unavailable to the majority of such patients, either as a result of tumor nonresectability, baseline liver function, restrictions of the Milan criteria (excluding recipients with more than one lesion ≥ 5 cm or more than three lesions ≤ 3 cm), or donor organ shortage.10,11,12,25,26,27
Results
There is substantial data supporting the role of percutaneous RFA as an effective, safe first-line therapy for cirrhotic patients with HCC < 3 cm who are not candidates for surgery or transplantation.12,25 In a large-scale series with long-term follow up, Tateishi et al performed 1000 RFA treatments in 2140 HCC nodules in 664 patients. Cumulative survival rates were compared between 319 patients who received RFA as a primary treatment and 345 patients who received RFA for recurrent tumor after previous treatment (including resection, percutaneous ethanol injection therapy [PEIT], microwave coagulation therapy, and transarterial embolization). Cumulative survival rates at 1, 2, 3, 4, and 5 years were favorable, at 94.7, 86.1, 77.7, 67.4, and 54.3% for patients treated with RFA alone and 91.8, 75.6, 62.4, 53.7, and 38.2% for patients treated with combination therapies, respectively.12 Recurrence following RFA, as assessed by monthly serum tumor markers and dynamic contrast-enhanced CTs every 3 to 4 months, was comparable to that encountered with PEIT and hepatectomy.28,29,30 The local tumor progression rate was low, at 2.4% during a median of 19 months of follow-up. Procedure-related morbidity was also low, with a 2.5% per treatment rate of major complications within 30 days after the procedure; there were no treatment-related deaths.
Lencioni et al evaluated 187 patients with early-stage HCC who were treated with percutaneous RFA as a sole first-line treatment and who were not surgical or transplantation candidates. Early-stage HCC was defined by having either a single HCC < 5 cm or up to three lesions each of which was < 3 cm. All patients had Child Class A or B cirrhosis.25 The survival in these patients was compared with a subset of 19 patients who were ineligible for RFA based upon tumor location and who were treated with PEIT or segmental transcatheter arterial chemoembolization (TACE). In the patients treated with RFA, overall survival rates were 97% at 1 year, 71% at 3 years, and 48% at 5 years, with the median survival being 57 months.25 Of note, there was no significant difference in survival between patients treated with RFA compared with those treated with PEIT or TACE.25
Histological evaluations following RFA of HCCs have also validated the efficacy of this technique. Lu et al treated 24 patients with 47 HCC nodules ranging in size from 0.4 to 5.5 cm with either single or double RFA sessions. Forty-four cases were treated once and three were treated twice in a 6-month period prior to liver transplantation.31 The explanted liver specimens were then examined with hematoxylin-eosin staining for viable tumor. Of the 47 ablated tumors, 74%, including 83% of tumors less than 3 cm, were found to be successfully treated on the basis of histological findings after a mean interval of 7.5 months between RFA and transplantation.31 Mazzaferro et al also evaluated the histological response rate after a single RFA treatment of small HCCs in patients awaiting liver transplantation. These investigators treated 60 HCCs, having a mean size of 3 cm, in 50 patients; all patients were treated with single-session RFA, with the mean time to transplant being 9.6 months.27 There was a 55% complete response rate that rose to 63% for lesions < 3 cm, providing additional evidence establishing the role of RFA as a safe and effective treatment of small (< 3 cm) HCCs.27
There is growing literature to suggest that percutaneous RFA is also a safe and effective method for treatment of large HCCs (> 3 cm). The treatment of larger lesions poses a challenge, and there is currently far less published experience using any ablative techniques for HCCs > 3 to 4 cm.26 Livraghi et al performed RFA on 114 patients with 126 HCCs greater than 3 cm in diameter to evaluate the treatment efficacy and complication rate in this population.26 Eighty tumors were 3.1 to 5 cm in size, and 46 were considered large, measuring 5.1 to 9.5 cm. The mean diameter for all tumors was 5.4 cm. Complete necrosis, as evidenced on follow-up contrast-enhanced CT scans, was obtained in 47.6% of patients; nearly complete (90 to 99%) necrosis was obtained in 31.7% of patients; and partial (50 to 89%) necrosis in the remaining 20.6%.26 A total of seven complications were observed, two of which were considered major, after a mean follow-up of 10.2 months. This complication rate is comparable to other ablation techniques.26
