ABSTRACT
Percutaneous abscess drainage is one of the most common and rewarding procedures performed by interventional radiologists. Technical success is immediately apparent by aspiration of purulent contents and is nearly always achieved, with rates exceeding 90% in most literature studies. Clinical success is typical even for many abscesses colonized with multidrug-resistant organisms. In patients presenting with sepsis, this procedure offers an immediate and minimally invasive solution to a life-threatening condition, often resulting in defervescence and restoration of hemodynamic stability within 1 to 2 days. Although complications of abscess drainage are uncommon, radiologists should be able to recognize and treat all adverse sequelae discussed in this article.
Keywords: Abscess drainage, aspiration, complications, empyema, pseudocyst
In the past three decades, percutaneous fluid drainage has had a profound affect on the management of a critically ill patient population and is arguably the most important procedure performed by radiologists. This procedure markedly reduces morbidity and mortality compared with open surgical drainage1 by offering precise noninvasive localization of fluid collections, minimally invasive therapeutic techniques, avoidance of general anesthesia in most cases, and shortening of the hospital stay. Open surgical drainage is now reserved for cases where percutaneous drainage fails to control sepsis, fails to close fistulae, or is impossible due to the presence of interposed structures such as bowel. Although complications of percutaneous drainage are infrequent, the sequelae may be profound. This article will address the basics of percutaneous fluid drainage and catheter management as well as pearls for minimizing risks and managing associated complications.
CATHETER PLACEMENT AND MANAGEMENT
In this section, preventive measures to avoid periprocedural and delayed complications will be discussed. A proactive approach to patient preparation, procedural planning, and catheter management can markedly reduce the risk of complications.
Choosing an Imaging Modality for Guidance
An appropriate choice of imaging modality for guidance is a critical first step as it provides information regarding feasibility, appropriate technique, and associated risks for the purposes of informed consent and procedural planning. The choice depends on physician preference and comfort as well as anatomic factors such as the size of the collection and its location in relationship to the surrounding structures. Computed tomography (CT) is the preferred modality to identify the extent of abscesses and to plan the route of access as distinction of the fluid collection from the adjacent normal structures can be exquisitely enhanced by the administration of oral and intravenous contrast agents. As a result, CT is also advantageous for guiding drainage, particularly in cases in which collections are small and deep, in close proximity to vital structures, and located in regions that are difficult to access (e.g., the transgluteal route for pelvic drainage). In addition, CT fluoroscopy promises to increase accuracy and decrease procedure time.
Despite the advantages of CT, ultrasonography (US) provides several advantages and is the preferred primary modality for specific drainage procedures by many radiologists. US is usually immediately available, provides excellent visualization of larger and more superficial collections, and provides the best visualization of direct needle advancement, septations, loculations, adjacent vascular structures (via Doppler imaging), and many pelvic collections (via transrectal or transvaginal US). One of the inherent disadvantages is the inability of US to penetrate extensive overlying soft tissue or air-filled structures such as lung, bowel loops, or gas-filled collections.
Preparing the Patient
Patient preparation requires evaluation and normalization of coagulation status, administration of periprocedural, culture-specific, or broad-spectrum antibiotics, and evaluation of the patient for tolerance of conscious sedation. Conscious sedation is critical to reduce patient pain and anxiety, increase physician ease and comfort, and thereby decrease the risk of complications. In patients with an inability to tolerate conscious sedation due to pain, cardiopulmonary instability, or allergies to local anesthetic agents, general anesthesia is usually required. For some patients, the procedure may be performed with only local anesthetic as the risk of sedation or anesthesia may be greater than the risk of the procedure. This may be the case for patients with the combination of tenuous cardiopulmonary status and a very large, superficial fluid collection.
Performing the Procedure
Because of the risks of complications such as sepsis, bleeding, and oversedation, abscess drainage should be performed during active monitoring of heart rate, blood pressure, and oxygen saturation. Regardless of the imaging modality used for guidance, two techniques for catheter placement are used: the Seldinger technique and the trocar technique. The choice is often based on physician experience, preference, and comfort level.
Seldinger technique can be used for most indications and carries the advantage of verification of successful access into the collection and successful avoidance of adjacent structures prior to the creation of a large-bore tract. Access is gained using a 20- to 22-G needle, and localization is confirmed by imaging, contrast injection, and aspiration of purulent contents. Subsequently, a 0.018-inch wire is advanced into the cavity, a coaxial dilator is used to transition from the 0.018-inch to a 0.035-inch wire, serial fascial dilatation is performed to accommodate the size of the drainage catheter (at least one French size larger than the catheter), and a locking-loop pigtail catheter with a metal or plastic stiffener is advanced over the wire into the collection. The stiffener and wire are removed and the catheter is locked. The principal disadvantages of this technique are the risk of loss of access and cross-contamination while exchanging dilators, during which abscess contents have the potential to spread to the bloodstream or adjacent spaces. For example, in the case of pulmonary abscess drainage, seeding or damage to the pleural space can lead to pneumothorax or empyema during fascial dilatation.
Trocar technique is used routinely by some practitioners, although the authors reserve this technique for transvaginal, transrectal, and cholecystostomy catheter placement and for the drainage of larger, more superficial collections. In this technique, direct CT or sonographic guidance is used to access the fluid collection by advancing the combination of the drainage tube, a metal stiffening cannula, and a sharp stylet. This technique facilitates rapid drainage of the collection and minimal potential for spreading the infection due to the absence of serial fascial dilatation. The principal disadvantage is the direct advancement of a large-bore catheter and sharp stylet, which can have serious consequences in cases of inadvertent nontarget access.2
The catheter is secured to the skin with an adhesive device or suture, sterilely dressed, and drained to a suction bulb. Dressings can be changed during rounds or by the nursing staff. In patients at high risk for accidental catheter removal, the catheter should be secured directly to the skin with suture. Catheter position should be noted and marked if necessary to detect accidental withdrawal.
