ABSTRACT
Mechanical thrombectomy devices are increasingly being used in selected patients with acute venous thromboembolic disease to rapidly decrease thrombus burden and establish venous patency. Hemolysis and hemoglobinuria secondary to red blood cell fragmentation is known to occur after the use of these devices. In this article we describe a case in which a 16-year-old female patient developed acute renal failure after use of a mechanical thrombectomy device for treatment of symptomatic iliocaval venous thrombosis.
Keywords: Mechanical thrombectomy, thrombolysis, venous, thrombosis, embolism
Venous thromboembolic disease remains an important cause of morbidity and mortality in the United States. Pulmonary embolism (PE), in and of itself, is an extremely common and highly lethal problem and remains a leading cause of death in all age groups. Unfortunately, the diagnosis of PE is often missed. If left untreated, approximately a third of patients who survive an initial PE die of a future embolic episode.1 This is true, whether the initial embolism is small or large.
Deep vein thrombosis (DVT) is also present in a large percentage of patients who present with PE. DVT, even in the absence of associated PE, can result in significant morbidity. Symptoms associated with acute lower extremity DVT primarily include pain and swelling. Venous valvular damage and venous reflux as sequelae of DVT can lead to chronic ambulatory venous hypertension and symptoms related to the post-thrombotic syndrome (PTS).
Traditional treatment of acute PE and/or DVT is long-term anticoagulation with heparin and warfarin, which prevents further thrombus formation and minimizes the risk of further PE. However, in cases of massive pulmonary thromboembolism or iliofemoral venous thrombosis with progressive symptoms, a body of evidence suggests that catheter-directed treatment options may provide both acute and chronic clinical and hemodynamic benefits.2,3 We present a case of a 16-year-old young woman who presented with PE and DVT and developed acute renal failure following use of a mechanical thrombectomy device during catheter-directed therapy.
CASE REPORT
A 16-year-old female patient presented to our institution on January 16, 2006, for a scheduled follow-up clinic visit for evaluation of pulmonary hypertension, a recent history of left lower extremity DVT and PE. Her past medical history was significant for pulmonary hypertension secondary to chronic recurrent thromboembolic disease, and a recent diagnosis of a hypercoagulable state due to antiphospholipid antibody disorder. She was being managed with chronic anticoagulation with warfarin. Four months earlier, she had undergone catheter-directed thrombolysis (CDT) for acute on chronic PE, and mechanical thrombectomy with the Possis AngioJet (Possis Medical, Minneapolis, MN) and CDT for acute left iliofemoral DVT. She also had an infrarenal inferior vena cava (IVC) filter placed during her initial intervention. At the time of her initial presentation, her pulmonary artery systolic pressures were as high as 75 mm Hg. Following thrombolysis and anticoagulation, her pulmonary artery systolic pressures had decreased to < 50 mm Hg, as estimated by echocardiography.
During her current clinic visit, she complained of a 2-week history of worsening shortness of breath with exertion and two episodes of “racing heart” while she was at rest. She was also experiencing some pain behind both of her knees. Her international normalized ratio was 2.7 and her serum creatinine was 0.8 mg/dL. A computed tomography (CT) pulmonary angiogram demonstrated new PE on the right, with some improvement in her left-sided PE (Fig. 1). A pelvic venogram and inferior vena cavagram (IVCgram) were performed and showed occlusive thrombus in the IVC, which extended cephalad to the IVC filter and inferiorly into the proximal right common iliac vein (Fig. 2). A selective right pulmonary angiogram was also obtained, which showed a large embolus in the right pulmonary artery, correlating well with the CT (Fig. 3). Pulmonary artery pressures were 83/38 mm Hg (mean pressure = 53 mm Hg). A retrievable IVC filter was then placed in a suprarenal location above the infrarenal filter to prevent further embolic events (Fig. 4).
Figure 1.
Computed tomography pulmonary angiogram shows a new pulmonary embolus (PE) on the right (white arrow) with some improvement in her left-sided PE (white arrowheads).
Figure 2.
Inferior vena cavagram shows an occlusive thrombus in the inferior vena cava (IVC), which extends cephalad to the IVC filter (black arrowhead) and inferiorly involves the whole infrarenal IVC and proximal right common iliac vein (black arrows).
Figure 3.
Emboli in the right pulmonary artery branches (black arrows) are also seen on pulmonary angiogram correlating with computed tomography findings.
Figure 4.
Second inferior vena cava filter was placed to the suprarenal location.