RFA as a Bridge to Liver Transplantation
In addition to results establishing the role of RFA in treating unresectable HCC, there is emerging data to suggest a role for RFA as an adjunct for patients awaiting transplantation (Fig. 1). Currently, long waiting times for cadaveric livers may lead to removal from the waiting list or a worsened posttransplant prognosis secondary to tumor progression.32 Lu et al evaluated the outcome of 52 pretransplant patients with 87 HCC nodules that were treated with RFA. A subset of seven patients were also treated with TACE and three with PEIT. On initial staging, the tumor burden exceeded the Milan criteria in 10 patients. Complete tumor response was observed in 89.6% treated exclusively with RFA based on postablation imaging.32 After a mean of 12.7 months, three patients (5.8%) had dropped out due to tumor progression, one of whom had exceeded the Milan criteria. Forty-one patients had undergone transplantation, with 1-, 2-, and 3-year survival rates of 85, 85, and 76%, respectively. No patients developed HCC recurrences after transplantation.32
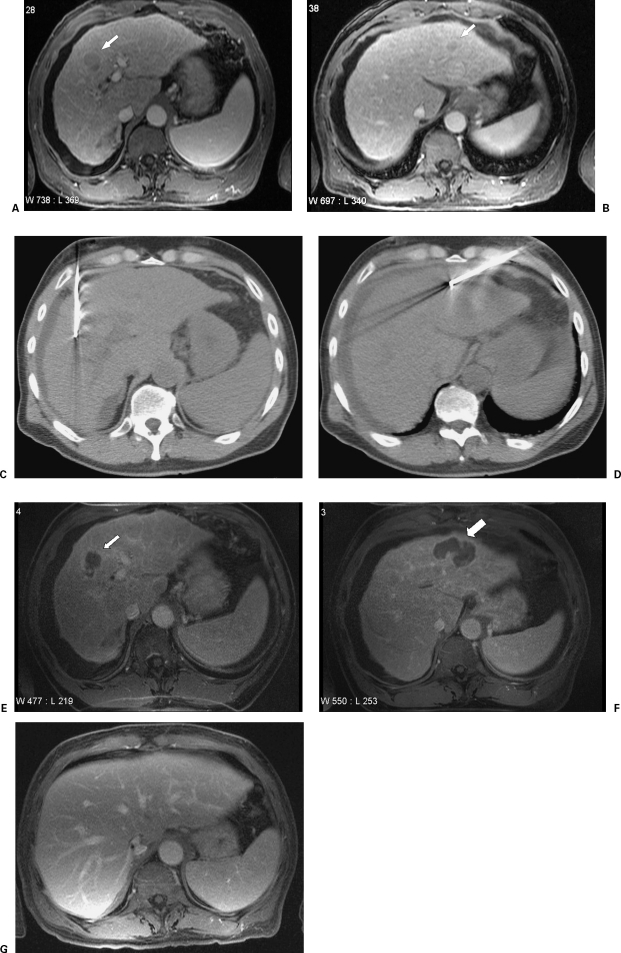
Figure 1.
A 67-year-old man with a history of hepatitis C–induced cirrhosis. An enhancing mass is noted in segment 8 (A, white arrow). An additional enhancing mass is noted in segment 2/4 (B, white arrow). These were both consistent with hepatocellular carcinoma based on their imaging appearance and AFP level. RFA was performed on both of these lesions using a straight-tip internally cooled electrode (C and D, respectively). Follow-up imaging 1 month later demonstrated no evidence for residual tumor (E and F, respectively, white arrows).
METASTATIC COLORECTAL CANCER
Colorectal cancer is the second most common cause of cancer death in the United States. In the year 2005, an estimated 145,290 people will be diagnosed with colorectal cancer, and ~56,290 will die of the disease.33 Hepatic metastases develop in 50% of patients and are the most common cause of morbidity and death.34 Although hepatectomy is a key therapeutic modality, it is practical for only a minority of patients. Similar limitations are encountered as in those patients with HCC: patients' tumor burden must be resectable with margins that are sufficient to affect a surgical cure without significant compromise of residual hepatic function.35 Only 10 to 20% of patients are surgical candidates, and of those the majority will develop new metastases after resection.36 For those who are not surgical candidates, systemic chemotherapy is an option; however, complete responses are rare and significant improvements in survival are difficult to achieve.37,38 Percutaneous RFA offers a new opportunity to treat liver metastases in patients who are not surgical candidates. In addition, the ability to combine RFA with either systemic or transarterial chemoembolization offers the potential to prolong survival in patients with unresectable cancers.39
Patient Selection
As in patients with HCC, surgical resection remains the treatment of choice for patients with colorectal metastases limited to the liver.13 However, percutaneous RFA is being increasingly used as an additional local therapy or as part of a combination of therapies employed with similar, curative intent.13 Under these circumstances, RFA may be considered in patients with a biopsy-proven hepatic metastasis or strong clinical suspicion based on imaging findings or an appropriate level of carcinoembryonic antigen. Such patients should have no evidence for underlying hepatic disease, residual hepatic disease following surgery, or systemic chemotherapy that is felt to be incurable.13 In patients without curable hepatic metastases, percutaneous RFA may still play a role in palliation and/or preservation of hepatic functional reserve (Fig. 2). Patients considered for palliative therapy include those with extrahepatic metastases, advanced age, disease nonresectability, clinical comorbidities, or refusal to consent for surgery.40
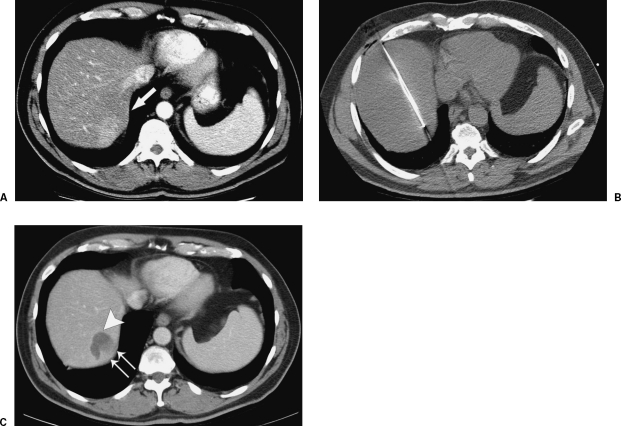
Figure 2.