Managing Catheters
Our clinical colleagues appreciate attentiveness in the management of abscess catheters.3 Close patient follow-up after catheter placement is essential to the timely detection and treatment of delayed complications associated with chronic drainage. Up to the point of catheter removal, the radiology team should perform daily clinical rounds on the patient to monitor the catheter output, evaluate the skin access site for leakage or infection, and evaluate the patient for laboratory and clinical signs of complications or failure of percutaneous drainage.
Normal catheter function is indicated by a gradual tapering of fluid output on a daily basis. If output has precipitously diminished or ceased over a 24-hour period, the catheter should be flushed with minimal (3 to 5 mL) saline to ensure that it has not clogged. Cessation of output may necessitate further imaging followed by catheter flushing, exchanging, or upsizing if a fluid collection remains. Persistent large volumes of fluid output, output of tube feeds, or output of fecal matter may indicate a fistulous connection to an enteric structure such as stomach, small bowel, or colon. In these cases, an “abscessogram” under fluoroscopy may identify the fistulous connection. Sudden output of large volumes of bloody fluid may indicate erosion of the catheter or collection (or retraction of a catheter side hole) into an adjacent vessel. Immediate capping and possible fluoroscopically guided exchange or repositioning of the catheter may be indicated. Catheter removal may be considered after clinical signs of abscess resolution, including gradual reduction of catheter output to less than 10 mL in a 24-hour period, defervescence, and resolution of leukocytosis. Prior to removal, repeat evaluation by cross-sectional imaging may be indicated to confirm resolution of the fluid cavity.
RECOGNIZING AND TREATING COMPLICATIONS
Even the most talented and compulsive interventional radiologist will experience complications of abscess drainage, including complications of sedation, drug-related allergic reactions, cardiopulmonary complications, infectious complications, bleeding, and nontarget access. The goal should be to maintain a complication rate at or below standards reported in the literature.
Infection
Despite the administration of preprocedural broad-spectrum antibiotics, infectious complications occur and, for the sake of discussion, can be divided into two categories: those occurring during the primary catheter placement, and those occurring as a result of continued catheterization.
During primary catheter placement, inherent risks include spread of abscess contents to the adjacent spaces or organs, transient bacteremia, and frank sepsis. Spread to adjacent structures or the bloodstream can result from nontarget puncture (e.g., inadvertent puncture of the colon; Fig. 1) or from prolonged serial fascial dilatation, and every attempt should be made to complete dilatation and catheter placement in a timely fashion. As indicated by contrast enhancement by CT and hyperemia by Doppler sonography, the wall of an abscess is quite vascular and may be surrounded by displaced or invaded normal vascular structures. Despite all efforts to localize collections by imaging guidance, transient bacteremia results from the spillage of infected contents into the bloodstream in up to 5% of cases. Except for verification of cavity access and guidance of catheter placement, the authors limit contrast injection during primary drainage as it provides little additional information not already gleaned from the diagnostic CT or US. Overdistention of the cavity with contrast may cause the contents to spill into the bloodstream or surrounding spaces causing complications such as bacteremia or peritonitis. If a fistulous communication to bowel or other structures (Fig. 2) is suspected, an abscessogram can be performed after a period of fluid drainage and appropriate antibiotic coverage. When multiple fluid collections are present and percutaneous drainage is requested, the use of fresh sterile preparation of each collection is necessary to avoid cross-contamination.4 Clinical presentation of acute sepsis related to abscess drainage includes rapid escalation of fever, chills, rigors, and in severe cases, cardiopulmonary collapse. Because most abscess patients present with various levels of this constellation of symptoms, unnecessary escalation of morbidity may be underreported in the literature, and only a few deaths have been described. Nevertheless, sepsis should be anticipated before every drainage procedure, and supportive care should be administered immediately, starting with assessment of heart rate, blood pressure, and oxygen saturation followed by administration of appropriate cardiopulmonary supportive care, intravenous antibiotics, and Demerol to treat persistent rigors.
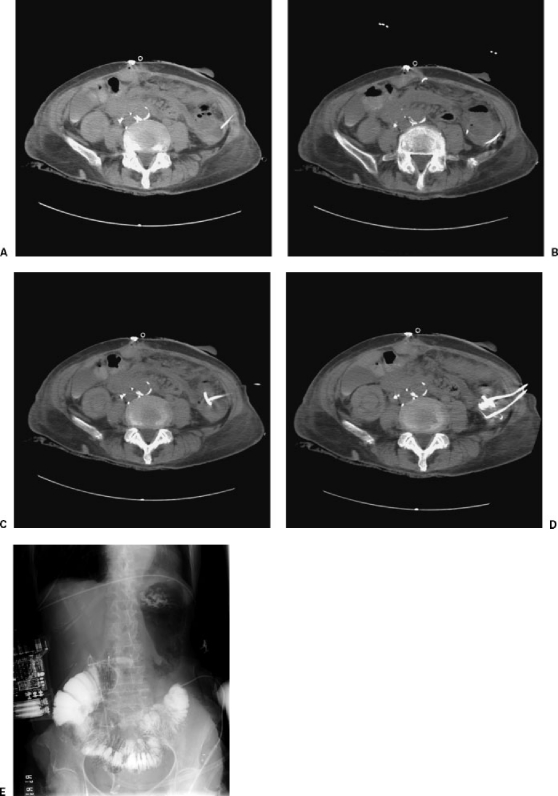
Figure 1.