Mechanical thrombectomy and CDT of the new IVC and iliac venous thrombosis were initiated. Four mg of tissue plasminogen activator (tPA) (Genentech, South San Francisco, CA) was diluted in 100 mL of normal saline. Utilizing the AngioJet DVX catheter (Possis Medical, Minneapolis, MN) and the tPA solution, the power-pulse spray technique was applied to the acute thrombus in the IVC and right iliac vein. During power-pulse spraying, the outflow lumen of the AngioJet catheter is occluded by a stopcock that allows the tPA containing fluid to be forcefully pulsed (0.6 mL/pulse) from the catheter into the thrombus, both mechanically disrupting the thrombus and causing a more diffuse penetration and mixing of the tPA in the thrombus. Approximately 30 minutes (dwell time) after power-pulse spraying the tPA solution into the acutely thrombosed segments, traditional mechanical thrombectomy of this area was performed with the AngioJet DVX catheter. A total of 5 minutes of mechanical thrombectomy was performed. Control venography was then performed and demonstrated partial recanalization of the infrarenal IVC and the right common iliac vein (Fig. 5). An infusion catheter with 20 cm of infusion side holes (Unifuse catheter; AngioDynamics, Glen Falls, NY) was then positioned in the IVC and right iliac vein. Infusion of tPA at a rate of 0.75 mg per hour was initiated through this catheter.
Figure 5.
Partial recanalization of the right common iliac vein and inferior vena cava (IVC) after power-pulse spray and mechanical thrombectomy. Black arrow demonstrates the existing infrarenal IVC filter.
A pigtail catheter was also embedded into the thrombus in the right pulmonary artery, and a tPA infusion was started via this catheter at a rate of 0.75 mg per hour (for a total of 1.5 mg/hour of tPA between the two infusions). The patient received intravenous hydration during the procedure and a total of 200 mL of iohexol 300 (Omnipaque 300; GE Healthcare, Princeton, NJ) during the CTPA and catheter-based procedures. The patient was also systemically heparinized to maintain the activated partial thromboplastin time between 60 and 80 seconds.
The evening of the procedure, her urine became “bloody” in appearance. The presumption was that she had developed hemoglobinuria from hemolysis related to the power-pulse spray mechanical thrombectomy procedure. Her hematocrit remained stable. A repeat venogram and limited pulmonary angiogram were performed the next day using a total of 30 mL of iohexol. There was no significant difference in the amount of thrombus in the pulmonary artery, with slight improvement in the iliocaval venous thrombus.
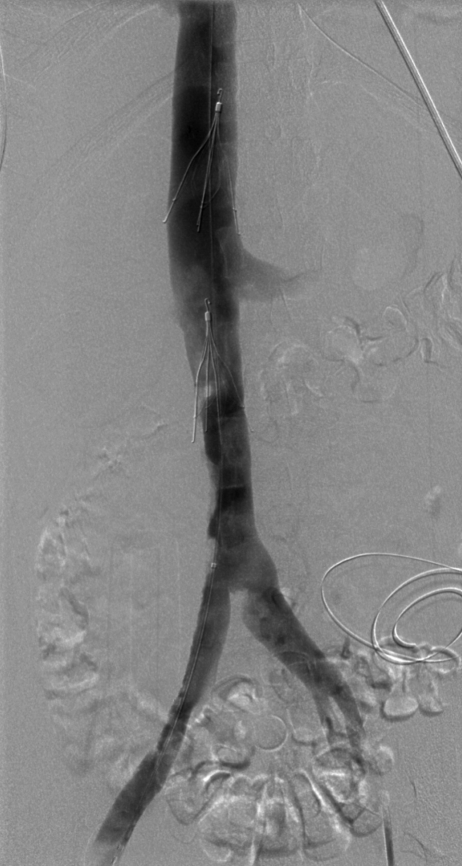
No further mechanical thrombectomy was performed due to concerns about inducing further hemolysis and hemoglobinuria. A sodium bicarbonate infusion was initiated as a renal protective measure, both for the hemoglobinuria and to minimize the risk of contrast-induced nephropathy. Gross hemoglobinuria persisted for 3 days. After 55 hours of pulmonary artery infusion of tPA, the pulmonary arterial pressures had decreased to 70/22 mm Hg, with a mean pressure of 44 mm Hg. The pulmonary artery catheter was removed at that time. To achieve optimum iliocaval recanalization, the patient received a total of 6 days of catheter-directed tPA infusion, with a good final result (Fig. 6). No further mechanical thrombectomy was performed. There was also improvement in the right pulmonary artery emboli with decreased thrombus burden and better visualization of peripheral branches (Fig. 7). The suprarenal IVC filter was removed 3 weeks later, at which time the iliocaval system was shown to be free of any residual thrombus (Fig. 8). The infrarenal IVC filter was left in place.