Palliative RFA with incomplete ablation in a patient with widely metastatic colorectal cancer. A 58-year-old man with a history of colorectal cancer metastatic to liver. A contrast-enhanced CT demonstrates the enhancing metastasis in segment VII (A, white arrow) abutting the liver capsule. This patient underwent RFA using a straight-tip internally cooled electrode (B). Follow-up contrast-enhanced CT performed 6 weeks later demonstrates a zone of ablation (C, large white arrow) that does not reach the liver capsule, indicating that there is likely residual tumor, even though no tumor enhancement is demonstrated (C, paired white arrows).
Results
The data concerning percutaneous RFA of small (≤ 5 cm) colorectal metastases compares well with postresection survival data.41 Gillams et al performed a prospective study of 167 patients with colorectal liver metastases treated with RFA; the patients had either been rejected or refused surgical resection. For patients with no extrahepatic disease and fewer than five metastases, each having a maximum diameter ≤ 5 cm, the 5-year survival rate from the time of diagnosis was comparable to the median 5-year survival for operable patients (30% versus 32%, respectively).41,42
In patients with solitary liver metastases, RFA compares even more favorably with operable survival rates. Oshowo et al treated solitary colorectal liver metastases in 25 patients with RFA, whose indications included extrahepatic disease, vessel contiguity, and comorbid factors.43 Their outcome was compared with that of 20 patient who had no evidence of extrahepatic disease and who were treated with hepatectomy for solitary metastases. Most patients in both groups also received systemic chemotherapy.43 The median survival after liver resection was 41 months with a 3-year survival rate of 55.4%; median survival after RFA was 37 months with a 3-year survival of 52.6%.43
Current data also suggest that RFA plays a useful role when used in combination with surgery. Livraghi et al evaluated the role of RFA during the interval between diagnosis and hepatic metastectomy as part of a “test of time” management approach. This approach, in which metastectomy is intentionally delayed to allow the identification of additional hepatic metastases that might be present but undetected, has been advocated by some surgeons.40 As noted by Livraghi et al, the rationale for this approach is based on the following considerations: (1) RFA may result in complete necrosis of lesions; (2) patients whose lesions are treated adequately with RFA and who do not develop additional lesions may avoid surgical resection; (3) patients whose lesions are not treated adequately with RFA and who do not develop additional lesions (which would make them no longer surgical candidates) can undergo surgical resection; and (4) patients who develop additional lesions after RFA would no longer be eligible candidates for surgery and can be spared an unnecessary operation.40 Livraghi et al treated 88 consecutive patients with 134 hepatic metastases from colorectal cancer with percutaneous RFA using single- or triple-probe treatments. At the time of the initial ablation, 56% of patients had one metastasis, 3% had two metastases, and 8% had three metastases; metastases ranged in size from 0.6 to 4 cm, with a mean size of 2.1 cm. A total of 119 RFAs were performed and patients were followed for a median of 33 months after treatment. Degree of necrosis was judged by a consensus interpretation of four readers who evaluated posttreatment contrast-enhanced CT scans performed at 3- to 6-month intervals. Complete necrosis was achieved in 60% of patients. Among these patients, 98% were spared surgical resection, 44% because they remained disease-free and 56% because they developed disease progression. Among the 35 patients in whom complete tumor necrosis was not achieved, 43% were spared surgical resection. Overall, 24% of patients underwent resection after RFA, 26% remained free of disease after successful RFA, and 64% developed untreatable disease progression. No patient treated with RFA became unresectable due to the growth of metastases.40
There is emerging data concerning the role of RFA in the palliation of colorectal carcinoma metastases. These data suggest a survival benefit over systemic chemotherapy, which was previously the sole mainstay of therapy in these patients. Berber et al performed laparoscopic RFA in 135 patients with colorectal liver metastases who were not candidates for resection, based upon unresectability of lesions, liver-predominant disease, enlarging liver lesions, worsening of symptoms, or failure to respond to prior treatment modalities. The median Kaplan-Meier survival for all patients was 28.9 months after RFA treatment, which compares favorably with historical survival data for chemotherapy alone, which ranges from 11 to 14 months.44 Predictors of survival included lesion size, lesion number and carcinoembryonic antigen level; the presence of extrahepatic disease did not affect survival.44 This study suggests that RFA has a positive impact on overall survival and that it serves as a useful adjunct to chemotherapy in those patients with liver-predominant disease. Additional studies in the future will likely demonstrate a similar survival benefit for percutaneous RFA in these patients.