Inadvertent transcolonic catheter placement. A 54-year-old woman presented for drainage of a diverticular abscess. (A) Preliminary CT showed localizing needle placed in fluid collection within the left paracolic gutter next to an adjacent bowel loop. (B) Wire successfully coiled within collection, avoiding bowel loop. (C) CT following catheter placement showed inadvertent placement within the bowel loop during serial dilatation and “blind” catheter placement. (D) Contrast injection confirmed catheter placement within the colon. A second catheter had been placed into the fluid collection. As this patient was a poor surgical candidate, the malpositioned catheter was left in place to allow a cutaneous fistula to develop. (E) Contrast injection of the enteric catheter demonstrated position within the colon. After the fluid collection was completely drained, the second catheter was removed. The originally malpositioned catheter was injected to rule out spillage of contents into the adjacent collapsed cavity. Following the development of a mature tract, the colonic catheter was removed.
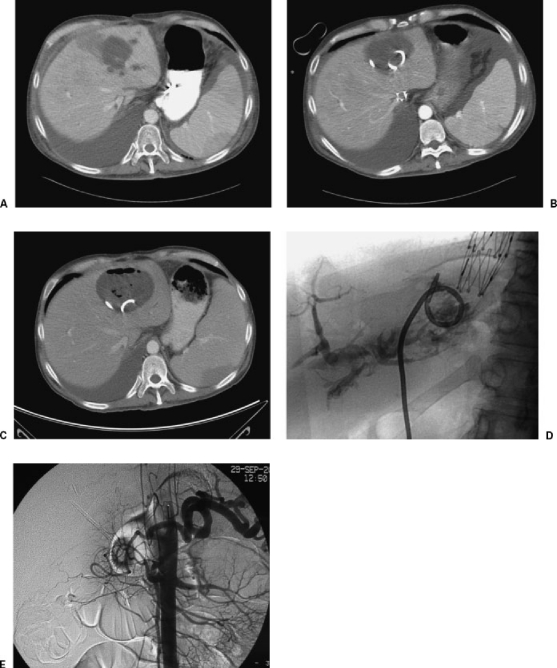
Figure 2.
Biliary communication to hepatic abscess. A 34-year-old man status post–liver transplant presented with fevers, chills, and a fluid collection within the liver. (A) CT showed left lobe fluid collection. (B) Successful drainage. (C) Two weeks postdrainage, the collection enlarged. (D) Abscessogram under fluoroscopy showed biliary communication. (E) Hepatic arteriogram showed absent flow in the hepatic artery resulting in biliary ischemia, abscess formation, and extended catheterization.
As a result of prolonged catheterization, local infections at the skin access site can occur. In such cases, if culture-specific or broad-spectrum antibiotic coverage and careful catheter management fail to resolve the skin infection, removal of the catheter and placement of a new catheter at a different site may be necessary, followed by appropriate wound care. In most cases, secondary skin infections heal with conservative management, but severe cases may require serial packing with iodoform gauze and wet-to-dry dressings, or even surgical management. Large, indurated, painful, or crepitant skin infections may be accompanied by an underlying abscess or necrotizing fasciitis and may require CT evaluation.
A final measure to prevent the inadvertent spread of infection during fluid drainage is the avoidance of nonsterile routes of drainage into potentially sterile collections, if possible. In some cases, diagnostic aspiration of a collection is recommended to establish that the contents of a cavity are infected prior to catheter placement through nonsterile routes (e.g., drainage of pelvic “cysts” or collections using the transvaginal or transrectal approach).
Bleeding
Fluid collections may be located behind, adjacent to, or surrounding vascular structures, within vascular structures such as the liver or spleen, or encased by a hypervascular, potentially angioinvasive wall of inflamed tissue. Therefore, abscess drainage carries an inherent risk of bleeding complications, including vessel laceration, pseudoaneurysm, and vascular fistula formation (Fig. 3). When bleeding occurs, it is important to remember to obtain follow-up imaging before moving or removing the instrument being introduced. Premature removal from a large vessel can result in catastrophic escalation in bleeding.2 In general, bleeding from small vessels is usually self-limited and can be managed conservatively with intravenous fluids, though some cases may require blood transfusion and observation in the intensive care unit. Temporary capping, upsizing, or repositioning of the catheter may tamponade acute bleeding, but persistent bleeding from small vessels, pseudoaneurysms, or fistulae may require treatment with transcatheter embolization. In the event of a large-vessel laceration, a surgical consult should be obtained as catastrophic cardiopulmonary collapse has been reported.2
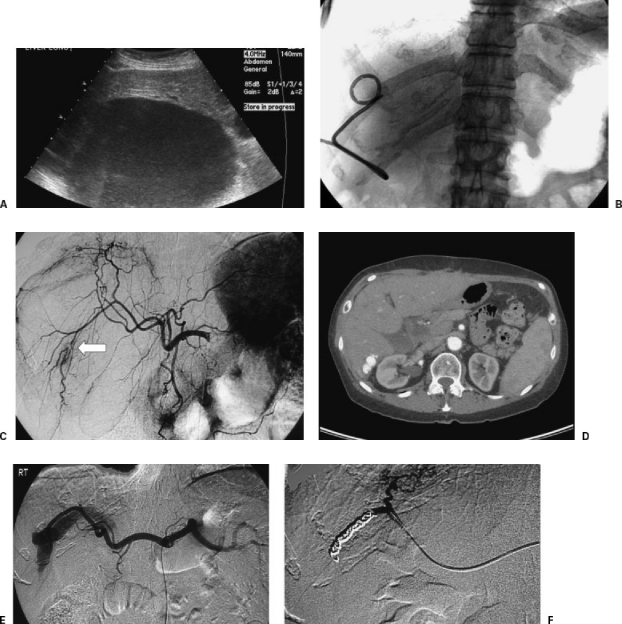
Figure 3.