Figure 6.
Significant improvement of right common iliac vein and inferior vena cava (IVC) thrombus with trace residual thrombus left within the infrarenal filter and at the right lateral wall of the IVC. This result was achieved on day 6 of the procedure.
Figure 7.
Follow-up pulmonary angiogram after catheter-directed thrombolysis shows improvement in right pulmonary artery embolus with better visualization of the peripheral pulmonary artery branches.
Figure 8.
(A) and (B) Three weeks later, inferior vena cavagram shows further resolution of trace infrarenal thrombus. The suprarenal filter was no longer needed and therefore removed. (C) Image shows capturing of the Tulip filter with a snare at its hook and pulled into a long 8F sheath during the process of removal.
The patient's creatinine level prior to the procedure was 0.8 mg/dL. Four hours after the initial power-pulse spray mechanical thrombectomy procedure, the creatinine was 1.1 mg/dL, and 10 hours later it was 1.7 mg/dL. The bicarbonate drip was initiated at that time. On day 3 after the procedure, the creatinine level had increased to 4.1 mg/dL, and peritoneal dialysis was initiated. The thrombolytic and heparin infusions were suspended for 24 hours for placement of the peritoneal dialysis catheter, and then reinitiated. Even after initiation of dialysis, the serum creatinine level continued to rise and was 9 mg/dL on day 9 of admission, despite renal protective efforts with hydration and continuous bicarbonate infusion.
Of note, the patient received a total of 200 mL of iohexol 300 on day 1 of the procedure and 30mL of iohexol 300 on day 2. She subsequently received 16 mL, 20 mL, 20 mL, and 20 mL of iodixanol (Visipaque 270; GE Healthcare, Princeton, NJ) on days 4,5, 6, and 7, respectively, during which she was receiving a continuous bicarbonate infusion at a rate of 125 mL/hour. Twenty days after the initial procedure, her renal function returned to normal, and the peritoneal dialysis catheter was removed. The patient was discharged to home on low molecular weight heparin injections with close follow-up with hematology and pediatric cardiology.
Routine follow-up at 6 months revealed that her pulmonary artery systolic pressure decreased to 45 mm Hg, as determined by echocardiography. She had no lower extremity or pulmonary symptoms. She was feeling well, working at a fast-food restaurant, and attending school.
DISCUSSION
Although the traditional management of acute DVT is anticoagulation, in the presence of acute iliofemoral DVT, some evidence indicates that catheter-directed therapy may be more effective in preventing morbidity related to the PTS.4,5 Anticoagulation may prevent the progression of DVT, but due to the large volume of thrombus associated with iliofemoral DVT, the endogenous lytic system is unlikely to reestablish venous patency or prevent the damage to venous valves caused by the unresolved thrombus. Prevention of the sequelae of the DVT may be possible by prompt removal of the thrombus and the more rapid establishment of antegrade flow and venous patency. In the National Venous Registry, there was a significant correlation between establishment of complete venous patency with CDT and subsequent long-term venous patency.2 However, CDT with thrombolytic agents alone often requires prolonged treatment times to establish complete venous patency, and the risk of bleeding complications increases as infusion duration increases. Patient discomfort and increasing hospital costs are additional problems related to increased infusion duration.