OTHER METASTASES
The success of percutaneous RFA in treating primary HCC and in metastatic colorectal cancer has prompted its application in the treatment of an increasing number of hepatic metastases. Indeed, the minimally invasive nature of this procedure and its potential to delay or even obviate surgery make it useful to consider in the treatment or palliation of many hepatic metastases. The most substantial of these data to date pertains to neuroendocrine tumor metastases and breast cancer metastases.
As noted by Berber et al, neuroendocrine malignancies with hepatic metastases can have an indolent course associated with prolonged patient survival. However, the clinical course may often be dominated by hormonal secretion with associated symptoms of diarrhea, nausea, and flushing, which can be the source of significant patient morbidity.45 Although hepatectomy is the gold standard in these patients, as in those with HCC or colorectal metastases, over 90% of these patients are not surgical candidates based either upon tumor nonresectability or comorbidities. Chemotherapy has limited value in these patients.46,47 Thus, percutaneous RFA may play a significant role by meeting these patients' need not by controlling tumor burden in these patients but by achieving palliation of their hormonal symptoms. In their 5-year experience with RFA in patients with neuroendocrine metastases, Berber et al treated 234 tumors in 34 patients, including carcinoid tumor in 18, medullary thyroid cancer in seven, secreting islet cell tumor in five, and nonsecreting islet cell tumor in four. Symptoms were improved in 95% of patients, with significant or complete symptom control in 80% of patients for a mean of 10 months (range 6 to 24 months). The mean follow-up time was 1.6 ± 0.2 years. During this period, new lesions developed in 28% of patients, with local liver recurrence in 13% and progression of existing liver lesions in another 13% of patients. Overall, 41% of patients showed no progression of their disease at the end of the follow-up period.45 These findings compare favorably with those obtained from surgical resection, which has been associated with a response rate of 90%. Medical management with somatostatin analogs, which is reserved for patients not responding to chemotherapy, has been associated with a response rate of 50 to 88%, and chemoembolization has been associated with a response rate of 63%.46,48
Elvin et al reported similarly favorable results following treatment of 42 patients with a total of 198 neuroendocrine metastases. These included carcinoid tumor, nonfunctioning endocrine pancreatic tumors, vasoactive intestinal peptide (VIP)omas, glucagonoma, medullary thyroid cancer, gastrinoma, and adrenal carcinoma.49 In total, they performed 109 RFA procedures, 84 percutaneously, 23 during open surgery, and two as an open procedure where only RFA was performed. Follow-up CT scans demonstrated successful treatment in 90% of patients, with a local recurrence rate of only 10% after a mean follow-up period of 3.2 years.49
Preliminary results in patients with metastatic breast cancer are also promising. Although metastatic breast cancer generally is managed with systemic rather than local therapy, there is evidence to suggest that those patients who undergo hepatectomy for breast cancer metastases limited to the liver experience median survivals up to three times those in comparable patients treated without surgery.50,51 Livraghi et al's experience in the treatment of 24 patients with breast cancer liver metastases achieved complete necrosis in 92% of lesions, requiring only a single treatment in the majority (92%) of cases.52 After 44 months of follow-up, 63% of 16 patients whose lesions were initially confined to the liver remained free of disease.52 The ultimate role of percutaneous RFA remains to be determined once results of larger cohorts of patients are available. However, for tumors that are of appropriate size and location for RFA, RFA can be considered, invoking the “test of time” rational if surgery is being considered.