Arterioportal fistula complicating drainage of a hepatic abscess. A 34-year-old woman with large, hepatic fluid collection presented for drainage. (A) Ultrasonography showed large hepatic fluid collection. (B) Catheter was placed using US guidance without any immediate complication. There was near complete resolution of the cavity by aspiration of the contents. Approximately 15 minutes after catheter placement, the patient became hypotensive and tachycardic. US imaging demonstrated echogenic contents within the previously drained abscess cavity. (C) Angiogram the same day showed segmental interruption of a right hepatic artery branch. In the absence of extravasation, no embolization was performed at this time. (D) Follow-up CT imaging 2 years later showed an arterioportal fistula in the same location. (E) The patient returned to the interventional radiology suite for right hepatic artery angiography, which clearly demonstrated the arterioportal fistula. (F) Following coil embolization, there was cessation of flow to the portal vein.
If the technical or clinical aspects of a case are associated with a high risk of bleeding despite preventive measures, the Seldinger technique may be preferred to perform initial access using a small needle rather than a large-bore catheter/stylet. In addition, a smaller catheter (6 to 8 French) may be advisable at the time of initial drainage if adequate evacuation can be achieved. The catheter can then be upsized after adequate tract formation. The use of Doppler ultrasound for imaging guidance is often helpful, even as an adjunct to CT-guided drainage, to avoid puncturing adjacent or involved vascular structures.
Nontarget Catheterization or Puncture
The majority of abscesses drained by the interventional radiology service are located within the abdomen, where nontarget catheterization can have disastrous clinical consequences. Inadvertent catheterization or traversal of the intestine, liver, spleen (Fig. 4), and stomach have all been reported. Prior to draining an abdominal abscess, administration of contrast into the mouth, rectum, or enteric stoma, depending on the location of the cavity to be drained, may minimize the risk of nontarget catheter placement. In our experience, puncture of bowel with a 21-G needle is inconsequential in the vast majority of cases. Inadvertent catheter placement through the small bowel can often be inconsequential and, when symptomatic, usually presents with high output of bowel contents from the catheter—easily recognized during clinical rounds. Alternatively, transenteric drainage may place the patient at risk for bowel obstruction. Inadvertent drainage through the colon (Fig. 2) places the patient at immediate risk for life-threatening peritonitis and sepsis and warrants immediate surgical consultation. In all cases of transenteric catheter placement, if peritoneal signs are not present, consideration can be given to bowel rest, supportive care, and a course of broad-spectrum antibiotics. Withdrawal of the catheter without surgery is possible after a mature tract has developed, which can be verified by contrast injection (“over-the-wire tractogram”). Slow withdrawal of the catheter over days to weeks is preferred by some practitioners to allow time for the enterocutaneous tract to close. If signs of peritonitis develop, emergent surgical consult is indicated.
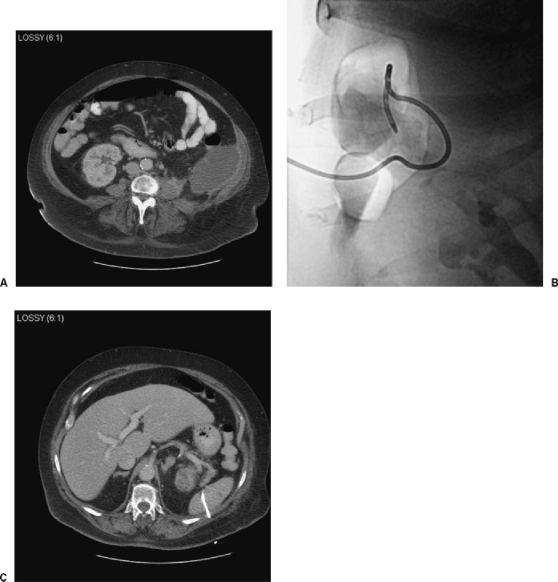
Figure 4.
Inadvertent splenic catheterization. A 52-year-old woman presented for drainage of a diverticular abscess. (A) CT showed an abscess within the left hemiabdomen. (B) After puncture by US guidance and catheter placement using Seldinger technique, fluoroscopy in the prone position showed catheter coiled within the left hemiabdomen. (C) CT showed the catheter coiling within the splenic capsule. Following successful placement of a second catheter and successful abscess drainage, the patient eventually underwent surgical removal of the nontarget catheter during a scheduled hemicolectomy for diverticulitis.
Catheter placement through solid parenchymal organs can result in parenchymal, subcapsular, or peritoneal bleeding, vascular fistula formation (Fig. 3), and pseudoaneurysm formation. These risks are minimal, as evidenced by literature studies describing percutaneous aspiration and drainage of abscesses located within the liver or spleen.5,6,7,8 Most cases of catheter placement through solid parenchymal organs of the abdomen can be confirmed by CT and treated by catheter removal following adequate tract formation or catheter removal with tract embolization using gelfoam pledgets. Rare cases require transarterial embolization or surgery to treat hemorrhagic complications.
DRAINAGE PROCEDURES BY LOCATION
Chest Drainage
Fluid collections in the chest include sterile pleural effusions, lung abscesses, empyemas, and mediastinal abscesses, and each of these entities has a unique set of associated considerations and potential complications. Despite the use of CT or fluoroscopic guidance, pneumothorax can occur in all forms of chest drainage. Depending on the size of the pneumothorax, management may include chest tube placement. Pneumothoraces that do not resolve despite proper chest tube placement may be the result of large pleural leaks or bronchopleural fistulas.
Pyogenic and fungal abscesses in the lung parenchyma can be drained percutaneously but often resolve with conservative management such as supportive care and appropriate antibiotic coverage selected after positive sputum or blood cultures. Therefore, in hemodynamically stable individuals responding to intravenous antibiotics, attempts at conservative management are typically indicated prior to percutaneous drainage. If patients worsen despite a trial of antibiotics, percutaneous drainage should be considered. The shortest trajectory to the collection that crosses the least number of pleural surfaces should be attempted to minimize the risk of pneumothorax and cross-contamination. Scant literature data are available for drainage of fungal collections in the chest but drainage of fungal collections often results in lower clinical success despite high technical success9 with poor outcomes predicted by collection complexity, history of malignancy, and admission to the intensive care unit.