Therefore, the concept of combining mechanical thrombectomy techniques with CDT to establish more rapid venous patency and reduce the duration of catheter-directed therapy is gaining more widespread acceptance.6,7,8 More recently, reports in which the use of the power-pulse spray technique was incorporated with mechanical thrombectomy for the treatment of acute iliofemoral DVT have shown clearance of the DVT in a single treatment session.6,7 With the power-pulse spray technique, the AngioJet system delivers the thrombolytic agent deeper into the thrombus under high pressure while mechanically disrupting the thrombus. Initially this technique was performed by modifying the pump set by closing the outflow port of the catheter tubing. The company has now developed a dedicated power-pulse spray delivery kit for use with Xpeedior catheter. The kit includes a Y–Spike set that allows the interventionalist to switch between heparinized saline and the thrombolytic agent solutions. By power-pulse spraying the thrombolytic agent directly into the thrombus with the AngioJet device, the total dose and duration of the thrombolytic infusion and the total procedure time may be reduced.6,7 After power-pulse spraying the thrombolytic agent into the thrombus, preliminary data suggest that if the lytic agent is allowed to dwell for at least 30 minutes, followed by traditional AngioJet thrombectomy, outcomes may be better.6,7
There are several mechanical thrombectomy devices (MTDs) currently available, most of which are approved by the Food and Drug Administration (FDA) for use in dialysis fistulas. The Possis AngioJet device, which was used in this case, is FDA approved for use in the peripheral arterial system, dialysis fistulas, and the venous system. The AngioJet thrombectomy system is designed to produce an area of extremely low pressure at the catheter tip by controlled high-velocity saline jets. Via this mechanism, thrombus surrounding the catheter tip is macerated and rapidly evacuated via an effluent lumen into a collection chamber. The system has three components, which include a drive unit, pump set, and a catheter. A newer, more compact drive unit system will become available in 2007. There is a variety of catheter choices, depending on the size of the target vessel. The XMI and XVG catheters have lower flow rates and low profiles and are designed for use in smaller vessels (2- to 4-mm diameters). The AVX catheter is 50 cm long and designed for dialysis interventions. The Xpeedior catheter, with a working length of 120 cm, is designed for peripheral applications with vessel diameters ranging 3 to 12 mm. The DVX catheter, with a 90-cm working length, is also designed for peripheral applications. It has a wider clearing profile and a more powerful jet and removed five times the thrombus volume as compared with the Xpeedior catheter during in vitro testing. However, hemolysis is known to occur with all of these devices and may be more substantial with the DVX device. Nephropathy has been seen in one series, but it is not clear whether or not the renal compromise was due to hemoglobinuria.7
In our case, we elected to use the DVX catheter due to its ability to remove larger volumes of thrombus burden in large-diameter vessels (i.e., the IVC), potentially in a shorter amount of time. Indeed, a completely occluded IVC was significantly recanalized in one treatment session using the power-pulse spray technique and the DVX catheter. Although we achieved a very good result in reducing thrombus burden acutely, significant hemoglobinuria, manifesting as dark red urine, was encountered as a complication. The hemoglobinuria was quite obvious for 3 full days and was associated with progressive oliguria and a rapidly rising serum creatinine. Despite efforts to alkalinize the urine for better elimination of the hemoglobin and reduce the risk for nephropathy, progressive renal failure ensued and significantly prolonged the patient's hospitalization. We believe that the acute renal failure was a result of massive hemolysis related to mechanical thrombectomy. We can only speculate that utilization of the significantly more powerful DVX catheter may have contributed to an increase in the amount of red cell lysis and more significant hemoglobinuria. In addition, antegrade venous flow was rapidly established, resulting in the lysed red blood cell products becoming systemic in a shorter period of time. In contrast, a closed or persistently occluded venous system would maintain the by-products in a local environment so that follow-up traditional AngioJet therapy would remove a significant proportion of the lysed red blood cells and free hemoglobin.
Iodinated contrast media is also a potential nephrotoxic agent, and the patient underwent both a computed tomographic pulmonary angiogram and catheter-based procedure on the same day. However, the patient had no history of renal problems and was very well hydrated prior to the procedure. Her initial serum creatinine was normal. She did receive a total of 200 mL of nonionic contrast (her weight was 55 kg) in a 24-hour period, but it is unlikely that the amount of contrast used during the procedures is sufficient to cause contrast-induced nephrotoxicity in a patient with no history of an underlying renal problem and no risk factors. It is possible that cell lysis could have led to a sudden increase in serum uric acid levels leading to uric acid nephropathy. Unfortunately, no blood or urine uric acid assays were obtained. Lastly, the combination of contrast, hemoglobinuria, and increased levels of uric acid could have led to the development of nephropathy. In any event, it is our belief that the extent of red cell lysis and hemoglobinuria was a significant factor in the development of nephropathy. Renal failure due to hemolysis as a result of mechanical thrombectomy has not been clearly described in the literature but rather vaguely implied in some reports.7 Nazarian described the hemolytic effect of the Amplatz thrombectomy device in nine dogs and nine patients.9 He reported no change in renal function; however, he recommended caution in patients with underlying renal insufficiency.
Our case demonstrates the risk of inducing red cell lysis, significant hemoglobinuria, and acute renal failure with aggressive utilization of the AngioJet mechanical thrombectomy device, especially with the use of the more powerful DVX catheter. In addition to known complications such as bradycardia, hemorrhage, embolism, dissection, and perforation, the risk for developing acute renal failure should also be considered by the interventionalists using these devices. Application of these mechanical thrombectomy devices in large volumes of thrombus, as in the venous system, is more likely to result in this complication. Establishment of antegrade flow during initial power-pulse spraying also carries a higher risk for this complication, whereas in a closed system with no antegrade flow, the by-products of red cell lysis may be contained and subsequently removed during traditional thrombectomy.