COMPLICATIONS
Knowledge of the potential complications associated with percutaneous RFA is crucial in assessing the risks of this procedure for a given patient. To categorize complications as minor or major, most investigators utilize the Standards of the Society for Interventional Radiology.53,54 The definition of a major complication under these criteria is one that, if left untreated, might threaten the patient's life, lead to substantial morbidity and disability, or result in a prolonged hospital stay. All other complications are considered minor. Livraghi et al reviewed the complication rate following treatment of 3554 lesions in 2320 patients involved in a multicenter study.55 They noted six deaths (0.3%), including two caused by multiorgan failure following intestinal perforation, one case of septic shock following Staphylococcus aureus peritonitis, massive hemorrhage following tumor rupture, liver failure following stenosis of the right intrahepatic bile duct, and one case of sudden death of unknown cause 3 days after the procedure. Fifty patients (2.2%) sustained additional major complications, and fewer than 5% sustained minor complications. The most common major complication was intraperitoneal hemorrhage, followed by neoplastic seeding, intrahepatic abscesses, and bowel perforation (Fig. 3).55 There was a significantly increased risk of procedural complications associated with an increased number of RFA sessions; additional variables such as tumor size and electrode type were related with an increased complication rate.55 Curley et al also evaluated the complication rate among patients undergoing RFA. Their group of 608 patients underwent RFA of 1225 malignant tumors, with 62.8% undergoing open intraoperative RFA and 37.2% undergoing percutaneous RFA.56 Their prospective analysis was performed to determine the rates of early versus late complications (< 30 days and > 30 days, respectively) that were associated with hepatic tumor RFA.56 The overall combined (early and late) complication rate was 9.5%. The periprocedural mortality rate was 0.5%, and causes of death included progressive liver failure, pneumonia complicated by respiratory failure and sepsis, and intraperitoneal hemorrhage leading to a fatal myocardial infarction.56 Additional early complications developed in 7.1% of patients and included a symptomatic pleural effusion, perihepatic abscess, RFA abscess, ascites requiring treatment, hemorrhage, biloma within the zone of ablation, hepatic insufficiency, hydropneumothorax, thermal injury to the stomach, biliary fistula, and one case of ventricular fibrillation.56 Late complications included formation of a biloma within the zone of ablation, biliary fistula, ascites requiring treatment, hepatic insufficiency, arteriovenous fistula, symptomatic pleural effusion, RFA abscess, and intractable pain.56 The authors found that early complications were more likely to occur in patients treated with open RFA compared with percutaneous RFA (8.6% versus 4.4%, respectively) and in patients with cirrhosis compared with those without cirrhosis (12.9% versus 7.5%, respectively). Late complications occurred with no difference between open and percutaneous RFA treatment. Although delayed complications occurred at a relatively low rate (2.4%), their incidence underlies the need for treating physicians to be mindful of these delayed procedural problems.54
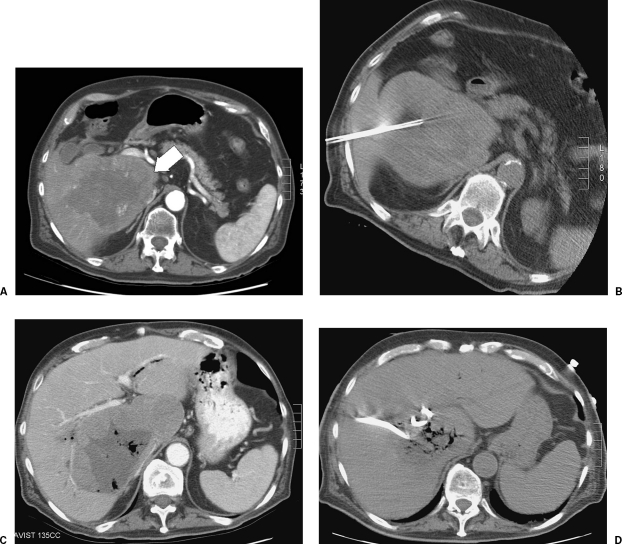
Figure 3.
A 76-year-old man with a history of colorectal carcinoma metastatic to liver. The patient's dominant metastasis was located in segment V/VI (A, white arrow). This lesion was treated with three separate RFAs over a 3-month period (B). The patient subsequently presented to the emergency department with fevers and right upper-quadrant pain. Contrast-enhanced CT of the abdomen demonstrated a large area of treated tumor, within which there were new foci of gas that demonstrated communication with the biliary tree. Although the presence of gas within the zone of ablation can be seen following RFA without indicating an underlying abscess, its presence, coupled with the patient's symptoms, prompted image-guided aspiration from the zone of ablation. This demonstrated purulent material consistent with abscess, and a percutaneous drain was placed.