Chronic empyema and organizing hemothorax are associated with a high risk of treatment failure after percutaneous drainage. Even with the use of large-bore catheters (14 French and greater) and suction drainage, chronic collections within the pleural space are often refractory to percutaneous drainage as they are often associated with high viscosity, septations, loculations, organizing clot, and bronchopleural fistula, which interferes with adequate suction. When catheter placement and aspiration alone fail to evacuate an empyema or subacute to chronic hemothorax, the contents may be too viscous or fibrotic to allow adequate drainage, and multidisciplinary cooperation may improve treatment outcome. In some cases of large, loculated empyemas and hemothoraces, thoracoscopic surgery decreases the rate of treatment failure and the length of hospital stay compared with catheter drainage.10 Alternatively, percutaneous catheter drainage and infusion of thrombolytic agents11,12,13,14 facilitates drainage by lysing the fibrinous septae and organizing blood clot. The authors instill 5 mg of recombinant tissue plasminogen activator in 40 mL of normal saline. The catheter is then capped and allowed to dwell for 2 to 3 hours, reopened, and allowed to drain, usually by bulb suction. This process can be repeated three to four times. Though literature regarding thrombolytic use in the pleural space is scant, complications related to the use of lytics have been presented in national meetings, including intracranial hemorrhage and other bleeding problems. Studies suggest that the complication rate is very low.15 If the collection persists despite the use of lytic agents, then surgical decortication is usually necessary. Reported complications specific to empyema drainage, in general, include bronchopleural fistula,11 and transgression of empyemas across the diaphragm or into the lung parenchyma has been described.16 In addition, mimickers of empyema such as diaphragmatic herniation of enteric structures should be excluded before catheter placement.17
Mediastinal abscesses usually result from cardiothoracic surgery and are typically covered by lung tissue or in close proximity to major vascular structures. The authors recommend the Seldinger technique under CT guidance, with the addition of Doppler US guidance if necessary. Injection of sterile saline to displace any intervening pleura and lung tissue may help avoid the complication of pneumothorax.
Fluid Collections within the Abdomen
Abscesses of the spleen and liver have been successfully drained percutaneously in published series with a low rate of complications. Perhaps the least literature precedent exists for splenic drainage. In the past, a high rate of treatment failure has been reported for percutaneous treatment of splenic abscesses, possibly resulting from the use of smaller-bore catheters or needle aspiration alone to avoid a theoretically high risk of bleeding complications.18 Limited series demonstrate that this procedure can be performed with a low or absent risk of bleeding.5,8,19
Fluid collections within the liver parenchyma can be drained with an ~4% risk of major complications and a 1 to 2% risk of minor complications. Although some radiologists have advocated needle aspiration rather than catheter drainage,20 a direct comparison of the two techniques shows improved cure rate and reduction of the abscess cavity with catheter drainage.21 Major complications have included hepatocolic fistula creation,22 sepsis due to communication of the abscess cavity with hepatic vascular structures,23 and death by biliary peritonitis.7 The most common minor complication is treatment failure resulting in repeat drainage or surgery and occurring in 16 to 18.5% of cases.20,24 Biliary communication may prolong catheterization, particularly in liver transplants with diminished hepatic arterial perfusion (Fig. 2), but does not preclude percutaneous drainage as a primary treatment.6,25,26 Rare complications include bleeding, sepsis, hemobilia, and arterioportal fistula formation (Fig. 3). Catheter drainage and scolicidal infusion of hydatid cysts has been described in large series27 with a risk of only minor complications, including secondary infection of the cyst, nausea, and minor skin reactions. For amebic abscesses, complications are uncommon and minor.28
Abdominal fluid collections resulting from perforated viscus due to such entities as Crohn's disease, previous operation, diverticulitis, and appendicitis can be drained with a less than 5% incidence of complications, as evidenced by large published series.29,30,31 The general complications of nontarget embolization (Fig. 1), bleeding, and infection described earlier in this article may occur infrequently, with an incidence of less than 3%. High rates of sepsis were reported in older studies,32 likely reduced secondary to improved antibiotic coverage, catheter design, and techniques for imaging guidance. Approaches to subphrenic collections include extrapleural and transpleural drainage; the latter is associated with a slightly higher rate of complications such as empyema and pneumothorax33 and should be reserved as a route of last resort. Patients with perforated appendicitis may benefit from catheter drainage to delay appendectomy until clinical presentation has improved; however, in the acute setting (typically less than 5 days), the first-line treatment of perforated appendicitis with peritoneal signs is surgical.34
Pancreatic, Renal, and Other Retroperitoneal Collections
Pancreatic collections resulting from pancreatitis or pancreatic duct injury are associated with a high incidence of treatment failure when drained by external radiological drainage.35 In cases involving ductal injury with direct communication to a pseudocyst, many surgeons are adamant about performing surgery as a first-line treatment to avoid delays and complications associated with percutaneous drainage, such as persistent enteric or cutaneous fistula formation. Nevertheless, literature precedent exists for careful selection of patients to provide the most appropriate treatment option. Patients with mature, homogeneous collections without communication to the duct by endoscopic or magnetic resonance pancreatography and patients with infected pseudocysts or frank abscesses typically benefit from percutaneous drainage as a first-line treatment.36 Patients with ductal involvement or thick, viscous collections (including necrosis) may benefit from surgical treatments such as debridement and cyst enterostomy.37 Mature pseudocysts with direct apposition to the gastric wall can be treated with endoscopic cystgastrostomy catheter placement. Reported complications of nonselective radiological and endoscopic management include hemorrhage, sepsis, persistent enteric or cutaneous fistula, tract infection, and a higher rate of mortality compared with open surgical management,37,38,39 though careful selection can markedly reduce the risk of these complications. In a large series of carefully selected pancreatic abscesses, van Sonnenberg demonstrated 86% cure rate with percutaneous drainage and antibiotic coverage, with non-life-threatening bleeding in 5% and spontaneous gastrointestinal fistula formation in 17%.36
Renal and perinephric fluid collections are a medical emergency that, despite aggressive surgical drainage, may result in a high mortality rate, as high as 56% in older studies.40 Despite antibiotics and drainage using both percutaneous and surgical techniques, the mortality rate in more recent studies has remained over 12%41 even in the most optimistic studies. Percutaneous drainage results in treatment failure requiring surgery, including nephrectomy, in over 30% of cases. Despite this finding, early recognition, percutaneous drainage of larger collections (over 3 cm), and vigorous follow-up is critical to patient survival, including catheter manipulations and changes to maintain drainage and aggressive surgical intervention when such efforts fail to produce an effective clinical response.41,42 Iliopsoas abscesses can be safely drained with a low-risk of periprocedural complications but a risk of treatment failure exceeding 30% in some studies.43 The risk of treatment failure or prolonged catheterization increases with tuberculous collections and viscous, debris-laden collections involving adjacent muscle necrosis.