In summary, we report a case in which hemolysis and hemoglobinuria following power-pulse spray mechanical thrombectomy with the Possis AngioJet may have led or significantly contributed to the development of acute oliguric renal failure in a patient with normal renal function and no apparent risk factors for the development of nephropathy. The effects of the hemoglobinuria on renal function could have been amplified by the use of iodinated contrast and the presence of a high concentration of serum uric acid, but the effect of hemoglobinuria was considered to be the primary culprit. Use of MTDs should be undertaken with caution in patients with underlying renal impairment while minimizing contrast volumes and maximizing patient hydration. Alkalinization of the urine with a sodium bicarbonate drip as a renal protective measure should also be employed when feasible.10 Lastly, the use of a MTD in a completely thrombosed venous segment in which there is no antegrade flow may be preferable to minimize the systemic distribution of the products of red cell lysis. Indeed, there is currently a MTD called Trellis (Bacchus Vascular, Santa Clara, CA) that provides a relatively closed environment for providing both mechanical thrombectomy and chemical lysis to minimize the systemic effects of the lytic agent and the by-products of cell lysis.11
ACKNOWLEDGMENT
The authors thank D. Laurie Persson, B.A., B.Sc., A.A.M., for his contributions in image preparation.
REFERENCES
- Dalen J E. Clinical diagnosis of acute pulmonary embolism: when should a V/Q scan be ordered? Chest. 1991;100:1185–1186. doi: 10.1378/chest.100.5.1185. [DOI] [PubMed] [Google Scholar]
- Mewissen M W, Seabrook G R, Meissner M H, et al. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211:39–49. doi: 10.1148/radiology.211.1.r99ap4739. [DOI] [PubMed] [Google Scholar]
- Fava M, Loyola S, Bertoni H, et al. Massive pulmonary embolism: percutaneous mechanical thrombectomy during cardiopulmonary resuscitation. J Vasc Interv Radiol. 2005;16:119–123. doi: 10.1097/01.RVI.0000146173.85401.BA. [DOI] [PubMed] [Google Scholar]
- Mewissen M W. Catheter-directed thrombolysis for lower extremity deep vein thrombosis. Tech Vasc Interv Radiol. 2001;4:111–114. doi: 10.1016/s1089-2516(01)90005-8. [DOI] [PubMed] [Google Scholar]
- Semba C P, Razavi M K, Kee S T, et al. Thrombolysis for lower extremity deep venous thrombosis. Tech Vasc Interv Radiol. 2004;7:68–78. doi: 10.1053/j.tvir.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Allie D E, Hebert C J, Lirtzman M D, et al. Novel simultaneous combination of chemical thrombolysis/rheolytic thrombectomy therapy for acute critical limb ischemia: the power-pulse spray technique. Catheter Cardiovasc Interv. 2004;63:512–522. doi: 10.1002/ccd.20216. [DOI] [PubMed] [Google Scholar]
- Cynamon J, Stein E G, Dym R J, et al. A new method for aggressive management of deep vein thrombosis: retrospective study of the power pulse technique. J Vasc Interv Radiol. 2006;17:1043–1049. doi: 10.1097/01.RVI.0000221085.25333.40. [DOI] [PubMed] [Google Scholar]
- Kasirajan K, Gray B, Ouriel K. Percutaneous AngioJet thrombectomy in the management of extensive deep venous thrombosis. J Vasc Interv Radiol. 2001;12:179–185. doi: 10.1016/s1051-0443(07)61823-5. [DOI] [PubMed] [Google Scholar]
- Nazarian G K, Qian Z, Coleman C C, et al. Hemolytic effect of Amplatz thrombectomy device. J Vasc Interv Radiol. 1994;5:155–160. doi: 10.1016/s1051-0443(94)71475-5. [DOI] [PubMed] [Google Scholar]
- Merten G J, Burgess W P, Gray L V, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328–2334. doi: 10.1001/jama.291.19.2328. [DOI] [PubMed] [Google Scholar]
- Ramaiah V, Del Santo P B, Rodriguez-Lopez J A, Gowda R G, Perkowski P E, Diethrich E B. Trellis thrombectomy system for the treatment of iliofemoral deep venous thrombosis. J Endovasc Ther. 2003;10:585–589. doi: 10.1177/152660280301000326. [DOI] [PubMed] [Google Scholar]