A similar overall complication rate was encountered by Mulier et al, who reviewed 82 independent reports of liver RFA in a total of 3670 patients.57 They found complications occurred in 8.9% of patients, with the range of complications being similar to those described by Curley et al. The most common complications included intraperitoneal bleeding and subcapsular hematomas, hepatic abscess and biliary stricture.57 The incidence of complications was slightly higher following RFA via laparoscopic and open approaches compared with the percutaneous route. The complication rates were 7.2, 9.5, 9.9, and 31.8%, respectively, following percutaneous, laparoscopic, simple open ablation, and combined open ablation procedures. The latter procedure involved open RFA combined with either cryotherapy or hepatic or extrahepatic resection.57
Complications overall are more likely to occur in cirrhotic patients than in those without cirrhosis.2 Technical complications that are most relevant in patients with HCC include intraperitoneal hemorrhage, portal vein thrombosis, hepatic abscess formation, and carcinoma seeding.2,12 Risk factors associated with seeding include previous needle biopsy, treatment of subcapsular lesions, patients treated in multiple sessions, and lesions requiring more than one electrode placement.56,57 The key challenge within this population is to minimize the likelihood of recurrence. Risk factors for local recurrence include tumor size and location relative to the liver surface, with larger tumors (> 2.5 cm) being more likely to recur. Some have advocated an aggressive multimodality ablative therapy, along with a short transplant waiting time, to optimize the use of transplant for curative intent.25 Fisher et al utilized such a regimen in 33 patients with T0 to T3 HCC, which included RFA and/or TACE, with PEIT, followed by transarterial chemoinfusion. Additional treatments were performed during the waiting period for transplant, based on the results of surveillance hepatic MRI. The waiting time was 9.1 ± 14.8 months, with a mean follow-up of 32 months. Twenty-eight patients received transplants, and 5 (12.2%) dropped out due to tumor progression. Posttransplant survival rates were favorable at 79% at 32 months of follow-up.25
Imaging Follow-up
The most accepted imaging finding that suggests complete treatment of HCC is the complete loss of previously seen vascular enhancement on contrast-enhanced cross-sectional images.59,60 Radiological-pathological correlations in both experimental and clinical studies have shown that the imaging findings with both contrast-enhanced CT and MRI are predictive of the nonperfusing area of coagulation to within 2 to 3 mm.59 Thus, baseline CT or MRI should be performed within 1 to 2 months prior to ablation to permit accurate comparisons with postablation images.10 CT or MRI is usually performed within 1 month of treatment to detect possible viable tumor that requires retreatment.60,61,62,63,64,65 Thereafter, it is generally recommended that close imaging follow-up should be performed every 2 to 4 months. Many experts advocate that this frequency of short interval imaging be continued indefinitely, given that local tumor recurrence has been observed more than 18 months after ablation.10
REFERENCES
- Bentrem D J, DeMatteo R P, Blumgart L H. Surgical therapy for metastatic disease to the liver. Annu Rev Med. 2005;56:139–156. doi: 10.1146/annurev.med.56.082103.104630. [DOI] [PubMed] [Google Scholar]
- Curley S A, Marra P, Beaty K, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450–458. doi: 10.1097/01.sla.0000118373.31781.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer D G, Rosve M H, Shaked A, et al. Current treatment modalities for hepatocellular carcinomas. Ann Surg. 1994;219:236–247. doi: 10.1097/00000658-199403000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y, Blumgart L H, Cohen A M. Surgical treatment of colorectal metastases to the liver. CA Cancer J Clin. 1995;45:50–62. doi: 10.3322/canjclin.45.1.50. [DOI] [PubMed] [Google Scholar]
- Fong Y, Kemedy N, Paty P, et al. Treatment of colorectal cancer: hepatic metastases. Semin Surg Oncol. 1996;12:219–252. doi: 10.1002/(SICI)1098-2388(199607/08)12:4<219::AID-SSU3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Franco D, Capussotti L, Smadja C, et al. Resection of hepatocellular carcinoma: results in 72 European patients with cirrhosis. Gastroenterology. 1990;98:733–738. [PubMed] [Google Scholar]
- Bismuth H, Majno P E. Hepatobiliary surgery. J Hepatol. 2000;32:208–224. doi: 10.1016/s0168-8278(00)80427-4. [DOI] [PubMed] [Google Scholar]
- Calvet X, Bruix J, Bru C, et al. Natural history of hepatocellular carcinoma in Spain: five year's experience in 249 cases. J Hepatol. 1990;10:311–317. doi: 10.1016/0168-8278(90)90138-h. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Saitoh S, Koida I, et al. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18:47–53. [PubMed] [Google Scholar]
- Rhim H, Goldberg S N, Dodd G D, et al. Essential techniques for successful radiofrequency thermal ablation of malignant hepatic tumors. Radiographics. 2001;21:S17–S39. doi: 10.1148/radiographics.21.suppl_1.g01oc11s17. [DOI] [PubMed] [Google Scholar]