Deep Pelvic Drainage
Abscesses that are deep in the pelvis are often technically challenging and require carefully planning. The sacrum, innominate bones, iliac crests, urinary bladder, and multiple bowel loops may be in the direct path of the abscess, and dense presence of pelvic vasculature further complicates the choice of route of access. Options include transgluteal catheter placement under CT guidance and transrectal or transvaginal catheter placement under US guidance.
From the transgluteal approach, there are a few landmarks that must be regarded to avoid complications.44 Entering the abscess as close to the sacrum or coccyx as possible avoids the sciatic nerve and sacral plexus, which may cause significant pain and paresthesia. Also, percutaneous access below the level of the piriformis muscle minimizes the complication of uncontrolled pain.44,45,46 When pain is not considered, the complication rate is as low as 2%,45 most commonly hemorrhage. Pseudoaneurysm of the inferior gluteal artery has been described as a consequence of transgluteal drainage,45,46 and the use of Seldinger rather than Trocar technique may minimize this risk by maximizing accuracy.44
Abscesses in close proximity to the rectum or vaginal cuff typically require drainage via the transrectal or transvaginal approach using US guidance.47,48,49 Using trocar technique, a drainage catheter can be advanced through a sheath attached to the ultrasound probe. Complications are rare, described in under 3% of cases in many published series. The most frequent complication of this type of placement is catheter dislodgement, as there is usually no ideal location to secure the catheter. Compared with transvaginal drainage, there is significantly less procedural pain, lifestyle disruption, and time of catheterization after transrectal drainage.50 A complication of transrectal catheter placement is introducing colonic flora to a potentially sterile fluid collection. For deep pelvic collections that may be sterile, avoidance of superinfection is an important consideration in procedural planning if drainage or aspiration is requested. Consideration should be given first to preliminary aspiration with a 21-G needle through a sterile route to verify the need for drainage. Alternatively, drainage via the transgluteal route can be considered as a first option, followed by the transvaginal route, and finally the transrectal route in this circumstance. Depending on the clinical scenario, a full course of prophylactic antibiotics may be beneficial after accessing a sterile collection from the transrectal or transvaginal approach.
CONCLUSION
Percutaneous fluid drainage is a routine procedure best performed in the hands of experienced radiologists with the availability of the complete range of imaging modalities for guidance. With adequate planning, careful technique, and thorough patient follow-up, the risks and consequences of complications can be minimized.
REFERENCES
- Brolin R E, Nosher J L, Leiman S, Lee W S, Greco R S. Percutaneous catheter versus open surgical drainage in the treatment of abdominal abscesses. Am Surg. 1984;50:102–108. [PubMed] [Google Scholar]
- Mueller P R, Berlin L. Complications of lung abscess aspiration and drainage. AJR Am J Roentgenol. 2002;178:1083–1086. doi: 10.2214/ajr.178.5.1781083. [DOI] [PubMed] [Google Scholar]
- Maher M M, Kealey S, McNamara A, O'Laoide R, Gibney R G, Malone D E. Management of visceral interventional radiology catheters: a troubleshooting guide for interventional radiologists. Radiographics. 2002;22:305–322. doi: 10.1148/radiographics.22.2.g02mr20305. [DOI] [PubMed] [Google Scholar]
- Heneghan J P, Everts R J, Nelson R C. Multiple fluid collections: CT- or US-guided aspiration-evaluation of microbiologic results and implications for clinical practice. Radiology. 1999;212:669–672. doi: 10.1148/radiology.212.3.r99se25669. [DOI] [PubMed] [Google Scholar]
- Chou Y H, Hsu C C, Tiu C M, Chang T. Splenic abscess: sonographic diagnosis and percutaneous drainage or aspiration. Gastrointest Radiol. 1992;17:262–266. doi: 10.1007/BF01888563. [DOI] [PubMed] [Google Scholar]
- Do H, Lambiase R E, Deyoe L, Cronan J J, Dorfman G S. Percutaneous drainage of hepatic abscesses with and without intrahepatic biliary communication. AJR Am J Roentgenol. 1991;157:1209–1212. doi: 10.2214/ajr.157.6.1719787. [DOI] [PubMed] [Google Scholar]
- Tazawa J, Sakai Y, Maekawa S, et al. Solitary and multiple pyogenic liver abscess: characteristics of the patients and efficacy of percutaneous drainage. Am J Gastroenterol. 1997;92:271–274. [PubMed] [Google Scholar]
- Gasparini D, Basadonna P T, DiDonna A. Splenic abscesses: their percutaneous treatment and the role of the interventional radiologist. Radiol Med (Torino) 1994;87:803–807. [PubMed] [Google Scholar]
- Varghese J C, Hahn P F, Harisinghani M G, et al. Fungus-infected fluid collections in thorax or abdomen: effectiveness of percutaneous catheter drainage. Radiology. 2005;236:730–738. doi: 10.1148/radiol.2362031044. [DOI] [PubMed] [Google Scholar]
- Coote N, Kay E. Surgical versus non-surgical management of pleural empyema. Cochrane Database Syst Rev. 2005;19:CD001956. doi: 10.1002/14651858.CD001956.pub2. [DOI] [PubMed] [Google Scholar]
- Basile A, Boullosa-Seoane E, Dominguez Viguera L, et al. Intrapleural fibrinolysis in the management of empyemas and haemothoraces: our experience. Radiol Med (Torino) 2003;105:12–16. [PubMed] [Google Scholar]
- Misthos P, Sepsas E, Konstantinou M, Athanassiadi K, Skottis I, Lioulias A. Early use of intrapleural fibrinolytics in the management of postpneumonic empyema: a prospective study. Eur J Cardiothorac Surg. 2005;28:599–603. doi: 10.1016/j.ejcts.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Gates R L, Hogan M, Weinstein S, Area M J. Drainage, fibrinolytics, or surgery: a comparison of treatment options in pediatric empyema. J Pediatr Surg. 2004;39:1638–1642. doi: 10.1016/j.jpedsurg.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Tasci S, Burghard A, Schafer H, Rabe C, Ewig S, Luderitz B. Long-term outcome of intrapleural fibrinolytic therapy via small bore catheter drainage in the management of complicated parapneumonic effusion and empyema: a case series. Med Klin (Munich) 2005;100:181–185. doi: 10.1007/s00063-005-1019-z. [DOI] [PubMed] [Google Scholar]
- Lahorra J M, Haaga J R, Stellato T, Flanigan T, Graham R. Safety of intracavitary urokinase with percutaneous abscess drainage. AJR Am J Roentgenol. 1993;160:171–174. doi: 10.2214/ajr.160.1.8416619. [DOI] [PubMed] [Google Scholar]
- Romijn S, Sturm M, der Schelling G van. Transphrenic fistulization of a subphrenic abscess to lung parenchyma. J Gastrointest Surg. 2005;9:716–717. doi: 10.1016/j.gassur.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Tsang J, Ryan F. Delayed diaphragmatic herniation masquerading as a complicated parapneumonic effusion. Can Respir J. 1999;6:361–366. doi: 10.1155/1999/357295. [DOI] [PubMed] [Google Scholar]
- Quinn S F, Sonnenberg E van, Cassola G, Wittich G R, Neff C. Interventional radiology in the spleen. Radiology. 1986;161:289–291. doi: 10.1148/radiology.161.2.3763890. [DOI] [PubMed] [Google Scholar]
- Lucey B C, Boland G W, Maher M M, Hahn P F, Gervais D A, Mueller P R. Percutaneous nonvascular splenic intervention: a 10-year review. AJR Am J Roentgenol. 2002;179:1591–1596. doi: 10.2214/ajr.179.6.1791591. [DOI] [PubMed] [Google Scholar]
- Baek S Y, Lee M G, Cho K S, Lee S C, Sung K B, Auh Y H. Therapeutic percutaneous aspiration of hepatic abscesses: effectiveness in 25 patients. AJR Am J Roentgenol. 1993;160:799–802. doi: 10.2214/ajr.160.4.8456667. [DOI] [PubMed] [Google Scholar]
- Rajak C L, Gupta S, Jain S, Chawla Y, Gulati Y, Suri S. Percutaneous treatment of liver abscesses: needle aspiration versus catheter drainage. AJR Am J Roentgenol. 1998;170:1035–1039. doi: 10.2214/ajr.170.4.9530055. [DOI] [PubMed] [Google Scholar]
- Satoh H, Matsuyama S, Mashima H, Imoto A, Hidaka K, Hisatsugu T. A case of hepatocolic fistula after percutaneous drainage for a gas-containing pyogenic liver abscess. J Gastroenterol. 1994;29:782–785. doi: 10.1007/BF02349288. [DOI] [PubMed] [Google Scholar]
- Chung Y F, Tay K H, Stan B, et al. Percutaneous drainage of liver abscess complicated by hepatovenous fistula. Singapore Med J. 2003;44:299–301. [PubMed] [Google Scholar]
- Bissada A A, Bateman J. Pyogenic liver abscess: a 7-year experience in a large community hospital. Hepatogastroenterology. 1991;38:317–320. [PubMed] [Google Scholar]
- Sugiyama M, Atomi Y. Pyogenic hepatic abscesses with biliary communication. Am J Surg. 2002;183:205–208. doi: 10.1016/s0002-9610(01)00869-8. [DOI] [PubMed] [Google Scholar]
- Bayraktar Y, Arslan S, Sivri B, et al. Percutaneous drainage of hepatic abscesses: therapy does not differ for those with identifiable biliary fistula. Hepatogastroenterology. 1996;43:620–626. [PubMed] [Google Scholar]
- Bosanac Z B, Lisanin L. Percutaneous drainage of hydatid cyst in the liver as a primary treatment: review of 52 consecutive cases with long-term follow-up. Clin Radiol. 2000;55:839–848. doi: 10.1053/crad.2000.0543. [DOI] [PubMed] [Google Scholar]
- Saraswat V A, Agarwal D K, Baijal S S, et al. Percutaneous catheter drainage of amoebic liver abscess. Clin Radiol. 1992;45:187–189. doi: 10.1016/s0009-9260(05)80639-7. [DOI] [PubMed] [Google Scholar]
- Flancbaum L, Nosher J L, Brolin R E. Percutaneous catheter drainage of abdominal abscesses associated with perforated viscus. Am Surg. 1990;56:52–56. [PubMed] [Google Scholar]
- Casola G, Sonnenberg E van, Neff C C, Saba R M, Withers C, Emarine C W. Abscesses in Crohn disease: percutaneous drainage. Radiology. 1987;163:19–22. doi: 10.1148/radiology.163.1.3823434. [DOI] [PubMed] [Google Scholar]