- Gillams A R. The use of radiofrequency in cancer. Br J Cancer. 2005;92:1825–1829. doi: 10.1038/sj.bjc.6602582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma: an analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- Parikh A A, Curley S A, Fornage B D, et al. RFA of hepatic metastases. Semin Oncol. 2002;29:168–182. doi: 10.1053/sonc.2002.31673. [DOI] [PubMed] [Google Scholar]
- White T J, Roy-Choudhury S H, Breen D J, et al. Percutaneous radiofrequency ablation of colorectal hepatic metastases: initial experience. Dig Surg. 2003;21:314–320. doi: 10.1159/000080886. [DOI] [PubMed] [Google Scholar]
- Choi D, Lim H K, Kim M J, et al. Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinoma: frequency and risk factors. AJR Am J Roentgenol. 2005;184:1860–1867. doi: 10.2214/ajr.184.6.01841860. [DOI] [PubMed] [Google Scholar]
- Dupuy D E, Goldberg S N. Image-guided radiofrequency tumor ablation: challenges and opportunities—part II. J Vasc Interv Radiol. 2001;12:1135–1148. doi: 10.1016/s1051-0443(07)61670-4. [DOI] [PubMed] [Google Scholar]
- Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radiofrequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119–126. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- Choi D, Lim H K, Kim M J, et al. Therapeutic efficacy and safety of percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the gastrointestinal tract. AJR Am J Roentgenol. 2004;183:1417–1424. doi: 10.2214/ajr.183.5.1831417. [DOI] [PubMed] [Google Scholar]
- Yamakado K, Nakatsuka A, Akeboshi M, et al. Percutaneous radiofrequency ablation of liver neoplasms adjacent to the gastrointestinal tract after balloon catheter interposition. J Vasc Interv Radiol. 2003;14:1183–1186. doi: 10.1097/01.rvi.0000086530.86489.05. [DOI] [PubMed] [Google Scholar]
- Chopra S, Dodd G D, Chanin M P, et al. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: feasibility and safety. AJR Am J Roentgenol. 2003;180:697–701. doi: 10.2214/ajr.180.3.1800697. [DOI] [PubMed] [Google Scholar]
- Bilchik A J, Wood T F, Allegra D, et al. Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg. 2000;135:657–662. doi: 10.1001/archsurg.135.6.657. [DOI] [PubMed] [Google Scholar]
- Onik G, Onik C, Medary I, et al. Life threatening hypertensive crises in two patients undergoing hepatic radiofrequency ablation. AJR Am J Roentgenol. 2003;181:495–497. doi: 10.2214/ajr.181.2.1810495. [DOI] [PubMed] [Google Scholar]
- Bosch F X, Ribes J, Cleries R, et al. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- El-Serag H B. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lencioni R, Cioni D, Crocetti L, et al. Early stage hepatocellular carcinoma in patients with cirrhosis: long term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Goldberg S N, Lazzaroni S, et al. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for treatment of hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Saitoh S, Tsubota A, et al. Risk factors for tumor recurrence and prognosis after curative resection of hepatocellular carcinoma. Cancer. 1993;71:19–25. doi: 10.1002/1097-0142(19930101)71:1<19::aid-cncr2820710105>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Okada S, Shimada K, Yamamoto J, et al. Predictive factors for postoperative recurrence of hepatocellular carcinoma. Gastroenterology. 1994;106:1618–1624. doi: 10.1016/0016-5085(94)90419-7. [DOI] [PubMed] [Google Scholar]
- Koike Y, Shiratori Y, Sato S, et al. Risk factors for recurring hepatocellular carcinoma differ according to infected hepatitis virus: an analysis of 236 consecutive patients with a single lesion. Hepatology. 2000;32:1216–1223. doi: 10.1053/jhep.2000.20237. [DOI] [PubMed] [Google Scholar]
- Lu D S, Yu N C, Raman S S, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954–960. doi: 10.1148/radiol.2343040153. [DOI] [PubMed] [Google Scholar]
- Lu D S, Yu N C, Raman S S. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130–1137. doi: 10.1002/hep.20688. [DOI] [PubMed] [Google Scholar]
- American Cancer Society Colorectal Cancer Facts and Figures, Special Edition. Atlanta, GA: American Cancer Society; 2005. pp. 1–22.
- Gillams A R, Lees W R. Radio-frequency ablation of colorectal liver metastases in 167 patients. Eur Radiol. 2004;14:2261–2267. doi: 10.1007/s00330-004-2416-z. [DOI] [PubMed] [Google Scholar]
- Hughes K S, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278–284. [PubMed] [Google Scholar]
- Solbiati L, Livraghi T, Goldbert S G, et al. Percutaneous radiofrequency ablation of hepatic metastases from colorectal cancer: long term results in 117 patients. Radiology. 2001;221:159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- LeVeen R F. Laser hyperthermia and radiofrequency ablation of hepatic lesions. Semin Intervent Radiol. 1997;14:313–324. [Google Scholar]
- Kemeny N, Huang Y, Cohen A M, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- Bloomston M, Binitie O, Fraiji E, et al. Transcatheter arterial chemoembolization with or without radiofrequency ablation in the management of patients with advanced hepatic malignancy. Am Surg. 2002;68:827–831. [PubMed] [Google Scholar]
- Livraghi T, Solbiati L, Meloni F, et al. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection, the “test of time” approach. Cancer. 2003;97:3027–3035. doi: 10.1002/cncr.11426. [DOI] [PubMed] [Google Scholar]
- Gillams A R, Lees W R. RFA of CRC in 167 patients. Eur Radiol. 2004;14:2261–2267. doi: 10.1007/s00330-004-2416-z. [DOI] [PubMed] [Google Scholar]
- Fong Y, Cohen A M, Fortner J G, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–994. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- Oshowo A, Gillams A, Harrison E, et al. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 2003;90:1240–1243. doi: 10.1002/bjs.4264. [DOI] [PubMed] [Google Scholar]
- Berber E, Pelley R, Siperstein A E. Predictors of survival after radiofrequency ablation of colorectal cancer metastasis: a prospective study. J Clin Oncol. 2005;23:1358–1364. doi: 10.1200/JCO.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Berber E, Flesher N, Siperstein A E. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases. World J Surg. 2002;26:985–990. doi: 10.1007/s00268-002-6629-5. [DOI] [PubMed] [Google Scholar]
- Oberg K. The use of chemotherapy in the management of neuroendocrine tumors. Endocrinol Metab Clin North Am. 1993;22:941–952. [PubMed] [Google Scholar]
- Siperstein A E, Berber E. Cryoablation, percutaneous alcohol injection, and radiofrequency ablation for treatment of neuroendocrine liver metastases. World J Surg. 2001;25:693–696. doi: 10.1007/s00268-001-0015-6. [DOI] [PubMed] [Google Scholar]
- Siperstein A, Garland A, Engle K, et al. Laparoscopic radiofrequency ablation of primary and metastatic liver tumors: technical considerations. Surg Endosc. 2000;14:400–405. doi: 10.1007/s004640000067. [DOI] [PubMed] [Google Scholar]
- Elvin A, Skogseid B, Hellman P. Radiofrequency ablation of neuroendocrine liver metastases. Abdom Imaging. 2005;30:427–434. doi: 10.1007/s00261-004-0257-5. [DOI] [PubMed] [Google Scholar]
- Elias D, Lasser P H, Montucolli D, et al. Hepatectomy for liver metastases from breast cancer. Eur J Surg Oncol. 1995;21:510–523. doi: 10.1016/s0748-7983(95)96972-1. [DOI] [PubMed] [Google Scholar]
- Schneebaum S, Walker M J, Young D, et al. The regional treatment of liver metastases from breast cancer. J Surg Oncol. 1994;55:26–31. doi: 10.1002/jso.2930550108. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Goldbert S N, Solbiati L, et al. Percutaneous radiofrequency ablation of liver metastases from breast cancer: initial experience in 24 patients. Radiology. 2001;220:145–149. doi: 10.1148/radiology.220.1.r01jl01145. [DOI] [PubMed] [Google Scholar]
- Burke D R, Lewis C A, Cardella J F, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. Society of Cardiovascular and Interventional Radiology. J Vasc Interv Radiol. 1997;8:677–681. doi: 10.1016/s1051-0443(97)70630-4. [DOI] [PubMed] [Google Scholar]
- Lewis C A, Allen T E, Burke D R, et al. Quality improvement guidelines for central venous access: the Standards of Practice Committee of the Society of Cardiovascular and Interventional Radiology. J Vasc Interv Radiol. 1997;8:475–479. doi: 10.1016/s1051-0443(97)70592-x. [DOI] [PubMed] [Google Scholar]
- Fisher R A, Maluf D, Cotterell A H, et al. Non-resective ablation therapy for hepatocellular carcinoma: effectiveness measured by intention-to-treat and dropout from liver transplant waiting list. Clin Transplant. 2004;18:502–512. doi: 10.1111/j.1399-0012.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- Curley S A, Marra P, Beaty K, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450–458. doi: 10.1097/01.sla.0000118373.31781.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulier S, Mulier P, Ni Y, et al. Complications of radiofrequency coagulation of liver tumors. Br J Surg. 2002;89:1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Solbiati L, Meloni M F, et al. Treatment of focal liver tumors with percutaneous radiofrequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- Goldberg S N, Gazelle S G, Mueller P R. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174:323–331. doi: 10.2214/ajr.174.2.1740323. [DOI] [PubMed] [Google Scholar]
- McGhana J P, Dodd G D., III Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001;176:3–16. doi: 10.2214/ajr.176.1.1760003. [DOI] [PubMed] [Google Scholar]
- Dodd G D, III, Soulen M, Kane R, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of major breakthrough. Radiographics. 2000;20:9–27. doi: 10.1148/radiographics.20.1.g00ja019. [DOI] [PubMed] [Google Scholar]
- Gazelle G S, Goldberg S N, Solbiati L, Livraghi T. Tumor ablation with radiofrequency energy. Radiology. 2000;217:633–646. doi: 10.1148/radiology.217.3.r00dc26633. [DOI] [PubMed] [Google Scholar]
- Rossi S, DiStasi M, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759–768. doi: 10.2214/ajr.167.3.8751696. [DOI] [PubMed] [Google Scholar]
- Solbiati L, Goldbert S N, Ierace T, et al. Hepatic metastases: percutaneous radiofrequency ablation with cool-tip electrodes. Radiology. 1997;205:367–373. doi: 10.1148/radiology.205.2.9356616. [DOI] [PubMed] [Google Scholar]
- Rhim H, Dodd G D., III Radiofrequency thermal ablation of liver tumors. J Clin Ultrasound. 1999;27:221–229. doi: 10.1002/(sici)1097-0096(199906)27:5<221::aid-jcu1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]