- Neff C C, Sonnenberg E van, Casola G, et al. Diverticular abscesses: percutaneous drainage. Radiology. 1987;163:15–18. doi: 10.1148/radiology.163.1.3823429. [DOI] [PubMed] [Google Scholar]
- Gerzof S G, Robbins A H, Johnson W C, Birkett D H, Nabseth D C. Percutaneous catheter drainage of abdominal abscesses: a five-year experience. N Engl J Med. 1981;305:653–657. doi: 10.1056/NEJM198109173051201. [DOI] [PubMed] [Google Scholar]
- McNicholas M M, Mueller P R, Lee M J, et al. Percutaneous drainage of subphrenic fluid collections that occur after splenectomy: efficacy and safety of transpleural versus extrapleural approach. AJR Am J Roentgenol. 1995;165:355–359. doi: 10.2214/ajr.165.2.7618556. [DOI] [PubMed] [Google Scholar]
- Nadler E P, Reblock K K, Vaughan K G, Meza M P, Ford H R, Gaines B A. Predictors of outcome for children with perforated appendicitis initially treated with non-operative management. Surg Infect (Larchmt) 2004;5:349–356. doi: 10.1089/sur.2004.5.349. [DOI] [PubMed] [Google Scholar]
- Gervais D A, Hahn P F, O'Neill M J, Mueller P R. Percutaneous abscess drainage in Crohn disease: technical success and short- and long-term outcomes during 14 years. Radiology. 2002;222:645–651. doi: 10.1148/radiol.2223010554. [DOI] [PubMed] [Google Scholar]
- Sonnenberg E van, Wittich G R, Chon K S, et al. Percutaneous radiologic drainage of pancreatic abscesses. AJR Am J Roentgenol. 1997;168:979–984. doi: 10.2214/ajr.168.4.9124154. [DOI] [PubMed] [Google Scholar]
- Heider R, Meyer A A, Galanko J A, Behrns K E. Percutaneous drainage of pancreatic pseudocysts is associated with a higher failure rate than surgical treatment in unselected patients. Ann Surg. 1999;229:781–789. doi: 10.1097/00000658-199906000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon W H, Walser E. Surgical management of complications associated with percutaneous and/or endoscopic management of pseudocyst of the pancreas. Ann Surg. 2005;241:948–957. doi: 10.1097/01.sla.0000164737.86249.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D B, Anderson M C. Percutaneous catheter drainage compared with internal drainage in the management of pancreatic pseudocyst. Ann Surg. 1992;215:571–576. doi: 10.1097/00000658-199206000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatierra O, Jr, Bucklew W B, Morrow J W. Perinephric abscess: a report of 71 cases. J Urol. 1967;98:296–302. doi: 10.1016/S0022-5347(17)62874-X. [DOI] [PubMed] [Google Scholar]
- Meng M V, Mario L A, McAninch J W. Current treatment and outcomes of perinephric abscesses. J Urol. 2002;168:1337–1340. doi: 10.1016/S0022-5347(05)64443-6. [DOI] [PubMed] [Google Scholar]
- Lang E K, Paolini R M, Pottmeyer A. The efficacy of palliative and definitive percutaneous versus surgical drainage of pancreatic abscesses and pseudocysts: a prospective study of 85 patients. South Med J. 1991;84:55–64. doi: 10.1097/00007611-199101000-00014. [DOI] [PubMed] [Google Scholar]
- Gupta S, Suri S, Gulati M, Singh P. Ilio-psoas abscesses: percutaneous drainage under image guidance. Clin Radiol. 1997;52:704–707. doi: 10.1016/s0009-9260(97)80036-0. [DOI] [PubMed] [Google Scholar]
- Woo J KH, Millward S F, Harisinghani M. Transgluteal approach for percutaneous drainage of deep pelvic abscesses: how to avoid injury to vital structures. Radiology. 2004;233:300–302. doi: 10.1148/radiol.2331040381. [DOI] [PubMed] [Google Scholar]
- Harisinghani M G, Gervais D A, Maher M M, et al. Transgluteal approach for percutaneous drainage of deep pelvic abscesses: 154 cases. Radiology. 2003;228:701–705. doi: 10.1148/radiol.2283020924. [DOI] [PubMed] [Google Scholar]
- Harisinghani M G, Gervais D A, Hahn P F, et al. CT-guided transgluteal drainage of deep pelvic abscesses: indications, technique, procedure-related complications, and clinical outcome. Radiographics. 2002;22:1353–1367. doi: 10.1148/rg.226025039. [DOI] [PubMed] [Google Scholar]
- Bennett J D, Kozak R I, Taylor B M, Jory T A. Deep pelvic abscesses: transrectal drainage with radiologic guidance. Radiology. 1992;185:825–828. doi: 10.1148/radiology.185.3.1438770. [DOI] [PubMed] [Google Scholar]
- Sonnenberg E van, D'Agostino H B, Casola G, Goodacre B W, Sanchez R B, Taylor B. US-guided transvaginal drainage of pelvic abscesses and fluid collections. Radiology. 1991;181:53–56. doi: 10.1148/radiology.181.1.1887056. [DOI] [PubMed] [Google Scholar]
- O'Neill M J, Rafferty E A, Lee S I, et al. Transvaginal interventional procedures: aspiration, biopsy, and catheter drainage. Radiographics. 2001;21:657–672. doi: 10.1148/radiographics.21.3.g01ma20657. [DOI] [PubMed] [Google Scholar]
- Hovsepian D M, Steele J R, Skinner C S, Malden E S. Transrectal versus transvaginal abscess drainage: survey of patient tolerance and effect on activities of daily living. Radiology. 1999;212:159–163. doi: 10.1148/radiology.212.1.r99jl23159. [DOI] [PubMed] [Google Scholar]